Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of autoantibodies. This review summarizes first the results obtained in the mouse that have revealed how B cell tolerance is breached in SLE. We then review the B cell subsets, in addition to the autoAb producing cells, which contribute to SLE pathogenesis, focusing on marginal zone B cells, B-1 cells and regulatory B cells. Finally, we review the interactions between B cells and other immune cells that have been implicated in SLE, such as dendritic cells, macrophages, neutrophils and T cells.
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of autoantibodies (autoAbs) (Ceppellini et al., 1957; Robbins et al., 1957). These autoAbs are produced by both long-lived plasma cells (PCs) and short-lived plasmasblasts (PBs) (Hoyer et al., 2004; Liu et al., 2011), some of which are generated through germinal centers (GCs) (Vinuesa et al., 2010) while others bypass GCs and differentiate into PBs in extrafollicular foci (Shlomchik, 2008). This review summarizes first the results obtained in the mouse that have revealed how B cell tolerance is breached in SLE. We will then review which B cell subsets, in addition to the autoAb producing cells, contribute to SLE pathogenesis. Finally, we will review the interactions between B cells and other immune cells that have implicated in SLE. This review will refer to several spontaneous mouse models of SLE which have distinct genetic backgrounds, and have provided different insights to the mechanism of lupus pathogenesis in general, including the role of B cells (Table 1).
Table 1.
Spontaneous Mouse Models of Lupus
| Strain | Parental strains | Lupus-like phenotype specific to the model | References |
|---|---|---|---|
| (NZB x NZW) F1 | NZB and NZW | Lymphadenopathy, splenomegaly, high level anti-dsDNA IgG, lupus nephritis (GN). Strong female bias | (Helyer and Howie, 1963) |
| NZM2410 NZM2328 |
NZM2410 and NZM2328 are 2 of 27 recombinant inbred strains between NZB and NZW | Similar to (NZB x NZW) F1 with less pronounced female bias | (Rudofsky et al., 1993) |
| B6.NZM2410.Sle1.Sle2.Sle3 | NZM2410 derived genetic loci, Sle1 - 3, are introduced to a B6 non-autoimmune background | Milder phenotypes than the parental NZM2410 | (Morel et al., 2000) |
| MRL/lpr | MRL strain is generated from inbreeding between several strains of mice. The lpr, lymphoproliferation, mutation is a loss of function in the pro-apoptotic Fas gene. | High level of autoAbs: anti-DNA, anti-Sm, rheumatoid factors, GN. Lymphadenopathy contributed mainly by accumulation of CD4− CD8− T cells. | (Cohen and Eisenberg, 1991) |
| MRL/gld | The gld, generalized lymphoproliferative disease, mutation is a loss of function mutation in the FasL gene. | Lymphadenopathy, autoAbs, GN. | |
| BXD2 | C57BL/6J x DBA/2J recombinant inbred strain | High level of IL - 17, autoAbs (anti-DNA, anti-histone, and rheumatoid factor), GN and arthritis. | (Hsu et al., 2008) |
| BXSB/Yaa | (B6 x SB/Le) F1 x SB/Le --> Inbreeding. Yaa, Y-linked autoimmune accelerator, refers to the translocation of 16 genes from the X chromosome, including TLR7 onto the Y chromosome | Only males are affected. AutoAbs skewed toward RNA-specificities, monocytosis. | (Murphy and Roths, 1979; Santiago-Raber et al., 2008) |
2. B cell Tolerance
Maintenance of B cell tolerance is essential for preventing the secretion of autoAbs with potential pathogenic specificities. In SLE, failure in B cell tolerance sits at the core of the disease process. Indeed, it is largely accepted that tissue injury results from the production of autoAbs which combine with self-antigens (self-Ags) to form immune complexes (ICs) that deposit into organs leading to inflammation and cellular damage. The mechanisms by which normal B cells from healthy subjects maintain tolerance against lupus-associated antigens follow the same general basic principles that have been described for generic antigens, which will be briefly reviewed below. In addition, more specific mechanisms are involved to prevent the production of lupus-associated autoAbs, due to the nature of the prevalent lupus autoAgs. Indeed, lupus-associated autoAgs are largely confined to nucleoprotein complexes that are released during cell death and that activate TLR7 and TLR9 (Marshak-Rothstein and Rifkin, 2007). These specific mechanisms will be reviewed in sections 2.1 and 2.2.
Given that 55–75% of B cell receptors (BCR) on human immature B cells are self-reactive, strict tolerance mechanisms are required to eliminate them from the B cell repertoire (Wardemann et al., 2003). Classic studies using BCR transgenic (Tg) mouse models have identified several tolerance checkpoints at which autoreactive B cells are regulated (Pillai et al., 2011). Central tolerance in the bone marrow (BM) eliminates self-reactive immature B cells primarily by receptor editing (Gay et al., 1993; Murphy and Roths, 1979; Tiegs et al., 1993). Failure in receptor editing results in the autoreactive B cells becoming either anergized or deleted depending on receptor affinity (Cambier et al., 2007). Immature B cells that pass the central tolerance checkpoint migrate to the spleen where they develop into mature B cells. At this stage, self-reactive B cells are regulated by peripheral checkpoints, such as deletion, anergy, follicular exclusion, and clonal ignorance (Shlomchik, 2008). In addition, recent work has shown that self-reactive B cells that arise from a GC reaction are tolerized if the self-Ag is expressed in large amounts and in close proximity to the GC (Chan et al., 2012). Elimination of autoreactive B cells has been a major therapeutic goal in SLE. This cannot be achieved without a thorough understanding of how these multiple tolerance mechanisms are affected in SLE. The knowledge gained in this field from mouse models will be reviewed in this section.
2.1 Breakdown of B cell tolerance in BCR tg mouse models of lupus
Studies crossing the classic BCR Tg tolerance models, such as HEL x anti-sHEL (Rathmell and Goodnow, 1994) or anti-MHCI (Rubio et al., 1996), to the MRL/lpr lupus-prone background did not reveal significant tolerance defects, which has been attributed to the lack of specificity of these models towards a lupus relevant self-Ag (Shlomchik, 2008). However, Tg mouse models targeting lupus-associated self-Ags such as DNA, RNA-containing particle such as Sm, and IgG have shown dysregulated B cell tolerance when crossed to an autoimmune background. A summary of the findings from these models is given in Table 2.
Table 2.
Self-Ag specific BCR transgenic models in a non-autoimmune versus lupus-prone background
| Specificity | Models | Non-autoimmune background | Lupus-prone background |
|---|---|---|---|
| Anti-dsDNA | 3H9, 3H9/Vκ8, 3H9/56R | anergized (Nguyen et al., 1997) or developmentally arrested after Ag encounter (Mandik-Nayak et al., 1997) | anti-dsDNA Ab secretion (Mandik-Nayak et al., 1999) or differentiation into MZB (Liu et al., 2007) |
| RF (anti-IgG) | AM14, AM14/Vκ8 | clonal ignorance (Hannum et al., 1996) | RF secretion and SHM at EF zones (Wang and Shlomchik, 1999; William et al., 2005) |
| Anti-Sm | 2-12, 2-12/Vκ8 | developmentally arrested or anergized (Santulli-Marotto et al., 1998) | accelerated anti-Sm response (Santulli-Marotto et al., 2001) |
2.1.1 Anti-DNA
Anti-dsDNA IgG is an important disease marker, given that it was detected in 55% of patient sera prior to disease diagnosis and in the great majority of them after diagnosis (Arbuckle et al., 2003). The 3H9 heavy chain (HC) Tg, derived from an MRL/lpr anti-DNA Ab, combines with several endogenous light chains (LC) to produce a BCR reactive to either ssDNA or dsDNA (Erikson et al., 1991). The HC 3H9 Tg model has been used extensively to study the mechanism of tolerance to DNA. Its main advantage is that it maintains a physiological polyclonal B cell repertoire, while nearly all the B cells in HC/LC double Tg mice are specific to a single Ag.
In the non-autoimmune BALB/c background, both the 3H9 HC tg and the 3H9/Vκ8 double Tg mice show high levels of B cells carrying DNA-specific BCRs, but anti-DNA autoAbs could not be detected. Therefore, in a healthy background tolerance mechanisms prevent the differentiation of self-reactive B cells into Ab forming cells (AFC). Further studies showed that the 3H9 B cells were Ag-experienced, yet developmentally arrested at the T-B cell interface of the splenic follicle (Mandik-Nayak et al., 1997). Meanwhile, the 3H9/Vκ8 B cells, which are primarily anti-ssDNA, had an anergic phenotype characterized by reduced proliferation in response to stimulation despite being long-lived (Nguyen et al., 1997).
