Abstract
Although conditioned fear can be effectively extinguished by unreinforced exposure to a threat cue, fear responses tend to return when the cue is encountered some time after extinction (spontaneous recovery), in a novel environment (renewal), or following presentation of an aversive stimulus (reinstatement). As extinction represents a context-dependent form of new learning, one possible strategy to circumvent the return of fear is to conduct extinction across several environments. Here, we tested the effectiveness of multiple context extinction in a two-day fear conditioning experiment using 3-D virtual reality technology to create immersive, ecologically-valid context changes. Fear-potentiated startle served as the dependent measure. All three experimental groups initially acquired fear in a single context. A multiple extinction group then underwent extinction in three contexts, while a second group underwent extinction in the acquisition context and a third group underwent extinction in a single different context. All groups returned 24 hours later to test for return of fear in the extinction context (spontaneous recovery) and a novel context (renewal and reinstatement/test). Extinction in multiple contexts attenuated reinstatement of fear but did not reduce spontaneous recovery. Results from fear renewal were tendential. Our findings suggest that multi-context extinction can reduce fear relapse following an aversive event – an event that often induces return of fear in real-world settings -- and provides empirical support for conducting exposure-based clinical treatments across a variety of environments.
Keywords: extinction, fear conditioning, anxiety, virtual reality, fear-potentiated startle
1. Introduction
The ability to predict aversive events from environmental cues serves a clear adaptive function. Nonetheless, it is also adaptive to override this acquired knowledge about fearful relationships with new learning once a cue no longer signals any danger, as this information allows an individual to disregard nonthreatening cues and thus spare energy resources. In laboratory studies, this new learning is referred to as extinction (Pavlov, 1927) and occurs through presentation of the previously learned threat cue (i.e. conditioned stimulus, CS) in the absence of an aversive unconditioned stimulus (US). Extinction procedures form the basis of exposure therapies (Milad and Quirk, 2012), which have proven effective in the treatment of anxiety disorders (Nemeroff, Bremner, Foa, Mayberg, North, and Stein, 2006). It is well known, however, that extinction learning is more fragile than initial fear learning. As evidence, conditioned fear expression tends to return over time, whether extinguished in laboratory experiments or treated by pharmacological and behavioral therapy in anxiety disorders. An important goal of clinical translational research is thus to understand what conditions reduce the return of extinguished fear in humans.
Laboratory studies of fear conditioning have identified three predominant ways in which conditioned fear returns (Bouton, 2004): spontaneous recovery, renewal, and reinstatement. Spontaneous recovery refers to the return of conditioned fear responding after some amount of time has elapsed since extinction; fear renewal refers to the return of conditioned fear observed when the threat cue is encountered outside the extinction context; and reinstatement refers to the return of conditioned fear following presentation of the aversive US or a related stressor. The role of spatiotemporal contexts is particularly relevant to understand many facets of these fear recovery phenomena. Human and non-human animal research has routinely demonstrated that extinction learning is typically bound to the context in which extinction occurred (Bouton, 2002). In clinical practice, the extinction context is the treatment environment (e.g. therapist’s office) where a patient is exposed to a fear-inducing stimulus or situation in the absence of an aversive consequence. While fear expression is reduced within the confines of the treatment context during exposure training, this inhibition often fails to generalize outside the treatment context, consistent with the laboratory models of extinction learning (Craske, Kircanski, Zelikowsky, Mystkowski, Chowdhury, and Baker, 2008). A theoretical interpretation for the specificity of extinction learning is that it is the second thing the animal learns regarding the CS (the first being that it predicts the US), and is thus an exception to an established rule (Bouton, 2004). In this regard, the context becomes highly relevant to this new information, as it may be a factor that determines why the CS no longer predicts the US. If, however, the animal is provided the opportunity to learn that the CS is safe across multiple different environments, then it may help break the context-specific grip of extinction learning.
