Abstract
Background
Gene polymorphisms that affect serotonin signaling modulate reactivity to salient stimuli and risk for emotional disturbances. Here, we hypothesized that these serotonin genes, which have been primarily explored in depressive disorders, could also have important implications for drug addiction, with the potential to reveal important insights into drug symptomatology, severity, and/or possible sequelae such as dysphoria.
Methods
Using an imaging genetics approach, the current study tested in 62 cocaine abusers and 57 healthy controls the separate and combined effects of variations in the serotonin transporter (5-HTTLPR) and monoamine oxidase A (MAOA) genes on processing of aversive information. Reactivity to standardized unpleasant images was indexed by a psychophysiological marker of stimulus salience (i.e., the late positive potential (LPP) component of the event-related potential) during passive picture viewing. Depressive symptomatology was assessed with the Beck Depression Inventory (BDI).
Results
Results showed that, independent of diagnosis, the highest unpleasant LPPs emerged in individuals with MAOA-Low and at least one ‘Short’ allele of 5-HTTLPR. Uniquely in the cocaine participants with these two risk variants, higher unpleasant LPPs correlated with higher BDI scores.
Conclusions
Taken together, these results suggest that a multilocus genetic composite of monoamine signaling relates to depression symptomatology through brain function associated with the experience of negative emotions. This research lays the groundwork for future studies that can investigate clinical outcomes and/or pharmacogenetic therapies in drug addiction and potentially other psychopathologies of emotion dysregulation.
Keywords: cocaine addiction, imaging genetics, depression, comorbidity, 5-HTTLPR, MAOA, event-related potentials
1. INTRODUCTION
Gene polymorphisms that modulate serotonin signaling may increase susceptibility to multiple psychopathologies marked by heightened emotional reactivity and poor affect regulation (Buckholtz and Meyer-Lindenberg, 2012). These symptoms characterize both drug addiction and major depression, highly comorbid psychiatric illnesses (Martins and Gorelick, 2011) that exhibit shared perturbations in brain regions and circuits mediating emotional regulation (Bogdan et al., 2013; Goldstein and Volkow, 2011). Of the candidate serotonin-associated genes that modulate serotonin neurotransmission and could influence emotional dysregulation in addiction, two genes likely to play prominent roles include those encoding the serotonin transporter (SLC6A4) and monoamine catabolic enzyme monoamine oxidase A (MAOA). The commonly studied risk variants in both genes are believed to exert their effects by modulating serotonin clearance from the synapse (Buckholtz and Meyer-Lindenberg, 2008, 2012; Cools et al., 2008). These include a functional insertion-deletion polymorphism (i.e., sequence variation) of the SLC6A4 promoter (5-HTTLPR), which produces “short” (S) and “long” (L) alleles and has been linked to depression (Kenna et al., 2012); and the repeat polymorphism (uVNTR, i.e., variable number of tandem repeats) upstream of the MAOA promoter, which produces common alleles with high activity (MAOA-H) and low activity (MAOA-L) and has been linked to impulsive aggression (Buckholtz and Meyer-Lindenberg, 2008) and depression (Fan et al., 2010).
Importantly, both of these polymorphisms modulate emotional reactivity, including responsiveness to aversive stimuli and experiences. In studies of 5-HTTLPR, study groups are often analyzed based on the presence of at least one S-allele. For example, compared with individuals homozygous for the L-allele, carriers of at least one 5-HTTLPR S-allele show increased startle response to noise bursts (Brocke et al., 2006). S-allele individuals also allocate more attention to fear-provoking stimuli (e.g., spiders) (Osinsky et al., 2008) and negative words (Kwang et al., 2010), and show a decreased ability to disengage attention from such stimuli (Beevers et al., 2009). A subsequent meta-analysis confirmed the association between the S-allele and attention bias to aversive stimuli (Pergamin-Hight et al., 2012). Neurally, S-allele carriers have enhanced event-related potential (ERP) responsiveness to unpleasant images (Herrmann et al., 2007) and enhanced functional magnetic resonance imaging (fMRI) response in the amygdala to aversive stimuli (meta-analysis: Murphy et al., 2013). Similarly, MAOA-L individuals show increased reactivity during aversive experiences, for example behaving more aggressively following provocation (Kuepper et al., 2013; McDermott et al., 2009) and showing greater dorsal anterior cingulate cortex activity (ACC) following social exclusion (Eisenberger et al., 2007). MAOA also modulates ERP reactivity (Williams et al., 2009) and fMRI activity in the amygdala and ACC (Alia-Klein et al., 2009; Lee and Ham, 2008; Meyer-Lindenberg et al., 2006) during the presentation of emotional faces and words. More recent research has aggregated these polymorphisms, thereby examining 5-HTTLPR and MAOA polygenic liability [defined as the aggregate burden of deleterious alleles harbored in each individual genome (Buckholtz and Meyer-Lindenberg, 2012)]. For example, the combined effects of 5HTTLPR-MAOA in interaction with negative life events increased risk for depression in adolescence (Priess-Groben and Hyde, 2013). In addition, 5-HTTLPR and MAOA interacted to modulate fMRI signal in the subgenual ACC during a go/no-go task in health (Passamonti et al., 2008).