In contrast, 3H9 B cells in the MRL/lpr autoimmune background were no longer developmentally arrested and entered the follicles (Mandik-Nayak et al., 1999). Furthermore, site-directed 3H9/Vκ8 B cells in the MRL/lpr background were activated, class-switched, and underwent somatic hypermutation (SHM) which led them to acquire specificity to other autoAbs (Brard et al., 1999). The effect of anti-dsDNA reactivity on receptor editing was studied in a 3H9 HC mouse with a site-directed mutation from aspartate to arginine at position 56 in the CDR2 region (3H9/56R) which resulted in a BCR with higher affinity for dsDNA when combined with most LCs (Li et al., 2001). In the non-autoimmune BALB/c background, the 3H9/56R B cells successfully underwent LC receptor editing to produce a BCR that did not bind to dsDNA. In contrast, this mechanism was defective in the MRL/lpr background as most B cells were still specific for dsDNA following receptor editing (Li et al., 2002). In addition, the NZM2410-derived Sle2 lupus susceptibility locus also breach tolerance of the 3H9/56R B cells by preferentially inducing their differentiation into marginal zone B cells (MZB) (Liu et al., 2007).
The 3H9 model has also provided insights into the dysregulation of DNA-specific B cells by comparing two non-autoimmune strains. While the BALB/c background prevents the secretion of anti-DNA autoAb, anti-DNA Abs are found in B6.3H9/56R mice (Tsao et al., 2008) due to defect in a post-GC checkpoint (Fukuyama et al., 2005). Therefore, some non-autoimmune genetic backgrounds already possess a predisposition to autoimmunity that is only apparent in the presence of high numbers of self-reactive B cells.
2.1.2 RF Specificity
Rheumatoid factors (RF) are autoAbs directed against self-IgG and the presence of serum RF has been associated with active SLE (Kessel et al., 2009). The AM14 HC, derived from an MRL/lpr hybridoma, was used to generate a RF specific Tg model in which the AM14 HC combines with endogenous Vκ8 LC to form a BCR specific for IgG2a of the “a” allotype (Shlomchik et al., 1993). Therefore, this system enables the study of B cell tolerance with a SLE-relevant specificity (RF) in the presence or absence of the IgG2aa autoAg. In the non-autoimmune BALB/c background, the moderate affinity of the AM14/Vκ8 BCR rendered the autoreactive B cells clonally ignorant rather than deleted or anergized in the presence of the autoAg (Hannum et al., 1996). However, in the MRL/lpr background, the AM14 B cells were spontaneously activated and differentiated into AFCs when IgG2aa was expressed (Wang and Shlomchik, 1999).
Further studies showed that MRL/lpr RF B cells underwent SHM in the extra-follicular (EF) zones bypassing GC reactions, and developed into short-lived plasmablasts (William et al., 2002; William et al., 2005). These results were validated in a site-directed AM14 HC model where the spleen and BM of MRL/lpr, but not BALB/c mice, contained activated and class-switched RF B cells located in EF clusters (Sweet et al., 2010). The presence of IgG2aa ICs found in MRL/lpr but not in BALB/c mice, led to the activation of the RF B cells (Rifkin et al., 2000) and in vitro studies have shown activation of AM14 B cells depended on dual ligation of the BCR and TLR7/TLR9 (Lau et al., 2005; Leadbetter et al., 2002). Finally, administration of anti-chromatin IgG2aa to either Tg MRL/lpr or BALB/c mice was sufficient to activate their AM14 B cells (Herlands et al., 2007). Therefore, the excess DNA/RNA ICs generated by lupus-prone mice leads to the activation of clonally ignorant RF B cells.
T cells are not required for differentiation of AM14 AFCs, class switching, and SHM when BCR and TLR7/TLR9 ligation was provided in vivo (Herlands et al., 2008; Sweet et al., 2011). However, CD40L and IL-21 signaling provides by CD4+ T cells increased the number of RF plasmablasts and the frequency of SHM. In addition, AM14 B cells can differentiate into memory B cells and provide secondary responses only with T-cell help (Sweet et al., 2013). Therefore, the activation of AM14 B cells is complex with other immune cells enhancing their pathogenic potential.
The AM14 model has been extensively studied in the MRL/lpr genetic background. A potential confounding factor arises with this model since the autoimmune background is primarily dependent on the Fas mutation (lpr). However, Fas deficiency in humans (Autoimmune Lymphoproliferative Syndrome) only shares some clinical manifestations with SLE (Teachey et al., 2010). We have crossed the AM14 HC with the Fas-sufficient C57BL/6-based B6.NZM2410.Sle1.Sle2.Sle3 triple congenic (B6.TC) mouse model of lupus (Morel et al., 2000). In this model, the B6.TC lupus background induced spontaneous activation of RF B cells in the presence of the autoAg in a TLR7/9 dependent manner. Just like in the MRL/lpr model, the activated AM14 B cells followed an EF response with high levels of SHM hypermutation (Sang et al., in preparation). Further studies will reveal the mechanisms by which RF B cell tolerance is broken in this autoimmune background.
2.1.3 Anti-Sm
AutoAbs against snRNPs, known as anti-Sm, are found in a large subset of SLE patients. To study Sm-specific B cells, a 2-12 HC Tg mouse was generated from an anti-Sm MRL/lpr hybridoma (Santulli-Marotto et al., 1998). The B cell repertoire of these mice is reactive towards Sm, ssRNA, as well as non-self Ags. Anti-Sm secretion could not be detected in 2-12 HC Tg B6 mice with anti-Sm B cells arrested at an immature stage with a shortened half-life. However, some anti-Sm B cells matured in an anergized state as immunization with murine snRNPs induced their activation and autoAb secretion. Meanwhile, crossing the 2-12 HC to the MRL/lpr background accelerated the anti-Sm response when compared to non-Tg MRL/lpr mice (Santulli-Marotto et al., 2001). A more thorough analysis of 2-12 HC Tg MRL/lpr mice revealed a defect in the differentiation of anti-Sm B cells to the B-1 lineage where they are tolerized (Santulli-Marotto et al., 2001). In non-autoimmune mice, 2-12 B cells preferentially differentiated into peritoneal B-1 cells that remained tolerant towards the self-Ag (Qian et al., 2001). This B-1 cell differentiation was dependent on a strong signaling threshold as lowering BCR signaling through CD19 deficiency resulted in differentiation to the B-2 compartment, and a breach of tolerance. In addition, 2-12/Vκ8 double Tg B cells have a low affinity for Sm Ags and only differentiated to the B-2 lineage and displayed an anergic phenotype (Borrero and Clarke, 2002). Furthermore, 2-12 B-1 as well as MZ B cells were clonally ignorant by being sequestered from self-Ag, as a deficiency in the clearance of apoptotic cells led to anti-Sm secretion from both B cell populations (Qian et al., 2004). Therefore, the 2-12 model illustrates how the signaling threshold as well as the availability of self-Ag to the different B cell lineages play a role in the maintenance of tolerance and how this complex mechanism is dysregulated in a lupus-prone background resulting in autoAb production.
2.2 Dysregulation of tolerance checkpoints are found in SLE patients
SLE patients show an abnormal distribution of B cell populations in comparison to healthy controls, which indicates defects in tolerance checkpoints. Studies using a dsDNA mimetope tetramer (DWEYS) showed that SLE patients had both naive and Ag-experienced B cells that were reactive against dsDNA. This observation was independent of disease activity suggesting a failure in both early and late selection checkpoints (Jacobi et al., 2009). Broader studies looking at multiple disease-associated autoAbs showed a defect in early tolerance mechanisms due to the increased presence of autoreactive mature naive B cells in SLE patients (Yurasov et al., 2005). Furthermore, patients in remission maintained elevated numbers of autoreactive mature naive B cells suggesting that the accumulation of self-reactive B cells can predispose individuals to disease (Yurasov et al., 2006).
In addition to early and peripheral tolerance checkpoints, a third checkpoint has been identified for Ag experienced B cells as self-reactive IgM+ CD27+ memory B cells are excluded from the circulation whereas B cells specific for common bacterial pathogens are expanded (Dunn-Walters et al., 1995; Tangye et al., 1998; Tsuiji et al., 2006). Surprisingly, IgG+ memory B cells produce self-reactive Abs, including anti-nuclear specificities, in the sera of healthy individuals (Tiller et al., 2007). The majority of these autoAbs are derived de novo through SHM during the differentiation of self-Ag activated B cells. These results point towards a breach in tolerance at the GC level. Autoreactive B cells were excluded from the GCs in the tonsils of healthy controls but not in SLE patients (Cappione et al., 2005). Therefore, defects regulating GC reactions could lead to the production of de novo autoreactive B cells which would explain the high levels of memory and plasma cells characteristically seen in SLE.