Potential explanations for why multiple-context extinction may promote generalization of extinction have been proposed by Bouton et al. (2006). First, this procedure increases the chance that contextual cues (i.e. features in the environment) related to the extinction context will be present when the CS is later encountered in a new context, which would help retrieve the extinction memory. Another possibility is that context switches during extinction maintain a heightened level of responding due to renewal. Higher levels of responding during extinction may be tied to better extinction learning, as emphasized by clinical models of exposure therapy (Foa and Kozak, 1986). Finally, extinction in a single context may promote the formation of an inhibitory association between the extinction context and the US, which “protects” the CS from receiving full extinction (Rescorla, 2003); in other words, the absence of the US is attributed primarily to the context and not to a change in the associative value of the CS. Switching between different contexts during extinction may consequently remove this inhibitory control, leading to better extinction that is less context-dependent.
A limited number of studies have tested the effects of extinction under multiple contexts on return of fear in humans or non-human animals (reviewed in Vervliet, Craske, and Hermans, 2013). These investigations have focused on fear renewal, as it pertains most directly to fear relapse following a change in the physical context between extinction and test. Relative to extinction in a single context, extinction in multiple contexts in rats attenuates the return of fear when the CS is later encountered in a novel context (Laborda and Miller, 2013; Thomas, Vurbic, and Novak, 2009) (but see Bouton et al., 2006). This finding has been extended to humans in a small number of fear conditioning studies (Balooch and Neumann, 2011; Balooch, Neumann, and Boschen, 2012) as well as clinical (Shiban, Pauli, and Muhlberger, 2013) and preclinical (Vansteenwegen, Vervliet, Iberico, Baeyens, Van den Bergh, and Hermans, 2007) investigations that do not use fear conditioning procedures per se. However, other human studies have not shown a reduction in fear renewal (Neumann, Lipp, and Cory, 2007) or only a modest reduction in renewal (Lang and Craske, 2000; Rodriguez, Craske, Mineka, and Hladek, 1999) following extinction in multiple contexts. One critique of the limited human conditioning literature on multiple context extinction is that return of fear has been assessed on the same day as fear acquisition. Thus, it is not clear whether these effects extend over a longer period of memory consolidation. Also, some studies have reported only explicit ratings of shock expectancy but have not reported psychophysiological markers of conditioned learning (Neumann et al., 2007). Finally, context manipulations have been limited thus far to changing only some key features (lights or sounds) within a testing room environment (Neumann et al., 2007) or a 2-D background image on a computer monitor (Balooch et al., 2012). As described below, these changes may not constitute effective contextual manipulations for human research subjects. Due to these methodological issues and inconsistency in the literature, more research is needed to determine the conditions under which multi-context extinction is effective in mitigating fear renewal.
In contrast to fear renewal studies, little human research has examined the factors that mitigate the other fear recovery paradigms. Although some studies have shown that spontaneous recovery can be modified by the delay intervals between acquisition and extinction testing (e.g. Huff, Hernandez, Blanding, and LaBar, 2009; Norrholm, Vervliet, Jovanovic, Boshoven, Myers, Davis, Rothbaum, and Duncan, 2008; Schiller, Cain, Curley, Schwartz, Stern, LeDoux, and Phelps, 2008), it is unknown whether multiple-context extinction has any effect on spontaneous fear recovery. Spontaneous recovery is typically tested in the extinction context following a delay (i.e. “extinction recall”). Recovery is context-dependent in the sense that the passage of time very likely changes the internal context of the animal between the time of extinction and the time of test, even if the physical features of the context are the same. Thus, if the time frame between extinction and test is held constant, then it is not clear that multiple-context extinction should afford any benefit over extinction in a single context on fear recovery in a previously encountered environment. Alternatively, if multiple-context extinction improves extinction learning by removing background inhibition, then this should be reflected in all forms of return of fear, including spontaneous recovery.