The goal of the current imaging-genetics study was to test whether these two serotonin gene polymorphisms modulate emotional reactivity in individuals with drug addiction, with whom these gene polymorphisms were previously associated (Bacher et al., 2011; Cao et al., 2013; Ehlers and Gizer, 2013; Fowler et al., 1996; Kenna et al., 2012). More specifically, we tested the separate and combined effects of 5-HTTLPR and MAOA on ERP-measured reactivity to unpleasant stimuli in individuals with cocaine use disorder (CUD) and healthy controls. Furthermore, to explore the possible clinical significance of these findings, we also tested whether such enhanced reactivity relates to higher depression symptomatology and/or cocaine use. Our primary ERP component of interest was the a priori defined late positive potential (LPP), thought to index stimulus salience (Hajcak et al., 2013, 2010; Hajcak and Olvet, 2008; Weinberg and Hajcak, 2010) and shown to be altered during passive picture viewing in CUD (Dunning et al., 2011). Drawing on the literature of these genes in healthy controls as described above, we hypothesized that (A) individuals with at least one 5-HTTLPR S-allele and/or MAOAL would show higher LPP response to aversive images. We additionally hypothesized that (B) such reactivity would correlate with higher depression symptomatology and/or cocaine use especially in the individuals with higher monoamine polygenic liability, who presumably are at higher risk for reactivity to unpleasant stimuli.
2. METHODS
2.1 Participants
Sixty-two CUD and 57 healthy controls, recruited through advertisements, local treatment facilities, and word of mouth, participated in this research. All provided written informed consent to participate in the study in accordance with the Stony Brook University Institutional Review Board. Exclusion criteria were: (A) head trauma (with a loss of consciousness for more than 30 min); (B) any psychiatric, medical, or neurological disorder requiring hospitalization or regular monitoring [except for highly frequently comorbid disorders in CUD, inclusive of additional substance use disorders, post-traumatic stress disorder (PTSD), and depression (with the latter being especially appropriate given our hypotheses)]; (C) current use of psychoactive medications (i.e., within the last six months); (D) current or past history of substance use disorder in the healthy controls (other than nicotine); and (E) positive urine screens for drugs of abuse (other than cocaine in CUD; any positive urine screens in controls).
All participants underwent a comprehensive clinical interview inclusive of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 1996; Ventura et al., 1998); (B) Addiction Severity Index (ASI; McLellan et al., 1992). (For complete description of this interview, see Supplementary Material 1). This interview determined that all 62 CUD met criteria for current cocaine dependence, 36 of whom tested positive for cocaine in urine (indicating use within 72 hours prior to the study). (For current and past psychiatric comorbidities, see Supplementary Material 2). Importantly, however, cocaine urine status did not differ by genotype (Table 1), and no participants were acutely intoxicated while performing the study procedures; these considerations broadly speak against a potential confounding influence of recent drug use on our results (but see Supplementary Materials for additional exploration of this variable 3). We also used the clinical interview, specifically the traumatic events section from the PTSD module of the SCID and the emotional/physical/sexual abuse section of the ASI, to explore for potential interactions of 5-HTTLPR and MAOA with stressful and traumatic life events (Caspi et al., 2002; Caspi et al., 2003; Karg et al., 2011). (For results of these analyses, which did not reveal any significant effects, see Supplementary Material4). Study groups were generally well-matched demographically, only differing on history of cigarette smoking (Table 1) for which we controlled in the analyses. Although race did not differ as a function of genotype and diagnosis (Table 1), we nonetheless also controlled for this variable because of the potential for population stratification in the current sample (Cardon and Palmer, 2003). Depression symptomatology, which was measured with the Beck Depression Inventory (BDI; Beck, 1996) and differed between the groups as expected (Table 1), was a key variable of interest (not a covariate).
Table 1.
Demographics and drug use of all study subjects as a function of diagnosis and number of monoaminergic risk variants (5-HTTLPR S-allele and/or MAOA-L).