3. Antigen-independent mechanisms of B cell tolerance
3.1 Endosomal Toll-like Receptors (TLRs) play an important role in the activation of pathogenic B cells
Recent studies have linked the endosomally localized TLR7 and TLR9 to the regulation of B cell tolerance. The dual ligation of BCR and TLR7 or TLR9 is necessary for the activation of AM14/Vk8 Tg B cells into RF secreting cells (Lau et al., 2005; Leadbetter et al., 2002), demonstrating that BCR-mediated internalization of the ICs delivers the TLR7/9 ligands into the endosomal compartment. In support of this hypothesis, lupus-prone MRL/lpr or MRL/gld mice lacking the TLR adaptor molecule MyD88 had reduced levels of autoAb. Furthermore, blocking endosomal TLR signaling decreased ANAs and improved survival in the B6.lpr and BXSB lupus-prone mice (Kono et al., 2009). Finally, the expression of TLR7 and TLR9 induces ANAs and RF production in a B-cell intrinsic manner (Koh et al., 2013; Teichmann et al., 2013). These nucleic acid sensing TLRs are also required for the production of pathogenic autoantibodies with non-nucleic acid specificities, most likely through dendritic cell activation (Koh et al., 2013).
TLR9 is essential for the development of anti-dsDNA and anti-chromatin Abs as TLR9 deficient MRL/lpr mice lacked autoAbs with these specificities but, unexpectedly, suffered from exacerbated lupus (Christensen et al., 2005). Meanwhile, TLR7 deficiency prevented the production of autoAbs against RNA-containing Ags and ameliorated disease in MRL/lpr mice. These opposing roles for TLR7 and TLR9 were replicated in the lupus-prone congenic strain B6.Nba2 (Santiago-Raber et al., 2010). Consistent with these results, the duplication of X-linked TLR7 gene results in a lupus-like phenotype in mice carrying the Y-linked autoimmune accelerating locus (yaa) or a Tg (Deane et al., 2007; Subramanian et al., 2006). Finally, genetic polymorphisms regulating TRL7 expression have been associated to SLE susceptibility in males (Deng et al., 2013; Shen et al., 2010).
The comparison of MRL/lpr mice deficient in TLR7, TLR9, and/or Myd88 revealed that TLR9 regulates TLR7 and suppresses the production of TLR7-dependent anti-RNA autoAbs (Nickerson et al., 2010). Furthermore, ANA production by MRL/lpr mice was solely attributed to TLR7/TLR9 signaling. Mechanistic studies have indicated that TLR9 restricts the survival of anergic anti-DNA B cells, while TLR7 requires type I IFN signaling to exacerbate disease symptoms (Nickerson et al., 2013a; Nickerson et al., 2013b). This suggests that TLR7 and TLR9 represent ideal therapeutic targets for SLE with TLR9 agonists used to eliminate anti-DNA Ab producing B cells and TLR7 antagonists used to dampen disease.
3.2 Dendritic Cells (DCs) modulate B cell responses via cytokine secretion
Alterations in cytokine levels are seen in SLE patients (Davis et al., 2011) and thus may play an important role in B cell mediated pathogenesis. Recent work showed that monocyte-derived DCs generated in the presence of serum from SLE patients promoted either naive and memory B cells to differentiate into IgG-secreting plasmablasts in a BAFF and IL-10 dependent manner (Joo et al., 2012). These results correlated with the elevated BAFF expression observed in blood DCs from SLE patients (Gerl et al., 2010).
In vitro studies showed that activated BM-derived DCs (BMDC) from the lupus prone B6.TC mice induced a greater B cell proliferation, Ab production, and PC differentiation than B6 BMDCs (Wan et al., 2008). The enhanced B cell response was mediated by soluble factors, including IL-6 and IFN-γ (Wan et al., 2008) and Sang et al. in preparation). In addition, DC deletion decreased autoAb titers and plasmablast numbers, which correlated with disease amelioration in MRL/lpr mice (Teichman et al., 2010). MyD88/TLR signaling in DCs contributes to the autoimmune pathology of MRL/lpr mice as deficient DCs secreted lower amounts of inflammatory cytokines. This, however, did not affect the production of pathogenic autoAbs (Teichman et al., 2013).
Finally, DC subsets regulate B cells differently. Immature BMDCs (iBMDC) as well as BM resident DCs (BM-RDC) inhibited TLR-induced B cell proliferation and differentiation whereas splenic resident DCs had no effect (Sindhava et al., 2012). Meanwhile, it is well accepted that IL-6, a cytokine produced at high levels by activated DCs, promotes B cell terminal differentiation into PCs. However, IL-6 secretion by DCs repressed LPS-induced Ab secretion in autoreactive B cells chronically exposed to self-Ag such as in the 2-12 anti-Sm or HEL-Ig X sHEL models (Kilmon et al., 2005). Therefore, cytokines secreted by DCs can play dual roles in promoting or repressing autoimmune responses.
4. Role of specific B cell subsets in lupus
Largely based on murine models of SLE, it has been proposed that marginal zone B (MZB) cells and B-1a cells contribute to the production of pathogenic autoAbs while B regulatory cells (Breg or B10) suppress these responses.
4.1 Marginal zone B cells expand and migrate to the follicle where they engage CD4+ T cells to promote autoantibody production
The vast majority of studies on MZBs have been conducted in the mouse, but there are important differences between the two species. In particular, human MZB cells are present in the spleen and circulation, but murine MZB cells are restricted to the MZ in the spleen (Steiniger et al., 2006). Because blood flow into the spleen initially passes through the MZ sinus, MZB cells are the first of B cells to encounter blood-borne Ag (Mebius and Kraal, 2005). Ag-activated MZB cells migrate toward the follicle (FO) where they can either receive CD4+ T cell help then become PCs or they can activate CD4+ T cells, which in turn activate cognant follicular B (FOB) cells (Förster et al., 1996; Lu and Cyster, 2002; MacLennan and Liu, 1991; Phan et al., 2005; Zhou et al., 2011). A weak affinity for self Ag suggests that MZB cells can become pathogenic in the context of lupus. The number of MZB cells expand with progression of disease in several lupus models, including NZB/W F1 mice (Wither et al., 2000), in which they generate more anti-dsDNA IgM than FOB cells (Zeng et al., 2000). NZB/W F1 MZB cells express CD80 at a high level equivalent to that of CD40-activated B cells (Wither et al., 2000). Because NZB/W F1 T cells express normal level of CD40L, this indicates that the expanded MZB cell population is intrinsically ‘active’, and is capable of activating autoreactive CD4+ T cells (Wither et al., 2000). Estrogen treatment of BALB/C mice carrying a dsDNA specific Tg BCR resulted in the expansion of the Tg MZB cells, which correlated with an increase in anti-dsDNA Ab titers (Grimaldi et al., 2001). The number of MZB cells also greatly expands in BAFF Tg mice (Enzler et al., 2006; Mackay et al., 1999). BAFF promotes B cell survival via the alternative NFκB pathway and induces class-switch and the production of autoAbs via the classical NFκB pathway. Disruption of either pathway reduces the MZB cell population, and disruption of the alternative NFκB pathway impairs the production of anti-dsDNA IgM (Enzler et al., 2006).
The expansion of MZB cells was also observed in the spontaneous triple congenic (TC) B6.Sle1.Sle2.Sle3 model (Duan et al., 2008). In addition, TC MZB cells breach follicular exclusion by migrating to the FO instead of staying in the MZ, and this is associated with high level of anti-dsDNA IgG (Duan et al., 2008). The breach of follicular exclusion occurs before autoAbs are detected in TC mice, suggesting that TC MZBs contribute to the production of pathogenic autoAbs. CD86-deficiency normalized both MZB cells location and anti-dsDNA IgG titers in TC mice (Duan et al., 2008), which suggested that T cells and MZB cells interact. Indeed, TC MZB cells were found co-localized with TC CD4+ T cells in the FO (Zhou et al., 2011). Furthermore, TC MZB cells proliferated more and secreted more anti-DNA IgM than B6 MZB cells in response to anti-CD40 stimulation (Zhou et al., 2011). TC MZB cells also induced B6 CD4+ T cells to proliferate more than did B6 MZB cells (Zhou et al., 2011). This suggests that autoreactive TC MZB cells contribute to disease by interacting with autoreactive CD4+ T cells in the follicles.