Reinstatement, on the other hand, is a context-dependent extinction effect that is subject to changes in the physical environment; reinstatement only occurs if the CS is tested in the same environment as the reinstatement US (e.g., LaBar & Phelps, 2005) (for related studies, see Bouton, 2002; Bouton and King, 1983). Bouton (2004) has proposed that reinstatement relies on contextual conditioning induced by the reinstatement US. Reinstatement increases the associative strength of the context, which then summates with residual fear from the extinguished CS to promote fear recovery. Importantly, reinstatement effects are not confined to the acquisition or extinction context, and can extend to novel contexts when the US and CS are both presented in that context (Westbrook, Iordanova, McNally, Richardson, and Harris, 2002). This feature makes reinstatement a clinically relevant phenomenon, as anxious individuals often experience strong return of fear when confronted directly with triggers or reminders in myriad environments after initial exposure. While it is unknown whether multi-context extinction attenuates fear reinstatement in a novel context in humans, reexposure to shock would provide a strong test of the effectiveness of this technique. That is, if, as a result of multiple context extinction, residual fear of the CS is low in the novel environment, then context conditioning induced by shock reexposure should afford little or no fear recovery.
One challenge in multiple-context extinction research is how to experimentally manipulate features of a human laboratory environment to provide impactful contextual changes. Prior efforts have largely used single unimodal cues (such as a visual change on a single feature of a static 2-D computer background) to manipulate contexts (e.g. Armony and Dolan, 2001; Kalisch, Korenfeld, Stephan, Weiskopf, Seymour, and Dolan, 2006; Pace-Schott, Milad, Orr, Rauch, Stickgold, and Pitman, 2009). However, such single-cue manipulations may produce weak context effects that do not invoke spatial contextual encoding mechanisms (see discussion in Huff, Hernandez, Fecteau, Zielinski, Brady, and LaBar, 2011). Moreover, they may not qualify as providing a genuinely new context, since other contextual cues in the laboratory are still present. Other studies have attempted to resolve this limitation by conducting different phases of the experiment in separate testing rooms (Huff et al., 2009; LaBar and Phelps, 2005); however, this approach limits the number of physical environments that can be used in multi-context extinction studies, and certain features of the context will undoubtedly overlap. To overcome these constraints, we used 3-D virtual reality technology to create ecologically-valid scenarios in order to enhance the sense that subjects were encountering the CSs while navigating through unique environments across the different stages of learning. Moreover, by presenting these virtual scenarios in a head-mounted display, participants are completely removed from the physical features of the laboratory setting in which the experiment takes place. 3-D presentation further enhances the sense of ‘presence’ or immersion in the context, which is important for integrating idiothetic cues with the spatial contextual information during navigation through an environment (Sanchez-Vives and Slater, 2005). These contexts were presented in a first-person perspective, and participants encountered virtual characters as social CSs while navigating forward down a virtual corridor containing three primary elements (floor, walls, and sky) that varied in both color and texture across the context manipulations.
To achieve the study goals, three experimental groups were run. Initially, all three groups underwent differential fear conditioning to two virtual characters in Context A. One CS (CS+) was paired with a mild electrical shock US and the other CS (CS−) served as unpaired control stimulus. Extinction training followed acquisition on the same day. The multiple-context extinction group was then exposed to the CS+ and the CS− in the absence of shock across three contexts -- Context A, B, and C. A single-context extinction group received the same number of extinction trials but solely in Context B, and a separate control group underwent extinction in the acquisition context (Context A). The length of training and duration between training and test was held constant across groups. All subjects returned 24 hours later to test for return of fear. Across all groups, we first tested recovery in a previously encountered context, and then tested renewal and reinstatement in a new context (Context D). We used fear-potentiated startle as a dependent measure of acquisition, extinction, and recovery. We predicted that the multiple-context extinction group would exhibit attenuated fear renewal and reinstatement but not spontaneous recovery on Day 2, given that the latter is less dependent on spatial contextual cues.