| Cocaine | Control | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Statistic X2, F, or H | 0 Risk N=14 | 1 Risk N=25 | 2 Risk N=21 | 0 Risk N=13 | 1 Risk N=23 | 2 Risk N=14 |
| Gender (Male/Female) | 3.5 | 12/2 | 24/1 | 17/4 | 12/1 | 22/2 | 13/1 |
| Self-Reported Race (African-American/Other) | 10.6 | 11/3 | 17/8 | 14/7 | 10/3 | 10/14 | 12/2 |
| History of Cigarette Smoking (Current or Past/Never) | 27.7*** | 12/2d,e,f | 18/7d,e,f | 14/7d,e,f | 2/11a,b,c | 8/16a,b,c | 3/11a,b,c |
| Cigarettes Per Day (Among Current Smokers: N=57) | 0.5 | 9.6 ± 5.9 | 6.7 ± 6.1 | 8.5 ± 4.6 | 10.0 ± 0.0 | 7.6 ± 6.8 | 5.0 ± 7.1 |
| Education (Years) | 1.1 | 12.6 ± 1.2 | 12.5 ± 1.3 | 13.4 ± 2.9 | 13.1 ± 1.9 | 13.7 ± 1.8 | 12.3 ± 3.8 |
| Age (Years) | 2.2 | 43.5 ± 8.9 | 44.9 ± 4.8 | 45.0 ± 7.1 | 42.4 ± 4.9 | 39.8 ± 7.6 | 40.4 ± 7.0 |
| Socioeconomic Status (Hollingshead Index) | 0.4 | 31.0 ± 11.4 | 33.9 ± 8.9 | 31.8 ± 9.7 | 30.9 ± 14.5 | 33.1 ± 10.7 | 35.6 ± 12.4 |
| Non-Verbal IQ (Matrix Reasoning) | 0.7 | 9.3 ± 3.7 | 9.6 ± 3.1 | 10.6 ± 3.1 | 9.1 ± 3.3 | 10.4 ± 3.3 | 9.6 ± 2.5 |
| Verbal IQ (Wide Range Achievement Test, Scaled Score) | 2.1 | 89.5 ± 14.0 | 92.7 ± 13.4 | 94.6 ± 11.0 | 97.7 ± 10.5 | 101.8 ± 11.0 | 92.7 ± 17.2 |
| Depression (BDI) | 36.6*** | 6.0 ± 4.2d,e,f | 9.4 ± 8.5d,e,f | 11.2 ± 10.1d,e,f | 2.9 ± 5.2a,b,c | 1.5 ± 2.1a,b,c | 1.4 ± 2.8a,b,c |
| Comorbidity History (No/Yes) †‡ | 0.3 | 6/8 | 9/16 | 9/12 | -- | -- | -- |
| Cocaine Urine Status (No/Yes) | 2.9 | 7/7 | 13/12 | 6/15 | -- | -- | -- |
| Cocaine Use: Age of Onset (Years) | 0.7 | 23.9 ± 6.0 | 26.4 ± 8.2 | 26.8 ± 8.3 | -- | -- | -- |
| Cocaine Use Duration (Years) | 0.3 | 16.2 ± 5.5 | 15.4 ± 7.5 | 14.6 ± 6.7 | -- | -- | -- |
| Current Use: Days Per Week Last 30 Days | 1.5 | 4.1 ± 2.8 | 3.0 ± 2.4 | 3.3 ± 2.8 | -- | -- | -- |
| Current $ Spent per Use; Last 30 Days (Min – Max, Median) | 3.4 | 0 – 300, 85 | 0-600, 40 | 0-200, 50 | -- | -- | -- |
| Duration of Current Abstinence (Min – Max, Median) | 0.1 | 0-62, 3 | 0-120, 4 | 0-330, 2 | -- | -- | -- |
| Cocaine Selective Severity Assessment: Withdrawal Symptoms (Total Score) | 0.4 | 20.2 ± 11.9 | 17.3 ± 10.5 | 15.8 ± 12.5 | -- | -- | -- |
| Severity of Dependence Scale (Total Score) | 1.1 | 7.1 ± 3.3 | 8.4 ± 3.3 | 6.8 ± 4.7 | -- | -- | -- |
| Cocaine Craving Questionnaire (Total Score) | 0.1 | 19.1 ± 11.7 | 18.4 ± 10.5 | 19.8 ± 14.7 | -- | -- | -- |
Note. Numbers are frequencies, or M ± SD as appropriate
p<0.05
p<0.001
differs from 0 Risk-Allele Cocaine Subjects
differs from 1 Risk-Allele Cocaine Subjects
differs from 2 Risk-Allele Cocaine Subjects
differs from 0 Risk-Allele Control Subjects
differs from 1 Risk-Allele Control Subjects
differs from 2 Risk-Allele Control Subjects
comorbidity history information missing for two cocaine subjects
because high lifetime comorbidity rates commonly occur in cocaine addiction, including subjects with these comorbidities enhances generalizability.
2.2 Genotyping
Using DNA extracted from peripheral blood, all participants were genotyped [by polymerase chain reaction as previously described (Shumay et al., 2011)] for the 5-HTTLPR and uVNTR MAOA polymorphisms. For 5-HTTLPR, individuals were grouped into those with the L/L genotype versus those with either L/S or S/S 5-HTTLPR genotypes; observed frequency of the major 5-HTTLPR genotypes were close to expected according to Hardy-Weinberg assumptions in both African Americans and Caucasians (χ2<0.56, ns). A different method of partitioning the groups, where the S/S genotype is considered particularly risky, is more common in pharmacogenomics studies examining response to antidepressants (Lesch and Gutknecht, 2005) [but see (Haase et al., 2013; Papousek et al., 2013)]. However, we decided to compare any S-allele carriers with the L/L genotype given the presumed dominant functional effects of the S-allele (Lesch et al., 1996) and following prior studies and meta-analyses (Brocke et al., 2006; Herrmann et al., 2007; Karg et al., 2011; Osinsky et al., 2008; Pergamin-Hight et al., 2012). Of particular mention is a study showing that carriers of one S-allele did not differ from those with the S/S genotype, and that both S-carrying groups differed from the L/L genotype (Kwang et al., 2010).
For MAOA, individuals were separately grouped into MAOA-L (high risk) versus MAOAH (low risk) genotypes; 4 individuals (3 of them women, 2 of them CUD) who had more complex MAOA genotypes were excluded from the MAOA analyses. Aside from these exclusions, women were otherwise retained in the analyses to maximize sample size and statistical power. Although the functional significance of the MAOA gene is less well-characterized in women, several studies have reported comparable effects between men and women in related paradigms. For example, there were no MAOA by gender interactions in studies examining impulsivity (Stoltenberg et al., 2012), reactive aggression following provocation (Kuepper et al., 2013), dorsal ACC activity during social exclusion (Eisenberger et al., 2007), or amygdala/subgenual ACC activity during the presentation of emotional faces (Meyer-Lindenberg et al., 2006; but see other studies that reported MAOA by gender interactions (Priess-Groben and Hyde, 2013) or excluded women from MAOA analyses entirely (Alia-Klein et al., 2009; Enge et al., 2011; McDermott et al., 2009)].