Expansion of MZB cells does not always correlate with disease in mouse models of lupus. NZM TAN mice only manifest a mild lupus-like phenotype although their MZB cell population is enlarged (Duan et al., 2007). NZM TAN MZB cells express high levels of CD5, a negative regulator of BCR signaling, which may be the reason why they do not respond to T cell-independent Ag stimulation and do not migrate to the FO (Duan et al., 2007; Duan et al., 2008). BXSB.Yaa mice represent a model which lupus develops in spite of a drastically reduced number of MZB cells in the spleen (Amano et al., 2003).
4.2 Autoreactive B1a cells contribute to glomerulonephritis and T cell activation
B-1 cells represent a separate lineage of B lymphocytes found mostly in the pleural and peritoneal cavities, and in lower numbers in the spleen. They have been subdivided into CD5+ B-1a and CD5− B-1b cells (Baumgarth, 2011; Godin et al., 1993; Hayakawa et al., 1985; Kantor, 1991). CD5 is a negative regulator of BCR signaling, which explains why CD5− B-1b, but not CD5+ B-1a cells undergo clonal expansion in response to Ag challenge (Alugupalli et al., 2003; Alugupalli et al., 2004; Bikah et al., 1996). As an alternative to BCR induced activation, B1a cells are activated by TLR signaling, which induce their migration from the peritoneum to the spleen or to sites of inflammation where they can class-switch and differentiate into PCs (Yang et al., 2007). B-1 cells are the main source of natural IgM, which are Abs generated in the absence of Ag exposure (Baumgarth, 2011; Baumgarth et al., 2005; Bouvet and Dighiero, 1998; Stewart, 1992), that have a low affinity polyreactivity and autoreactivity (Avrameas, 1991; Baumgarth et al., 1999; Casali and Schettino, 1996; Choi and Baumgarth, 2008; Coutinho et al., 1995). The autoreactivity of natural Abs has suggested that B-1 cells contribute to autoimmune pathogenesis (Duan and Morel, 2006). Accordingly, the removal of peritoneal B-1 cells from NZB/W F1 mice correlated with disease attenuation (Mihara et al., 1988), and osteopontin-induced B-1 cell expansion paralleled an increased anti-dsDNA Ab titers (Iizuka et al., 1998). On the other hand, B-1a cells are not responsible for autoAb production in Fas-deficient mice (Reap et al., 1993), and IL-5 induced expansion of B-1a cells in NZB/W F1 was associated with disease protection (Wen et al., 2004).
B-1a cells have been shown to contribute to the development of GN in several murine models of lupus. In NZB/W F1 mice, class switched B-1a cells travel to the spleen (Enghard et al., 2010) or kidneys where they secrete anti-dsDNA IgG (Ishikawa et al., 2002; Ishikawa et al., 2001; Ito et al., 2004). A correlation between the expansion of B-1a cells and development of GN has been linked to the NZM2410-derived Sle2 lupus susceptibility locus (Xu et al., 2005), in which Sle2c1 provides the most significant contribution (Xu et al., 2011). Sle2c1 contains a SNP in the promoter of the Cdkn2c gene that encodes for the cyclin-dependent kinase inhibitor p18INK4c. This SNP creates a second binding site for YY-1 that represses p18 transcription. Contrary to conventional B cells, B-1 cells are maintained by self-renewal in which p18 is critical to regulate cell cycle. The decreased expression of p18 promotes cell division hence the observed B-1a cell expansion in mice carrying Sle2c1 (Potula et al.; Xu et al.). B-1a cells can also exacerbate lupus in mice by engaging CD4+ T cells and promoting Th17 differentiation (Zhong et al., 2007). IL-17 has been implicated in lupus in mice and humans (Crispín and Tsokos, 2010).
Human B1a cells have been recently been described (Griffin et al., 2011), SLE patients have an enlarged population of B1 cells that are activated and induced the expansion of CD4+ T cells (Griffin and Rothstein, 2011). Even though B-1a cells do not contribute to pathology in all mouse models of lupus, the fact that B1 cells are expanded in SLE patients warrants further investigation of the mechanisms by which B-1a cells expand and contribute to systemic autoimmunity.
4.3 Regulatory B cells can suppress lupus before disease onset
A subset of B cells that share surface markers, including CD5 and CD1d, with MZB and B-1a cells possesses regulatory function by their production of IL-10 (Blair et al., 2010). In the Palmerston North mouse model of lupus, TLR9 activated MZB cells secrete high level of IL-10, which is associated with a reduction of the pro-inflammatory cytokine subunit IL-12p40 (Lenert et al., 2005). B cell depletion before disease onset accelerated the development of proteinuria in NZB/W F1 mice, indicating that Bregs have a protective role early in the disease process (Haas et al., 2010). However, depletion of B cells from NZB/W F1 mice during disease onset reduced disease severity (Haas et al., 2010), indicating that when pathogenic autoAbs are produced, Bregs have a very limited, if any, protective role. Adoptive transfers of CD1dhi CD5+ B cells into CD19− deficient NZB/W F1 mice significantly prolonged their survival, possibly through the expansion of regulatory T cells (Watanabe et al., 2010). Tim-1 deficient mice lacked IL-10 expression in B cells, and this resulted in systemic autoimmunity that was enhanced by Fas-deficiency (Xiao et al., 2012). On the other hand, B cell-specific depletion of IL-10 had no protective effect on disease progression in the MRL/lpr mice, indicating that B10 cells were not involved, at least in this model (Teichmann et al., 2012).
A very small numbers of B cells that secrete IL-10 in vitro in response to CpG have been found in the blood of some SLE patients in a higher amount than in healthy controls (Iwata et al., 2011). CD40-stimulated CD19+ CD24hi CD38hi B cells have also been found to suppress human Th1 differentiation, partly via IL-10. Furthermore, the suppressive capacity of CD19+ CD24hi CD38hi is defective in SLE patients (Blair et al., 2010). Overall, these studies suggest that regulatory B cells may play a protective role in the early stages of lupus, but the mechanisms may differ between mice and humans (Fujio et al., 2013).
4. Interactions of B cells with other immune cells that contribute to lupus
5.1 Neutrophils can activate autoreactive B cells
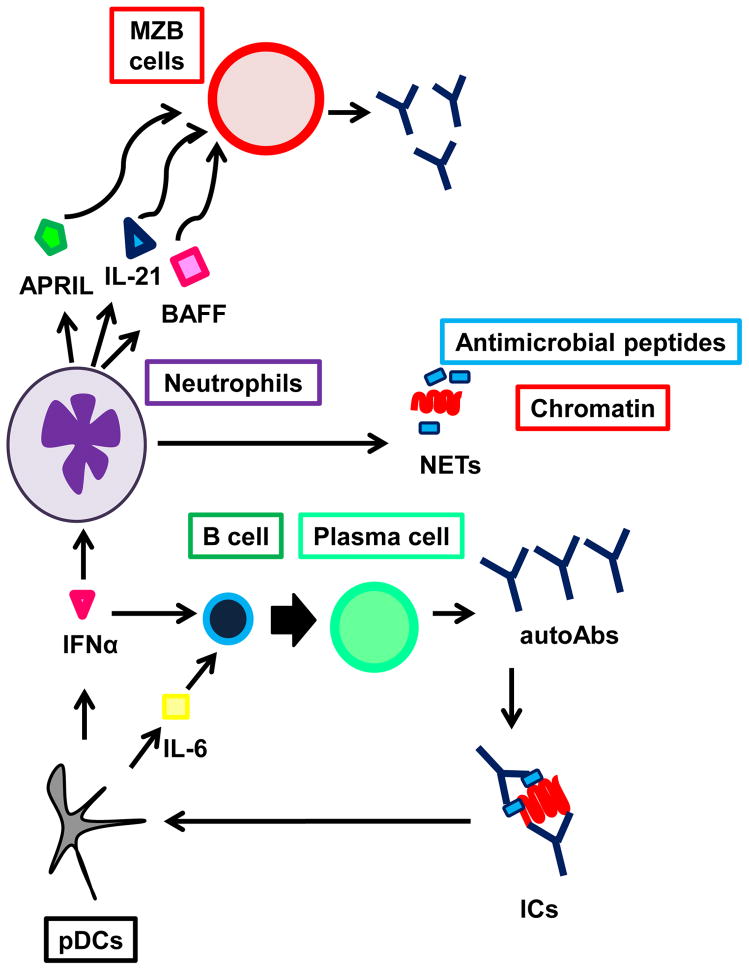
Neutrophils (PMNs) offer protection from pathogens by secreting neutrophil extracellular traps (NETs) that contain antimicrobial peptides and chromatin. NETs are not quickly cleared in SLE patients, leading to the formation of autoAbs-DNA ICs (Knight and Kaplan, 2012). In addition to leading to organ damage, ICs stimulate plasmacytoid dendritic cells (pDCs) to secrete IFNα, which re-stimulate PMNs to produce more NETs, thereby creating a positive feedback loop which exacerbates disease (Knight and Kaplan, 2012; Lande et al., 2011). Furthermore, IFNα in combination with IL-6 induce B cell differentiation into PCs (Jego et al., 2003). IFNα also stimulate myeloid DCs (mDCs) that activate and induce class-switching in autoreactive B cells either directly or indirectly through autoreactive T helper cells (Banchereau and Pascual, 2006; Caux et al., 1997). A recent study has unveiled a subset of splenic B cell helper neutrophils (NBH), that, when activated by microbial products, secrete BAFF, APRIL and IL-21 to stimulate Ab production and class-switch by MZB cells (Puga et al., 2012). Although the NBH subset has been found to be defective in immune deficiencies (Puga et al., 2012), its role in lupus has not yet been explored. These findings have been summarized in Figure 1.