2. Materials and Methods
2.1. Participants
Forty-three psychologically healthy adult volunteers (female = 22; median age = 22 yrs; range = 18–40 yrs) provided written informed consent in accordance with the Duke University Medical Center Institutional Review Board guidelines. Subjects were randomly assigned to a group receiving extinction under multiple contexts (n = 15), a group receiving extinction under a single novel context (n = 14), and a group receiving extinction in the same context as acquisition (n = 14). For clarity, we refer to the multiple-context extinction group as Group ABC, the single, novel-context extinction group as Group B, and the single, acquisition-context extinction group as Group A – where the letters denote the extinction context(s).
2.2. Materials
2.2.1. Contexts
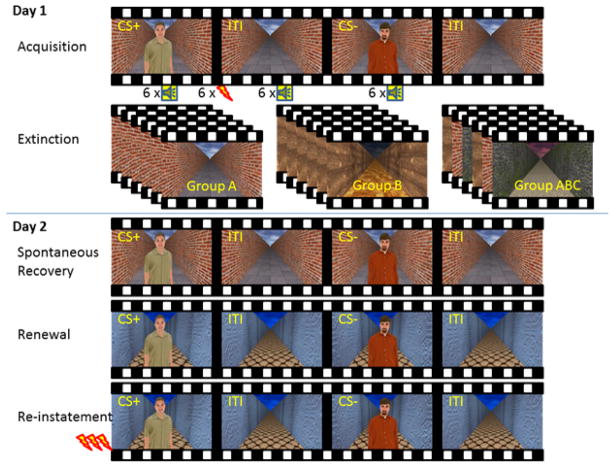
Four 3D environments that varied in color and texture were created in VirTools (Dassault Systeme, Paris, France). The virtual environments consisted of a floor, two walls, and a sky (intended to resemble an alleyway) presented in a first-person perspective through 3-D (stereoscopic) head-mounted goggles (eMagin Z800 3DVisor, Bellevue, Washington) (See Figure 1). The features of this context were manipulated to construct four unique environments that varied in both color and texture (A, B, C, and D). Participants traveled through the virtual environments on a forward-facing, straight path in first-person perspective at an average velocity of 0.3 m/s. Each run ended when the first-person path perspective stopped moving forward and faded to a black screen. This transition occurred between each phase (e.g. between acquisition and extinction). The contexts serving as A, B, C, and D were counterbalanced across subjects.
Figure 1.
Experimental design. Fear conditioning (Acquisition) was performed identically across three experimental groups on Day 1. During Extinction on the same day, one group (ABC) received extinction training in three virtual contexts, whereas the two other groups extinguished in a single virtual context -- either in the fear conditioning context (Group A) or a novel context (Group B). On Day 2, which took place 24 hrs following Extinction, participants came back for tests of Spontaneous Recovery, Renewal and Reinstatement. Numbers below Acquisition indicate the number of trials that were reinforced by a shock and those that were not. The conditioned stimuli were never reinforced during the other portions of the experiment. CS+ = reinforced conditioned stimulus, CS− = unreinforced conditioned stimulus, ITI = inter-trial interval.
2.2.2. CS and US
Two 3-D virtual characters, presented at a distance of 1 m in the virtual environments, served as CSs. Due to our interest in translational models of clinical anxiety, and to add to the ecological validity of the contextual manipulation, social stimuli were used rather than affectively neutral inanimate objects (e.g. shapes). Which of the characters served as the CS+ and CS− was counterbalanced across subjects. In each experimental phase, the characters appeared for 6 s, during which time forward navigation down the alleyway was paused. An inter-trial interval (ITI) followed each CS, during which time participants were passively guided down the alleyway on a fixed rate and forward path with no CSs present for 6–14 s. Participants were told prior to the experiment that they could learn to predict the US, but were not told which character was the CS+.