Finally, we created a monoamine risk-allele profile: individuals with L/L 5-HTTLPR and MAOA-H were coded to have 0 risk variants; individuals with L/S 5-HTTLPR, S/S 5-HTTLPR, or MAOA-L were coded to have 1 risk variant; and individuals with either L/S 5-HTTLPR or S/S 5-HTTLPR and MAOA-L were coded to have 2 risk variants. Initial multiplicative analyses that tested the two genotypes separately in the same analyses did not reveal any MAOA × 5-HTTLPR interactions on any dependent variables reported below (all p>0.1), suggesting that an additive approach is appropriate. Importantly, all analyses reported below, whether split by 5-HTTLPR, MAOA, or their aggregation, always contained groups with at least 13 participants, which is not unlike other LPP studies in clinical populations [e.g., 15 individuals with generalized anxiety disorder (MacNamara and Hajcak, 2010), 13 individuals with anorexia nervosa (Horndasch et al., 2012), or 10 individuals with the 9R-allele of the dopamine transporter gene who tested positive for cocaine in urine (Moeller et al., 2013)], suggesting that the current study was sufficiently powered. Although study investigators were not blinded to genotype or participant grouping during analysis, they were blinded to genotype during study conduct and data collection [note that complete blinding of all relevant participant groupings would have been impractical (e.g., given the extensive cocaine information collected throughout the study, which was important for guaranteeing validity and quality assurance of the data)].
2.3 ERPs
ERPs were collected via electroencephalography (EEG) as participants passively viewed standardized pleasant, unpleasant, and neutral images that were selected from the International Affective Picture System (IAPS; Lang et al., 2008); and matched cocaine pictures (2000 ms per picture; 30 pictures per category; Moeller et al., 2009). Continuous recordings of the EEG (Neuroscan Inc., Sterling USA) and electro-oculogram were obtained throughout using a 64 silver-silver chloride electrode cap positioned according to the International 10/20 System (Klem et al., 1999). All recordings were performed using a fronto-central electrode as ground. Electrodes were placed above and below the left eye to record vertical eye movements, and placed on the outer canthi of both eyes to record horizontal eye movements; note that eye movements were recorded for artifact rejection purposes, not as a tool for eye-tracking or data analysis. The EEG was digitized at a rate of 500 Hz and amplified with a gain of 250, and a band-pass filter of 0 to 70 Hz. The amplifiers were calibrated prior to each recording. Electrode impedances did not exceed 10 kΩ for any electrodes used in the analysis.
All bioelectric signals were analyzed off-line using Statistical Parametric Mapping (SPM8) for MEG/EEG (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm/) and custom MATLAB code (The MathWorks). Data were filtered with low and high cutoffs of 0.01 and 30 Hz, respectively, and were then re-referenced to the averaged electrical activity from all 64 scalp sites. The artifact rejection procedure identified a voltage step of more than 75 μV between sample points and a peak-to-peak voltage difference of 150 μV within an epoch. Additional artifacts were identified and subsequently rejected through visual inspection or robust averaging (Wager et al., 2005). Following previous principle components analysis (Foti et al., 2009) and our prior studies in CUD (Dunning et al., 2011; Moeller et al., 2012, 2013), the entire LPP component was defined as the activity between 400-2000 ms that was localized at the Cz, FCz, FC1, FC2, and Fz electrodes; the average activity in the 200 ms window prior to picture onset served as the baseline (Figure 1A).
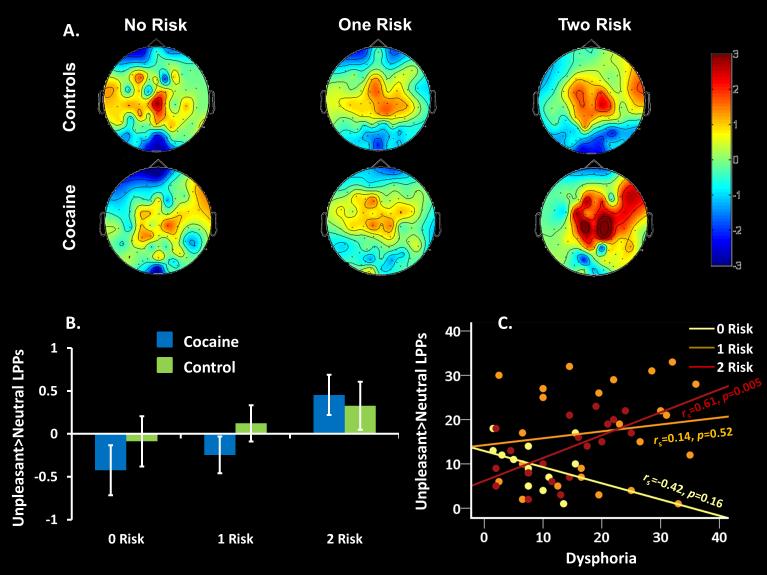
Figure 1.