Figure 1.
Activated neutrophils produce neutrophil extracellular traps (NETs). This mechanism of immune protection exposes large amounts of autoantigens that form immune complexes (IC) with autoAbs. The IC can induce plasmacytoid dendritic cells (pDCs) to secrete IFNα, which in turn stimulates neutrophils to generate more NETs. Furthermore, pDC-derived IFNα and IL6 induce B cell differentiation into plasma cells. In the spleen, TLR activation triggers neutrophils to differentiate into B cell helper neutrophils (NBH), which are located in the perifollicular region and secrete APRIL, IL–21, and BAFF to induce MZB cells to secrete antibodies. In addition, NBH can induce MZB cells to express Activation Induced Cytidine Deaminase (AID) and undergo class-switch.
5.2 Marginal zone macrophages are necessary for the clearance of apoptotic cell debris and arrest of autoreactive marginal zone B cells in the marginal zone
MZ macrophages (MZMφ) retain MZB cells within the MZ by processing apoptotic cell (AP) debris, preventing them to activate autoreactive B cells which migrate to the FO (Karlsson, 2003; Wermeling et al., 2007). Another way to retain MZB cells in the MZ is via direct contact via MARCO receptor on MZMφ and unknown receptor on MZB cells (Chen et al., 2005; Yokota et al., 1998). Both mechanisms work together to prevent release of Ag-activated autoreactive MZB cells into the FO where they can initiate the process that leads to autoAbs production.
In BXD2 mice, the MZMφ population gradually decreases with progression of disease, and they are inherently unable to clear AP debris (Li et al., 2013). This exposes autoreactive MZB and MZB precursor (MZP) cells to autoAgs. BXD2 MZP cells upload more AP debris than MZB cells, and only MZP cells migrate to the FO (Li et al., 2013). Mice deficient in scavenger receptors SR–A and MARCO, which are used by MZMφ to bind apoptotic debris (Kraal and Mebius, 2006; Peiser and Gordon, 2001; Platt et al., 1996; Wermeling et al., 2007) produce high titer of DNA specific Abs in response to APs (Wermeling et al., 2007) (see Figure 2). Furthermore, blocking scavenger receptor mediated signaling increased the anti-DNA Ab titer in FcγRIIB−/− and NZB/W F1 mice (Wermeling et al., 2007). Finally, MARCO has been proposed as a lupus susceptibility gene in the BXSB.Yaa model (Rogers et al., 2009).
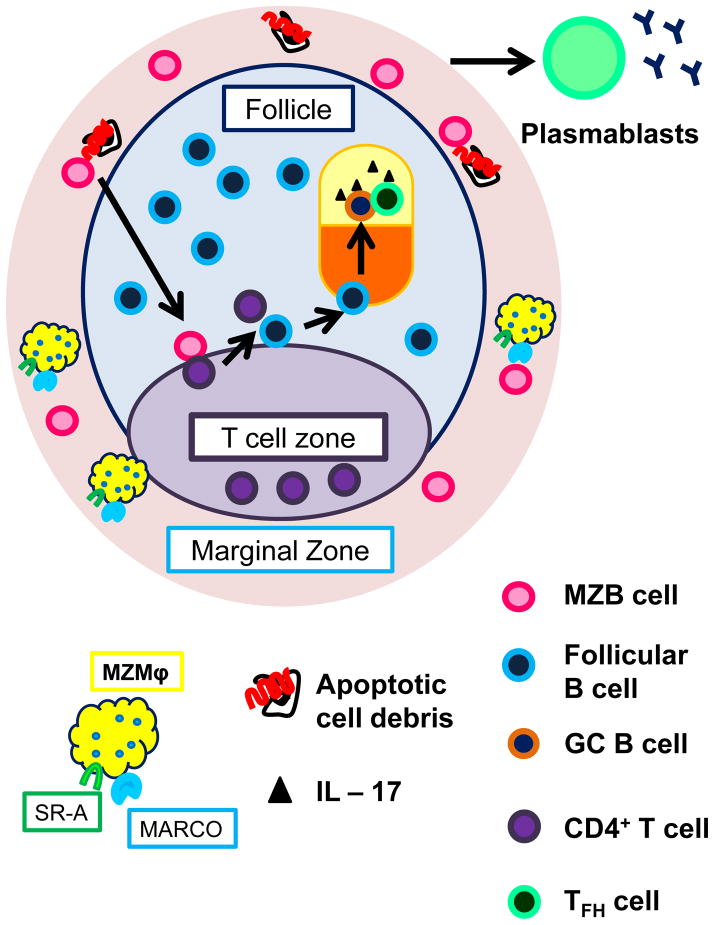
Figure 2.
Marginal zone macrophages (MZMϕ) retain MZB cells in the marginal zone and clear apoptotic cell debris. In their absence, such as in the BXD2 mouse, exposes autoreactive MZB cells to autoantigens from apoptotic cells. Such antigen-activated autoreactive MZB cells can either migrate to the red pulp and become short-lived plasmablasts or migrate into the follicle where they engage cognate CD4+ T cells from the T cell zone. Those activated CD4+ T cells can activate cognate follicular B cell, which proliferate in the follicle to form a germinal center (GC). Proliferating GC B cells undergo affinity maturation in the dark zone, then enter the light zone where it encounters follicular helper T (TFH) cells. TFH help induce the engaged B cell to undergo class switch and become either long lived antibody secreting plasma cells or memory B cells. Mountz’s group have shown in the BXD2 model that IL – 17 signaling arrests both TFH cells and GC B cells in the GC, and thereby prolongs GC reaction and promote antibody production.
Functional MZMφ can also contribute to murine lupus by supporting the expansion of MZB cells via direct contact. Splenic Mφ and most likely MZMφ express delta-like 1 (DL1) ligand, which when engaged with Notch2 receptor (Notch2R) on transitional B cells or MZPs to promote MZB cell differentiation (Moriyama et al., 2008). While anti-DL1 monoclonal Ab (mAb) treatment of young non-autoimmune B6 and NZB/W F1 mice successfully depleted MZB cells, the same treatment of diseased NZB/W F1 mice was not able to reduce their MZB cell population (Moriyama et al., 2008). This suggests that the expansion of MZB cells in old NZB/W F1 mice may result from an enhanced Notch2R signaling, and possibly an increased expression of DL1.