The shock US was presented to the subjects’ wrist via pre-gelled disposable snap electrodes (EL503 BIOPAC Systems, Goleta, CA), and was calibrated to each subject’s tolerance prior to the start of the experiment using an ascending staircase procedure, so that the shocks were rated as ‘annoying’ but not ‘painful’ (see Dunsmoor, Mitroff, and LaBar, 2009). US duration was 6 ms. Shock delivery was controlled with the STM100C module connected to the STM200 constant voltage stimulator (BIOPAC Systems, Goleta, CA).
2.3. Procedure
The experiment included two assessments separated by 24 hrs. Participants were assigned to one of three groups, which differed only in regard to the context in which extinction learning occurred on Day 1. In all experimental phases, stimulus order was pseudorandomized such that the CS+ (and CS−) did not appear more than twice in a row. We used three stimulus presentation orders to counterbalance CS order across subjects.
Day 1 included four phases that occurred in the following order: startle-probe habituation, CS habituation, fear acquisition, and fear extinction. Startle-probe habituation included 9 presentations of a 50 ms white-noise stimulus (see Fear-potentiated startle procedures below) presented binaurally through headphones (ATH-M45, Audio Technica, Stow, Ohio) while subjects viewed a monochromatic, 2-D gray screen without depth perception. CS habituation involved two presentations of each CS in the acquisition context. These habituation phases (data not reported) were included to reduce initial orienting responses. Fear acquisition occurred in one testing run that included 12 presentations of the CS+ and 12 presentations of the CS−. Six of the CS+ presentations co-terminated with presentation of the US (50% partial reinforcement schedule). Startle probes were delivered on 6 of 12 presentations of each CS type. So as to avoid a correlation between startle-probes and shock on CS+ trials, half the startle-probes occurred on US paired trials and the other half occurred on US unpaired trials. Six noise-alone startle probes were also delivered during the ITIs of each phase so that probe presentations were uncorrelated to CS presentations. This phase was identical for all three groups.
Acquisition training was immediately followed by extinction training. Extinction occurred over 6 runs, with each run separated by a transition (fade to black with forward movement paused). Each run contained 3 presentations of the CS+ and 3 presentations of the CS−, for a total of 36 extinction trials overall. In each run, startle probes were delivered on 1 out of 3 CS presentations and during 1 ITI. For Group ABC, the extinction context alternated between the acquisition context (A) and two unique contexts (B and C). Each of the three contexts was presented twice in one of three fixed orders that were counterbalanced across subjects (i.e., ABCABC, BCABCA, CABCAB). Group B underwent 6 runs of extinction in one repeated novel context (BBBBBB), while Group A underwent 6 runs of extinction in the acquisition context (AAAAAA). To be clear, a transition occurred between each run of extinction training for all three groups, and each group received the same overall number of exposures to the CSs across the 6 extinction training runs.
Subjects returned 24 hrs later to test for return of fear. Day 2 contained 4 phases that occurred in the following order: startle-probe habituation, spontaneous recovery, fear renewal, and fear reinstatement. Subjects were reconnected to the shock electrodes and the shock level was set to the level calibrated on the previous day. The shock was not recalibrated so as to avoid reinstatement prior to extinction recall. Spontaneous recovery was tested in the extinction context and included 4 presentations of the CS+ and 4 presentations of the CS−. Startle probes occurred on half of the CS presentations and during 2 ITIs. For Group ABC, spontaneous recovery was assessed in context A. Renewal and reinstatement were tested in a context that was novel for all three groups (D). Renewal included 4 presentations of the CS+ and 4 presentations of the CS−. Startle probes occurred on half of the CS presentations and during 2 ITIs. Reinstatement occurred in context D and began with three unsignaled shocks separated by 2 s. The first reinstatement test trial occurred 20 s following the last reinstatement US. The reinstatement test included 4 presentations of the CS+ and 4 presentations of the CS−. Startle probes occurred on every trial, including 2 ITI probes. The low number of CS presentations and startle-probes during each phase on Day 2 were included to help mitigate habituation effects and over-learning that the CS+ was still safe, since multiple return of fear phenomena were being assessed. The overall design is similar to that used in a 1-day treatment-analogue study by Vansteenwegen et al. (2007) investigating multiple-context exposure in spider-anxious individuals.