Effects of serotonin-associated risk variants on aversive processing and correlations with depression symptomatology. (A) Event-related potential scalp maps for unpleasant images (versus neutral images) during a passive viewing task, separately by diagnosis and number of risk alleles. (B) Across all study participants, individuals with both monoaminergic risk variants (MAOA-L and at least one ‘short’ allele of 5-HTTLPR) showed the greatest LPP response to unpleasant images (versus neutral images), which (C) was associated with total score on the Beck Depression Inventory (BDI) in the cocaine participants (with a correlation magnitude higher than the other groups; see Results). Data from healthy controls are not presented in the scatterplot.
2.4 Statistical Analyses
Our primary, a priori analytic goal was to examine associations between MAOA, 5-HTTLPR, and their aggregation in CUD and healthy controls in response to aversive stimuli. Other analyses were meant to complement and clarify this primary goal. An important set of correlational analyses tested for possible behavioral associations of these risk alleles (i.e., depression- and drug use symptoms). In addition, we conducted supplementary analyses to rule out potential confounds and alternative explanations (Supplementary Material): modulation of effects by stressful life events, gender, alcohol use disorder comorbidity, or recent drug use.
2.4.1 ERP analyses
Prior to analysis, and following the literature linking risk variants of 5-HTTLPR and MAOA to negative emotionality, each participant's neutral (baseline) LPP was subtracted from the unpleasant LPP, yielding the contrast unpleasant>neutral. [For results exploring the parallel pleasant>neutral contrast, which did not result in any significant results and therefore establishes specificity to the unpleasant>neutral contrast, see Supplementary Materials 5; also note that results of the cocaine-related contrasts are reported elsewhere (Moeller et al., 2013).] We then performed two-way analyses of covariance (ANCOVAs) (three total ANCOVAs), with diagnosis (CUD, control) as the first between-group factor and genetics as the second between-group factor; cigarette smoking history (yes/no) and race were included in these ANCOVAs as dummy covariates. These three ANCOVAs included one for 5-HTTLPR, one for MAOA, and one for their aggregation. The dependent variables in these ANCOVAs were unpleasant>neutral LPPs. Effects were considered significant at p<0.05.
2.4.2 Correlation Analyses
Correlations of LPPs with depression and cocaine use were meant to test for possible behavioral associations of these risk alleles. We performed correlations between the LPP variables showing significant differences as a function of genotype with the well-validated BDI (Beck, 1996), and with select drug use variables in Table 1 (specifically, those reflecting current use frequency and severity: days per week of cocaine use and amount spent per use on cocaine). Because we also wanted to inspect correlations separately by diagnosis and genotype, significance for correlations was set at p<0.01 to minimize Type I error.
3. RESULTS
3.1 MAOA
A 2 (Diagnosis: CUD, control) × 2 (MAOA: H, L) ANCOVA (controlling for cigarette smoking history and race) revealed no main effect of Diagnosis (p>0.1) and no interaction (p>0.1). There was, however, a main effect of MAOA in the expected direction (L>H) [F(1,105)=5.86, p=0.017, d=0.47], indicating that MAOA-L is associated with increased reactivity to unpleasant stimuli relative to neutral stimuli across diagnostic groups.
3.2 5-HTTLPR
Results of 2 (Diagnosis: CUD, control) × 2 (5-HTTLPR: L/L, S-allele) ANOVAs revealed no significant main effects or interactions – although we note that the main effect of 5-HTTLPR was in the expected direction (S-allele>L/L; p>0.15).
3.3 Monoamine Polygenic Liability
To test the hypothesis of incrementally increased reactivity to unpleasant>neutral stimuli as a function of monoamine gene polygenic liability, we performed a 2 (Diagnosis: CUD, control) × 3 (Risk Variant: 0, 1, 2) ANCOVA (controlling for cigarette smoking history and race). This ANCOVA revealed no main effect of Diagnosis (p>0.4) and no interaction (p>0.5). However, there was a main effect for Risk Variant factor [F(2,99)=3.21, p=0.045, d=0.51]. A follow-up ascending linear contrast analysis for the Risk Variant factor reached significance, demonstrating a stepwise increase in unpleasant>neutral reactivity as a function of the number of monoaminergic risk variants across diagnostic groups (p=0.018;Figure 1B).
3.4 Correlation Analyses
We next correlated the unpleasant>neutral LPPs with the BDI total score and current drug use severity variables, separately by MAOA and the monoamine risk score. These analyses showed that the higher the unpleasant>neutral LPP, the higher was the depression symptomatology only in CUD with 2 risk variants (Spearman r=0.61, p=0.005; Figure 1C) [but not in any of the other subgroups (all other p>0.1)]. A subsequent test of correlations showed that this subgroup (CUD with 2 risk variants) significantly differed from all other groups when combined (all other participants: r=-0.11, p>0.3; correlation difference, z=2.99, p=0.003), indicating specificity of the correlation to this subgroup. Controlling for cigarette smoking history and race in a partial correlation did not attenuate this correlation in the CUD with two risk variants (p=0.002). Thus, individuals with the highest genetic and environmental risk toward negative emotionality (i.e., CUD with 2 risk variants) also showed the strongest relationship between reactivity to unpleasant stimuli and depression symptomatology compared with all other subgroups. We did not observe any correlations with drug use at p<0.01.