5.3 T cells activate autoreactive B cells
Several T cell subsets contribute to lupus by activating autoreactive B cells. Follicular helper T (TFH) cells can be divided into two subsets: CXCR5+ TFH cells are attracted to CXCL13 in the GC where they induce B cells to undergo class switch and produce Abs, meanwhile, CXCR4+ extrafollicular T cells (THEF) are attracted by CXCL12 to extrafollicular sites in lymphoid organs where they induce differentiation of cognate B cells into short-lived plasmablasts (Breitfeld et al., 2000; Chan and Brink, 2012; Craft, 2012; Goodnow et al., 2010; Kim et al., 2001; Kim et al., 2005; Schaerli et al., 2000). Some SLE patients show elevated numbers of circulating TFH cells that positively correlate with levels of autoAbs, circulating GC B cells and disease severity (Feng et al., 2012; Simpson et al., 2010; Terrier et al., 2012). The Sanroque mutation results in an increased ICOS expression, which results in expansion of IL-21-secreting TFH cells, the spontaneous formation of GCs and lupus-like phenotypes (Luzina et al., 2001; Vinuesa et al., 2005). IL-21, CD40L, and ICOSL are the key mediators of the interactions between TFH cells and GC B cells in the differentiation of long-lived PCs producing high affinity class-switched Abs. Blockade of each of these three pathways reduced anti-dsDNA IgG titers and alleviated renal pathology in lupus-prone mice (Daikh et al., 1997; Herber et al., 2007; Iwai et al., 2003; Ma et al., 1996). Anti-CD40L treatment has been shown to be effective in SLE patients (Grammer et al., 2003), but was not further pursued due to thromboembolic side effects caused by the aggregation of activated platelets expressing CD40L (Peters et al., 2009). In the MRL/lpr lupus-prone mice, THEF cells secrete IL-21 and induce B cells outside the FO to undergo SHM, class-switch, and differentiate into short-lived autoAb producing PCs (Odegard et al.; Rankin et al.). The same phenomenon was reported for the AM14Tg BCR producing rheumatoid factor (William et al., 2002), although in this model T cell help is not necessary to, but enhances autoAb production (Sweet et al., 2011). Nevertheless, both TFH and THEF produced IL-21 stimulates B cells to differentiate into autoAb-secreting cells (Odegard et al.; Vinuesa et al.), and polymorphisms in the IL21 and IL21R genes have been associated with human SLE disease (Sawalha et al., 2008; Webb et al., 2009).
TH17 cells represent another B cell activating T cell subset. Increased serum IL-17 in SLE patients is correlated with disease severity (Doreau et al., 2009; Garrett-Sinha et al., 2008). IL-17 in combination with BAFF promotes autoreactive human B cell survival and differentiation into PCs (Doreau et al., 2009). BXD2 mice have large populations of IL17 receptor expressing (IL17-R+) B cells and Th17 cells (Hsu et al., 2008). IL-17 induces BXD2 B cells to up-regulate the expression of regulator of G signaling proteins 16, which leads to a decreased sensitivity of G protein coupled chemokine receptors on B cells to chemokine gradients and thereby maintain GC stability (Xie et al., 2010). This explains how IL-17 activated B cells are retained in GCs where they receive prolonged TFH help in BXD2 mice (Hsu et al., 2008) (see Figure 2).
Finally, in vitro assays have shown that NK T cells engage MZB and B-1 cells with CD1d and CD40L (Takahashi and Strober, 2008). This interaction induced MZB and B-1 cells from 12 weeks old NZB/W F1 to secrete anti-dsDNA IgM, and B-1 cells, to a lesser extent than MZB cells, from older NZB/W F1 to class witch to anti-dsDNA IgG (Takahashi and Strober, 2008).
B cells producing pathogenic autoantibodies are the primary effector cells in systemic lupus erythematosus
B cell tolerance to self antigens is breached through multiple mechanisms
B cell subsets such as marginal zone B cells, B1-a cells and regulatory B cells, modulate autoimmune pathogenesis
Interactions between B cells and other immune cell types such as T cells, dendritic cells, macrophages and neutrophils, are important to sustain autoantibody production.
Abbreviations
- Ab
antibody
- AFC
antibody forming cells
- BCR
B cell receptor
- DC
dendritic cell
- GC
germinal center
- IC
immune complex
- ICOS
inducible T cell co-stimulator
- PC
plasma cell
- SLE
systemic lupus erythematosus
- Tg
transgenic
- Ag
antigen
- MZ
marginal zone
- FO
follicle
- MZB cells
marginal zone B cells
- FOB cells
follicular B cells
- TC
triple congenic
- GN
Glomerulonephritis
- SNP
single nucleotide polymorphism
- PMN
polymorphonuclear neutrophil
- NET
neutrophil extracellular traps
- pDCs
plasmacytoid dendritic cells
- mDC
myeloid DC
- AID
activation induced (cytidine) deaminase
- MZMφ
marginal zone macrophage
- AP
apoptotic cell
- DL1
delta-like 1
- mAb
monoclonal antibody
- TFH
follicular helper T cells
- THEF
extrafollicular T cells
- SHM
somatic hypermutation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–27. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–90. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Amano H, Amano E, Moll T, Marinkovic D, Ibnou-Zekri N, Martinez-Soría E, Semac I, Wirth T, Nitschke L, Izui S. The Yaa mutation promoting murine lupus causes defective development of marginal zone B cells. J Immunol. 2003;170:2293–301. doi: 10.4049/jimmunol.170.5.2293. [DOI] [PubMed] [Google Scholar]
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol Today. 1991;12:154–9. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–92. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–5. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–62. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–9. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- Bouvet JP, Dighiero G. From natural polyreactive autoantibodies to à la carte monoreactive antibodies to infectious agents: is it a small world after all? Infect Immun. 1998;66:1–4. doi: 10.1128/iai.66.1.1-4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brard F, Shannon M, Prak EL, Litwin S, Weigert M. Somatic mutation and light chain rearrangement generate autoimmunity in anti-single-stranded DNA transgenic MRL/lpr mice. J Exp Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–43. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappione A, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–16. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–79. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–70. [PubMed] [Google Scholar]
- Ceppellini R, Polli E, Celada F. A DNA-reacting factor in serum of a patient with lupus erythematosus diffusus. Proc Soc Exp Biol Med. 1957;96:572–4. doi: 10.3181/00379727-96-23544. [DOI] [PubMed] [Google Scholar]
- Chan TD, Brink R. Affinity-based selection and the germinal center response. Immunol Rev. 2012;247:11–23. doi: 10.1111/j.1600-065X.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- Chan TD, Wood K, Hermes JR, Butt D, Jolly CJ, Basten A, Brink R. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37:893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pikkarainen T, Elomaa O, Soininen R, Kodama T, Kraal G, Tryggvason K. Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J Immunol. 2005;175:8173–80. doi: 10.4049/jimmunol.175.12.8173. [DOI] [PubMed] [Google Scholar]
- Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–64. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S, Kashgarian M, Alexopoulou L, Flavell R, Akira S, Shlomchik M. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–31. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–47. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispín JC, Tsokos GC. IL-17 in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:943254. doi: 10.1155/2010/943254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–8. [PubMed] [Google Scholar]
- Davis LS, Hutcheson J, Mohan C. The role of cytokines in the pathogenesis and treatment of systemic lupus erythematosus. J Interferon Cytokine Res. 2011;31:781–9. doi: 10.1089/jir.2011.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–10. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Zhao J, Sakurai D, Kaufman KM, Edberg JC, Kimberly RP, Kamen DL, Gilkeson GS, Jacob CO, Scofield RH, Langefeld CD, Kelly JA, Ramsey-Goldman R, Petri MA, Reveille JD, Vilá LM, Alarcón GS, Vyse TJ, Pons-Estel BA, Freedman BI, Gaffney PM, Sivils KM, James JA, Gregersen PK, Anaya JM, Niewold TB, Merrill JT, Criswell LA, Stevens AM, Boackle SA, Cantor RM, Chen W, Grossman JM, Hahn BH, Harley JB, Alarc n-Riquelme ME, Brown EE, Tsao BP Group AC and networks BaG. MicroRNA-3148 modulates allelic expression of toll-like receptor 7 variant associated with systemic lupus erythematosus. PLoS Genet. 2013;9:e1003336. doi: 10.1371/journal.pgen.1003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Bérard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- Duan B, Croker BP, Morel L. Lupus resistance is associated with marginal zone abnormalities in an NZM murine model. Lab Invest. 2007;87:14–28. doi: 10.1038/labinvest.3700497. [DOI] [PubMed] [Google Scholar]
- Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Duan B, Niu H, Xu Z, Sharpe AH, Croker BP, Sobel ES, Morel L. Intrafollicular location of marginal zone/CD1d(hi) B cells is associated with autoimmune pathology in a mouse model of lupus. Lab Invest. 2008;88:1008–20. doi: 10.1038/labinvest.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182:559–66. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enghard P, Humrich JY, Chu VT, Grussie E, Hiepe F, Burmester GR, Radbruch A, Berek C, Riemekasten G. Class switching and consecutive loss of dsDNA-reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur J Immunol. 2010;40:1809–1818. doi: 10.1002/eji.200940050. [DOI] [PubMed] [Google Scholar]
- Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–15. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–4. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang D, Chen J, Lu L, Hua B, Li X, Tsao BP, Sun L. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PLoS One. 2012;7:e51982. doi: 10.1371/journal.pone.0051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio K, Okamura T, Sumitomo S, Yamamoto K. Regulatory cell subsets in the control of autoantibody production related to systemic autoimmunity. Ann Rheum Dis. 2013;72:ii85–ii89. doi: 10.1136/annrheumdis-2012-202341. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–47. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–25. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl V, Lischka A, Panne D, Grossmann P, Berthold R, Hoyer BF, Biesen R, Bruns A, Alexander T, Jacobi A, Dörner T, Burmester GR, Radbruch A, Hiepe F. Blood dendritic cells in systemic lupus erythematosus exhibit altered activation state and chemokine receptor function. Ann Rheum Dis. 2010;69:1370–7. doi: 10.1136/ard.2009.111021. [DOI] [PubMed] [Google Scholar]
- Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–8. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, Illei GG, Lipsky PE. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112:1506–20. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. The J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2591–8. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–90. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: implications for the origins of autoantibodies in autoimmune disease. J Exp Med. 1996;184:1269–78. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–68. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197:197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–30. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- Herlands R, Christensen S, Sweet R, Hershberg U, Shlomchik M. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–60. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol. 2007;37:3339–51. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–84. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- Iizuka J, Katagiri Y, Tada N, Murakami M, Ikeda T, Sato M, Hirokawa K, Okada S, Hatano M, Tokuhisa T, Uede T. Introduction of an osteopontin gene confers the increase in B1 cell population and the production of anti-DNA autoantibodies. Lab Invest. 1998;78:1523–33. [PubMed] [Google Scholar]
- Ishikawa S, Nagai S, Sato T, Akadegawa K, Yoneyama H, Zhang YY, Onai N, Matsushima K. Increased circulating CD11b+CD11c+ dendritic cells (DC) in aged BWF1 mice which can be matured by TNF-alpha into BLC/CXCL13-producing DC. Eur J Immunol. 2002;32:1881–7. doi: 10.1002/1521-4141(200207)32:7<1881::AID-IMMU1881>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, Lipp M, Matsushima K. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ishikawa S, Sato T, Akadegawa K, Yurino H, Kitabatake M, Hontsu S, Ezaki T, Kimura H, Matsushima K. Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J Immunol. 2004;172:3628–34. doi: 10.4049/jimmunol.172.6.3628. [DOI] [PubMed] [Google Scholar]
- Iwai H, Abe M, Hirose S, Tsushima F, Tezuka K, Akiba H, Yagita H, Okumura K, Kohsaka H, Miyasaka N, Azuma M. Involvement of inducible costimulator-B7 homologous protein costimulatory pathway in murine lupus nephritis. J Immunol. 2003;171:2848–54. doi: 10.4049/jimmunol.171.6.2848. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi AM, Zhang J, Mackay M, Aranow C, Diamond B. Phenotypic characterization of autoreactive B cells--checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS One. 2009;4:e5776. doi: 10.1371/journal.pone.0005776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Joo H, Coquery C, Xue Y, Gayet I, Dillon SR, Punaro M, Zurawski G, Banchereau J, Pascual V, Oh S. Serum from patients with SLE instructs monocytes to promote IgG and IgA plasmablast differentiation. J Exp Med. 2012;209:1335–48. doi: 10.1084/jem.20111644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunol Today. 1991;12:389–91. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- Karlsson Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel A, Rosner I, Halasz K, Grushko G, Shoenfeld Y, Paran D, Toubi E. Antibody clustering helps refine lupus prognosis. Semin Arthritis Rheum. 2009;39:66–70. doi: 10.1016/j.semarthrit.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–81. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Lim HW, Kang SG, Hillsamer P, Kim CH. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol. 2005;6:3. doi: 10.1186/1471-2172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24:441–50. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, Beutler BA, Theofilopoulos AN, Kono DH. Role of Nucleic Acid-Sensing TLRs in Diverse Autoantibody Specificities and Anti-Nuclear Antibody-Producing B Cells. J Immunol. 2013;190:4982–90. doi: 10.4049/jimmunol.1202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono D, Haraldsson M, Lawson B, Pollard K, Koh Y, Du X, Arnold C, Baccala R, Silverman G, Beutler B, Theofilopoulos A. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–6. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–57. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- Li H, Wu Q, Li J, Yang P, Zhu Z, Luo B, Hsu HC, Mountz JD. Cutting Edge: defective follicular exclusion of apoptotic antigens due to marginal zone macrophage defects in autoimmune BXD2 mice. J Immunol. 2013;190:4465–9. doi: 10.4049/jimmunol.1300041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196:1543–52. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li L, Kumar K, Xie C, Lightfoot S, Zhou X, Kearney J, Weigert M, Mohan C. Lupus susceptibility genes may breach tolerance to DNA by impairing receptor editing of nuclear antigen-reactive B cells. J Immunol. 2007;179:1340–52. doi: 10.4049/jimmunol.179.2.1340. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zou Y, Davidson A. Plasma cells in systemic lupus erythematosus: the long and short of it all. Eur J Immunol. 2011;41:588–91. doi: 10.1002/eji.201041354. [DOI] [PubMed] [Google Scholar]
- Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–12. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–84. [PubMed] [Google Scholar]
- Ma J, Xu J, Madaio MP, Peng Q, Zhang J, Grewal IS, Flavell RA, Craft J. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–26. [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC, Liu YJ. Marginal zone B cells respond both to polysaccharide antigens and protein antigens. Res Immunol. 1991;142:346–51. doi: 10.1016/0923-2494(91)90089-2. [DOI] [PubMed] [Google Scholar]
- Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J Exp Med. 1997;186:1257–67. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999;189:1799–814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–41. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Mihara M, Ohsugi Y, Saito K, Miyai T, Togashi M, Ono S, Murakami S, Dobashi K, Hirayama F, Hamaoka T. Immunologic abnormality in NZB/NZW F1 mice. Thymus-independent occurrence of B cell abnormality and requirement for T cells in the development of autoimmune disease, as evidenced by an analysis of the athymic nude individuals. J Immunol. 1988;141:85–90. [PubMed] [Google Scholar]
- Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–5. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Sekine C, Koyanagi A, Koyama N, Ogata H, Chiba S, Hirose S, Okumura K, Yagita H. Delta-like 1 is essential for the maintenance of marginal zone B cells in normal mice but not in autoimmune mice. Int Immunol. 2008;20:763–73. doi: 10.1093/intimm/dxn034. [DOI] [PubMed] [Google Scholar]
- Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979;22:1188–94. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Mandik L, Bui A, Kavaler J, Norvell A, Monroe JG, Roark JH, Erikson J. Characterization of anti-single-stranded DNA B cells in a non-autoimmune background. J Immunol. 1997;159:2633–44. [PubMed] [Google Scholar]
- Nickerson K, Christensen S, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik M. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–8. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson KM, Christensen SR, Cullen JL, Meng W, Luning Prak ET, Shlomchik MJ. TLR9 promotes tolerance by restricting survival of anergic anti-DNA B cells, yet is also required for their activation. J Immunol. 2013a;190:3889–94. doi: 10.4049/jimmunol.1202115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson KM, Cullen JL, Kashgarian M, Shlomchik MJ. Exacerbated autoimmunity in the absence of TLR9 in MRL.Fas lpr mice depends on Ifnar1. J Immunol. 2013b;190:3889–94. doi: 10.4049/jimmunol.1203525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–86. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001;3:149–59. doi: 10.1016/s1286-4579(00)01362-9. [DOI] [PubMed] [Google Scholar]
- Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Gardam S, Basten A, Brink R. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. J Immunol. 2005;174:4567–78. doi: 10.4049/jimmunol.174.8.4567. [DOI] [PubMed] [Google Scholar]
- Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Curr Opin Immunol. 2011;23:721–31. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–60. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula HH, Xu Z, Zeumer L, Sang A, Croker BP, Morel L. Cyclin-dependent kinase inhibitor Cdkn2c deficiency promotes B1a cell expansion and autoimmunity in a mouse model of lupus. J Immunol. 2012;189:2931–40. doi: 10.4049/jimmunol.1200556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baró T, de Heredia CD, Torán N, Català A, Torrebadell M, Fortuny C, Cusí V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Aróstegui JI, Juan M, Yagüe J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Santiago C, Borrero M, Tedder TF, Clarke SH. Lupus-specific antiribonucleoprotein B cell tolerance in nonautoimmune mice is maintained by differentiation to B-1 and governed by B cell receptor signaling thresholds. J Immunol. 2001;166:2412–9. doi: 10.4049/jimmunol.166.4.2412. [DOI] [PubMed] [Google Scholar]
- Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–35. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, Keegan S, Senices M, Stedman N, Ryan M, Bloom L, Medley Q, Collins M, Nickerson-Nutter C, Craft J, Young D, Dunussi-Joannopoulos K. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188:1656–67. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Goodnow CC. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J Immunol. 1994;153:2831–42. [PubMed] [Google Scholar]
- Reap EA, Sobel ES, Cohen PL, Eisenberg RA. Conventional B cells, not B-1 cells, are responsible for producing autoantibodies in lpr mice. J Exp Med. 1993;177:69–78. doi: 10.1084/jem.177.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, Marshak-Rothstein A. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. J Immunol. 2000;165:1626–33. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- Robbins WC, Holman HR, Deicher H, Kunkel HG. Complement fixation with cell nuclei and DNA in lupus erythematosus. Proc Soc Exp Biol Med. 1957;96:575–9. doi: 10.3181/00379727-96-23545. [DOI] [PubMed] [Google Scholar]
- Rogers NJ, Lees MJ, Gabriel L, Maniati E, Rose SJ, Potter PK, Morley BJ. A defect in Marco expression contributes to systemic lupus erythematosus development via failure to clear apoptotic cells. J Immunol. 2009;182:1982–90. doi: 10.4049/jimmunol.0801320. [DOI] [PubMed] [Google Scholar]
- Rubio CF, Kench J, Russell DM, Yawger R, Nemazee D. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J Immunol. 1996;157:65–71. [PubMed] [Google Scholar]
- Rudofsky U, Evans B, Balaban S, Mottironi V, Gabrielsen A. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68:419–26. [PubMed] [Google Scholar]
- Santiago-Raber M, Dunand-Sauthier I, Wu T, Li Q, Uematsu S, Akira S, Reith W, Mohan C, Kotzin B, Izui S. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–48. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Santiago-Raber ML, Kikuchi S, Borel P, Uematsu S, Akira S, Kotzin BL, Izui S. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181:1556–62. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- Santulli-Marotto S, Qian Y, Ferguson S, Clarke SH. Anti-Sm B cell differentiation in Ig transgenic MRL/Mp-lpr/lpr mice: altered differentiation and an accelerated response. J Immunol. 2001;166:5292–9. doi: 10.4049/jimmunol.166.8.5292. [DOI] [PubMed] [Google Scholar]
- Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–19. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J, Wakeland EK, Li QZ, Wandstrat AE, Karp DR, James JA, Merrill JT, Lipsky P, Harley JB. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:458–61. doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, Fu Q, Deng Y, Qian X, Zhao J, Kaufman KM, Wu YL, Yu CY, Tang Y, Chen JY, Yang W, Wong M, Kawasaki A, Tsuchiya N, Sumida T, Kawaguchi Y, Howe HS, Mok MY, Bang SY, Liu FL, Chang DM, Takasaki Y, Hashimoto H, Harley JB, Guthridge JM, Grossman JM, Cantor RM, Song YW, Bae SC, Chen S, Hahn BH, Lau YL, Tsao BP. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107:15838–43. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5:1329–41. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, Goodnow CC, Vinuesa CG, Cook MC. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- Sindhava VJ, Tuna H, Gachuki BW, DiLillo DJ, Avdiushko MG, Onami TM, Tedder TF, Cohen DA, Bondada S. Bone marrow dendritic cell-mediated regulation of TLR and B cell receptor signaling in B cells. J Immunol. 2012;189:3355–67. doi: 10.4049/jimmunol.1101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiniger B, Timphus EM, Barth PJ. The splenic marginal zone in humans and rodents: an enigmatic compartment and its inhabitants. Histochem Cell Biol. 2006;126:641–8. doi: 10.1007/s00418-006-0210-5. [DOI] [PubMed] [Google Scholar]
- Stewart J. Immunoglobulins did not arise in evolution to fight infection. Immunol Today. 1992;13:396–9. doi: 10.1016/0167-5699(92)90088-O. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–5. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Christensen SR, Harris ML, Shupe J, Sutherland JL, Shlomchik MJ. A new site-directed transgenic rheumatoid factor mouse model demonstrates extrafollicular class switch and plasmablast formation. Autoimmunity. 2010;43:607–18. doi: 10.3109/08916930903567500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Cullen JL, Shlomchik MJ. Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. J Immunol. 2013;190:1974–81. doi: 10.4049/jimmunol.1202816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Ols ML, Cullen JL, Milam AV, Yagita H, Shlomchik MJ. Facultative role for T cells in extrafollicular Toll-like receptor-dependent autoreactive B-cell responses in vivo. Proc Natl Acad Sci U S A. 2011;108:7932–7. doi: 10.1073/pnas.1018571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38:156–65. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey D, Seif A, Grupp S. Advances in the management and understanding of autoimmune lymphoproliferative syndrome (ALPS) Br J Haematol. 2010;148:205–16. doi: 10.1111/j.1365-2141.2009.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Kashgarian M, Weaver CT, Roers A, Muller W, Shlomchik MJ. B cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J Immunol. 2012;188:678–85. doi: 10.4049/jimmunol.1102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–78. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–40. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, Klatzmann D, Saadoun D, Cacoub P. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol. 2012;39:1819–28. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–13. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181:7770–7. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–8. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- Wan S, Zhou Z, Duan B, Morel L. Direct B cell stimulation by dendritic cells in a mouse model of lupus. Arthritis Rheum. 2008;58:1741–50. doi: 10.1002/art.23515. [DOI] [PubMed] [Google Scholar]
- Wang H, Shlomchik MJ. Autoantigen-specific B cell activation in Fas-deficient rheumatoid factor immunoglobulin transgenic mice. J Exp Med. 1999;190:639–49. doi: 10.1084/jem.190.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder T, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–9. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD, Ziegler J, Kimberly RP, Edberg JC, Ramsey-Goldman R, Petri M, Reveille JD, Alarcón GS, Vilá LM, Alarcón-Riquelme ME, James JA, Gilkeson GS, Jacob CO, Moser KL, Gaffney PM, Vyse TJ, Nath SK, Lipsky P, Harley JB, Sawalha AH. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009;60:2402–7. doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang D, Kikuchi Y, Jiang Y, Nakamura K, Xiu Y, Tsurui H, Takahashi K, Abe M, Ohtsuji M, Nishimura H, Takatsu K, Shirai T, Hirose S. Transgene-mediated hyper-expression of IL-5 inhibits autoimmune disease but increases the risk of B cell chronic lymphocytic leukemia in a model of murine lupus. Eur J Immunol. 2004;34:2740–2749. doi: 10.1002/eji.200425267. [DOI] [PubMed] [Google Scholar]
- Wermeling F, Chen Y, Pikkarainen T, Scheynius A, Winqvist O, Izui S, Ravetch JV, Tryggvason K, Karlsson MC. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J Exp Med. 2007;204:2259–65. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–70. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–87. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- Wither JE, Roy V, Brennan LA. Activated B cells express increased levels of costimulatory molecules in young autoimmune NZB and (NZB x NZW)F(1) mice. Clin Immunol. 2000;94:51–63. doi: 10.1006/clim.1999.4806. [DOI] [PubMed] [Google Scholar]
- Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, Yeste A, Quintana FJ, Ichimura T, Sobel RA, Bonventre JV, Kuchroo VK. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc Natl Acad Sci U S A. 2012;109:12105–10. doi: 10.1073/pnas.1120914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, Smythies LE, Mountz JD. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184:2289–96. doi: 10.4049/jimmunol.0903133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Duan B, Croker BP, Wakeland EK, Morel L. Genetic dissection of the murine lupus susceptibility locus Sle2: contributions to increased peritoneal B-1a cells and lupus nephritis map to different loci. J Immunol. 2005;175:936–943. doi: 10.4049/jimmunol.175.2.936. [DOI] [PubMed] [Google Scholar]
- Xu Z, Potula HH, Vallurupalli A, Perry D, Baker H, Croker BP, Dozmorov I, Morel L. Cyclin-dependent kinase inhibitor Cdkn2c regulates B cell homeostasis and function in the NZM2410-derived murine lupus susceptibility locus Sle2c1. J Immunol. 2011;186:6673–6682. doi: 10.4049/jimmunol.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci U S A. 2007;104:4542–6. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Ehlin-Henriksson B, Hansson GK. Scavenger receptors mediate adhesion of activated B lymphocytes. Exp Cell Res. 1998;239:16–22. doi: 10.1006/excr.1997.3876. [DOI] [PubMed] [Google Scholar]
- Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, Nussenzweig MC. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006;203:2255–61. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–4. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–4. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Niu H, Zheng YY, Morel L. Autoreactive marginal zone B cells enter the follicles and interact with CD4+ T cells in lupus-prone mice. BMC Immunol. 2011;12:7. doi: 10.1186/1471-2172-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]