4. DISCUSSION
The present study identified additive effects of 5-HTTLPR and MAOA risk variants on LPP reactivity to unpleasant stimuli in both healthy controls and CUD. Because the LPP indexes stimulus salience (Hajcak et al., 2010), our results support the hypothesis that individuals with greater monoamine polygenic liability have higher sensitivity to aversive events. These results extend a framework that has been robust in elucidating depressive disorders – that is, an association between serotonin gene polymorphisms and emotional reactivity – to the study of drug addiction. By targeting aversive processing specifically, our results also extend a growing literature that has provided evidence for impaired salience and emotional responsiveness in CUD, but has mainly focused [until recently (Ersche et al., 2014)] on responsiveness to pleasant (Asensio et al., 2010; Lubman et al., 2009) or drug-related (Jasinska et al., 2014) stimuli. In the current study, the lack of monoamine gene effects for the pleasant>neutral LPP (see Supplementary Material 6) serves the dual function of establishing specificity to the unpleasant stimuli and reducing the possibility that our results were driven by the less evocative stimuli (e.g., neutral; Canli et al., 2005).
We interpret our findings according to the perspective that these two “risk” alleles render individuals more reactive to aversive stimuli and events in their social environments. For example, individuals with the S/S genotype of 5-HTTLPR responded more negatively to marital conflict: uniquely in this genotype, the higher the conflict during a marital discussion, the greater the martial dissatisfaction over time (Haase et al., 2013). In a related paradigm that involved couples discussing their marriages, individuals with an S-allele, compared with the L/L genotype, were more influenced by their partners’ pre-interaction emotional states (Schoebi et al., 2012). For MAOA, this polymorphism only correlated with reactive aggression following provocation (i.e., not with dispositional or instrumental anger; Kuepper et al., 2013; McDermott et al., 2009), suggesting a greater reactivity upon being confronted with negative social environmental stimuli. To concretely attribute our results to the neurochemical influence of serotonin as we anticipate, future experimental studies could manipulate this neurotransmitter directly. For example, studies employing tryptophan depletion often find that depletion, which temporarily decreases serotoninergic tone, is associated with more sensitivity to aversive stimuli (Feder et al., 2011; Robinson et al., 2013; Wang et al., 2009) [but see (Beacher et al., 2011)]. An important caveat is that it is difficult to directly compare studies of serotonin depletion (occurring during a single experiment) and genetic modulation [occurring over the lifetime, with the largest effects ostensibly exerted during early development (Buckholtz and Meyer-Lindenberg, 2008, 2012)]. Speaking to this complexity, crossing these two factors (genetics and serotonin depletion) in psychiatric populations has yielded higher-order interactions that are difficult to interpret (Neumeister et al., 2006; Roiser et al., 2012).
Although in the current study there were no diagnosis by genotype interactions on the unpleasant>neutral LPPs directly as one might have anticipated, differences between the groups nonetheless emerged vis-à-vis how unpleasant>neutral LPPs correlated with depression symptomatology. Specifically, CUD with the greatest monoamine polygenic liability displayed the tightest coupling between LPP reactivity to unpleasant stimuli and depression symptomatology. Because this correlation was specific to the CUD group with 2 risk alleles – despite this group not having more depression symptoms than CUD with 0 or 1 risk alleles (Table 1) – it speaks against the idea that higher unpleasant>neutral LPPs are simply redundant with depression scores. Also speaking against the conflation of LPPs and depression symptoms is that while healthy controls, by design, had lower BDI scores than CUD, controls with 2 risk alleles nonetheless had higher responsiveness to the unpleasant>neutral stimuli (Figure 1B). It would be interesting for future studies to evaluate whether CUD with two risk alleles have an elevated propensity toward poorer clinical outcomes. For instance, one could hypothesize that these CUD may be at increased risk for developing depression symptoms especially when confronted with aversive experiences (e.g., stress), which together with the genes may modulate relapse propensity (Sinha, 2013); conversely, CUD without these risk alleles may be better shielded from the effects of stress and/or other negative environmental stimuli on mood regulation that could derail abstinence.
The present study has several limitations pertaining to the sample: (A) the sample size was relatively small for a genetics study that partitioned the groups by two genotypes and diagnosis. Importantly, however, no genotype-diagnosis subgroup ever contained fewer than 13 participants (see Methods). This is a reasonable sample size for clinical ERP research (Horndasch et al., 2012; MacNamara and Hajcak, 2010; Moeller et al., 2013), further evidenced by the fact that we were able to detect a significant main effect of genotype that had respectable, medium effect sizes (Cohen's d). Since gene polymorphisms typically explain a limited amount of variance in highly complex diseases such as addiction and depression, these medium effect sizes are in fact expected. (B) Although our results appeared to be quite comparable between men and women (Supplementary Material 7), which speaks against the idea that these effects are operating differently in women, our effects nonetheless should be replicated with samples that include more women. (C) Given that multiple races were studied, there is a possible concern of population stratification, which can occur even in well-designed studies (Freedman et al., 2004). However, there were no genotype × diagnosis group differences on race (Table 1), and all results controlled for the effects of race, together reducing concern about this potential issue. (D) Because the current sample did not have high depression scores, it will important for future studies to include a group of CUD with comorbid depression to fully validate the clinical significance of these findings.
In conclusion, this study provides novel evidence for additive effects of the 5-HTTLPR and MAOA polymorphisms on unpleasant picture reactivity in health and cocaine addiction. Uniquely in CUD with two risk variants, heightened unpleasant LPPs also tracked depression symptomatology. Thus, beyond a potential impact of these risk alleles to initiate illness, in the presence of disease (e.g., addiction) these risk alleles may alter illness severity by modulating sensitivity to aversive cues (Alia-Klein et al., 2011). Reducing such aversive reactivity could be especially important during early abstinence/detoxification, when difficulties with emotion regulation in addicted individuals are accentuated (Fox et al., 2007). Results of this study help forge an initial foundation for the study of genes modulating serotoninergic functioning in addiction, complementing the valuable work on dopamine gene polymorphisms (Sweitzer et al., 2012). Taken together, our results support the important idea that neuroimaging is well-positioned to bridge genetic risk and psychopathology (Savitz and Drevets, 2009). Future clinical intervention studies can aim to leverage the combined power of genetic, neuroimaging, and possibly also clinical symptomatology to investigate long-term outcomes and/or pharmacogenetic therapies in drug addiction and other psychopathologies of emotion dysregulation (e.g., anxiety, eating disorders, intermittent explosive disorder, and/or borderline personality).
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of Thomas Maloney, Patricia A. Woicik, Dardo Tomasi, Ruiliang Wang, and Gene-Jack Wang for help with study coordination.
Role of Funding Source
This study was supported by grants from the National Institute on Drug Abuse: 1R01DA023579 (RZG), 1F32DA030017-01 (SJM), and 1F32DA033088-01 (MAP). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
S.J.M., M.A.P., E.S., N.A.-K., and R.Z.G. designed the research; M.A.P. and A.B.K. conducted the research; S.J.M., M.A.P., E.S., S.W., N.B.-W., and M.M. analyzed data; S.J.M. and R.Z.G. wrote the paper. All authors contributed to and have approved the final manuscript.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Conflict of Interest
No conflict declared.
REFERENCES
- Alia-Klein N, Goldstein RZ, Tomasi D, Woicik PA, Moeller SJ, Williams B, Craig IW, Telang F, Biegon A, Wang GJ, Fowler JS, Volkow ND. Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion. 2009;9:385–396. doi: 10.1037/a0015904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch. Gen. Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict. Biol. 2010;15:504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Bacher I, Houle S, Xu X, Zawertailo L, Soliman A, Wilson AA, Selby P, George TP, Sacher J, Miler L, Kish SJ, Rusjan P, Meyer JH. Monoamine oxidase A binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch. Gen. Psychiatry. 2011;68:817–826. doi: 10.1001/archgenpsychiatry.2011.82. [DOI] [PubMed] [Google Scholar]
- Beacher FD, Gray MA, Minati L, Whale R, Harrison NA, Critchley HD. Acute tryptophan depletion attenuates conscious appraisal of social emotional signals in healthy female volunteers. Psychopharmacology (Berl.) 2011;213:603–613. doi: 10.1007/s00213-010-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The Beck Depression Inventory (BDI-II) The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J. Abnorm. Psychol. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, Pizzagalli DA. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol. Dis. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP, Kirschbaum C, Strobel A. Serotonin transporter gene variation impacts innate fear processing: Acoustic startle response and emotional startle. Mol. Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Hudziak JJ, Li D. Multi-cultural association of the serotonin transporter gene (SLC6A4) with substance use disorder. Neuropsychopharmacology. 2013;38:1737–1747. doi: 10.1038/npp.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users – an ERP study. Eur. J. Neurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR. Evidence for a genetic component for substance dependence in native americans. Am. J. Psychiatry. 2013;170:154–164. doi: 10.1176/appi.ajp.2012.12010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: clues from the brain's response to social exclusion. Biol. Psychiatry. 2007;61:1100–1108. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Reif A, Strobel A. Serotonergic modulation in executive functioning: linking genetic variations to working memory performance. Neuropsychologia. 2011;49:3776–3785. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Hagan CC, Smith DG, Abbott S, Jones PS, Apergis-Schoute AM, Doffinger R. Aberrant disgust responses and immune reactivity in cocaine-dependent men. Biol. Psychiatry. 2014;75:140–147. doi: 10.1016/j.biopsych.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Liu B, Jiang T, Jiang X, Zhao H, Zhang J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr. Genet. 2010;20:1–7. doi: 10.1097/YPG.0b013e3283351112. [DOI] [PubMed] [Google Scholar]
- Feder A, Skipper J, Blair JR, Buchholz K, Mathew SJ, Schwarz M, Doucette JT, Alonso A, Collins KA, Neumeister A, Charney DS. Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol. Psychiatry. 2011;69:804–807. doi: 10.1016/j.biopsych.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007;89:298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, Patterson N, Gabriel SB, Topol EJ, Smoller JW, Pato CN, Pato MT, Petryshen TL, Kolonel LN, Lander ES, Sklar P, Henderson B, Hirschhorn JN, Altshuler D. Assessing the impact of population stratification on genetic association studies. Nat. Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase CM, Saslow LR, Bloch L, Saturn SR, Casey JJ, Seider BH, Lane J, Coppola G, Levenson RW. The 5-HTTLPR polymorphism in the serotonin transporter gene moderates the association between emotional behavior and changes in marital satisfaction over time. Emotion. 2013;13:1068–1079. doi: 10.1037/a0033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Foti D, Ferri J, Keil A. The dynamic allocation of attention to emotion: simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biol. Psychol. 2013;92:447–455. doi: 10.1016/j.biopsycho.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 2010;35:129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Huter T, Muller F, Muhlberger A, Pauli P, Reif A, Renner T, Canli T, Fallgatter AJ, Lesch KP. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cereb. Cortex. 2007;17:1160–1163. doi: 10.1093/cercor/bhl026. [DOI] [PubMed] [Google Scholar]
- Horndasch S, Heinrich H, Kratz O, Moll GH. The late positive potential as a marker of motivated attention to underweight bodies in girls with anorexia nervosa. J. Psychosom. Res. 2012;73:443–447. doi: 10.1016/j.jpsychores.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Roder-Hanna N, Leggio L, Zywiak WH, Clifford J, Edwards S, Kenna JA, Shoaff J, Swift RM. Association of the 5-HTT gene-linked promoter region (5-HTTLPR) polymorphism with psychiatric disorders: review of psychopathology and pharmacotherapy. Pharmacogenomics Pers. Med. 2012;5:19–35. doi: 10.2147/PGPM.S23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1999;52:3–6. [PubMed] [Google Scholar]
- Kuepper Y, Grant P, Wielpuetz C, Hennig J. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behav. Brain Res. 2013;247:73–78. doi: 10.1016/j.bbr.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Kwang T, Wells TT, McGeary JE, Swann WB, Jr., Beevers CG. Association of the serotonin transporter promoter region polymorphism with biased attention for negative word stimuli. Depress. Anxiety. 2010;27:746–751. doi: 10.1002/da.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-8. University of Florida; Gainsville, FL: 2008. [Google Scholar]
- Lee BT, Ham BJ. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. Neuroreport. 2008;19:515–519. doi: 10.1097/WNR.0b013e3282f94294. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Pharmacogenetics of the serotonin transporter. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1062–1073. doi: 10.1016/j.pnpbp.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch. Gen. Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depress. Anxiety. 2010;27:234–243. doi: 10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the U.S. population. Drug Alcohol Depend. 2011;119:28–36. doi: 10.1016/j.drugalcdep.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott R, Tingley D, Cowden J, Frazzetto G, Johnson DD. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2118–2123. doi: 10.1073/pnas.0808376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Hajcak G, Parvaz MA, Dunning JP, Volkow ND, Goldstein RZ. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain. 2012;135:3481–3494. doi: 10.1093/brain/aws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol. Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, Volkow ND, Goldstein RZ. Gene x abstinence effects on drug cue reactivity in addiction: multimodal evidence. J. Neurosci. 2013;33:10027–10036. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, Munafo MR. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol. Psychiatry. 2013;18:512–520. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, Herscovitch P, Goldman D, Drevets WC, Charney DS. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch. Gen. Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- Osinsky R, Reuter M, Kupper Y, Schmitz A, Kozyra E, Alexander N, Hennig J. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Papousek I, Reiser EM, Schulter G, Fink A, Holmes EA, Niederstatter H, Nagl S, Parson W, Weiss EM. Serotonin transporter genotype (5-HTTLPR) and electrocortical responses indicating the sensitivity to negative emotional cues. Emotion. 2013;13:1173–1181. doi: 10.1037/a0033997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, Fera F. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage. 2008;40:1264–1273. doi: 10.1016/j.neuroimage.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biol. Psychiatry. 2012;71:373–379. doi: 10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Priess-Groben HA, Hyde JS. 5-HTTLPR X stress in adolescent depression: moderation by MAOA and gender. J. Abnorm. Child Psychol. 2013;41:281–294. doi: 10.1007/s10802-012-9672-1. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Allen PS, Letkiewicz A, Vytal K, Pine DS, Grillon C. The role of serotonin in the neurocircuitry of negative affective bias: serotonergic modulation of the dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit. Neuroimage. 2013;78:217–223. doi: 10.1016/j.neuroimage.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Goldman D, Hodgkinson CA, Hasler G, Sahakian BJ, Drevets WC. Serotonin transporter genotype differentially modulates neural responses to emotional words following tryptophan depletion in patients recovered from depression and healthy volunteers. J. Psychopharmacol. 2012;26:1434–1442. doi: 10.1177/0269881112442789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164:300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoebi D, Way BM, Karney BR, Bradbury TN. Genetic moderation of sensitivity to positive and negative affect in marriage. Emotion. 2012;12:208–212. doi: 10.1037/a0026067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, Chen J, Fowler JS, Volkow ND. Genotype and ancestry modulate brain's DAT availability in healthy humans. PLoS ONE. 2011;6:e22754. doi: 10.1371/journal.pone.0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling relapse situations in the human laboratory. Curr. Top Behav. Neurosci. 2013;13:379–402. doi: 10.1007/7854_2011_150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Christ CC, Highland KB. Serotonin system gene polymorphisms are associated with impulsivity in a context dependent manner. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39:182–191. doi: 10.1016/j.pnpbp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Hariri AR. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug Alcohol Depend. 123 Suppl. 2012;1:S59–71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P). Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wang L, Mullette-Gillman OA, Gadde KM, Kuhn CM, McCarthy G, Huettel SA. The effect of acute tryptophan depletion on emotional distraction and subsequent memory. Soc. Cogn. Affect. Neurosci. 2009;4:357–368. doi: 10.1093/scan/nsp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg AU, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, Palmer DM, Paul RH, Song L, Costa PT, Schofield PR, Gordon E. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.