2.4. Fear-potentiated startle (FPS)
Psychophysiological recordings and shock administration were controlled with the MP-150 BIOPAC system and collected using AcqKnowledge software (BIOPAC Systems, Goleta, CA). Fear-potentiated startle was measured by electromyography (EMG) and served as the primary dependent measure of fear conditioning. EMG was continuously recorded from the right orbicularis oculi muscle at 1000 Hz using two cup electrodes filled with electrolyte gel. A ground electrode was attached to the left hand. Startle probes were 100-dB 50-ms white-noise bursts with near-instantaneous rise time presented binaurally through headphones and jittered between 5100 and 5500 ms post-CS onset. Presentations of ITI probes were jittered to occur at 6000 or 7000 s following the offset of the CS. Startle was quantified as the maximum EMG response 20–120 ms post-probe onset subtracted from the average EMG response during the immediate 500 ms preceding probe delivery. Responses were transformed to T-scores within each phase (T-scores = z-scores*10 + 50).
2.5. Data analysis
Repeated measures analysis of variance (ANOVA) was performed in SPSS 18 (IBM Corporation, New York, USA) to evaluate the main effect of stimulus (CS−, CS+) and the stimulus x Group interaction within each phase. Due to the low number of startle probe trials for each event type in each phase, data were collapsed across all similar events within each phase. Because the extinction phase was much longer than the other phases and was experimentally manipulated, patterns of extinction may be different across groups. We therefore examined extinction by dividing the data into early (first three runs) late (last three runs) trials. Planned two-sample t-tests were used to compare differential startle magnitudes between the CS+ and the CS−. Comparisons between the CS+ and ITI during each experimental phase are included as a low-level baseline contrast (see Supplemental Results). Statistical significance was set to α = .05.
3. Results
3.1. Acquisition and extinction (Day 1)
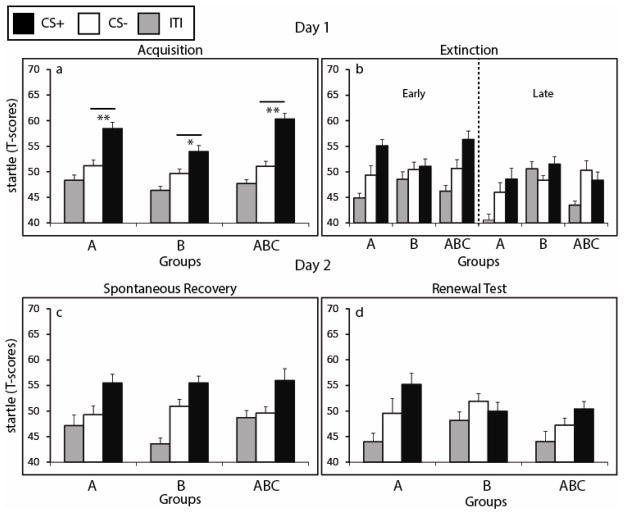
All three groups exhibited successful acquisition of conditioned fear, as revealed by a main effect of CS type (F1,40 = 60.52, P < .001) but no CS x Group interaction (F2,40 = 2.68, P = .08) (Figure 2a). Post-hoc t-tests showed greater responses to the CS+ versus the CS− in each group during acquisition: Group ABC, t14 = 6.61, P < .001; Group B, t13 = 2.27, P = .041, and Group A, t13 = 5.61, P < .001. By early extinction (Figure 2b), participants had not yet fully extinguished, as demonstrated by a main effect of CS type (F1,40 = 11.17, P = .002), but no CS x Group interaction (F2,40 = 1.92, P = .15). Although there was no group interaction, Group B showed only a minimal difference between the CS+ and CS−, suggesting that this group did not possess strong retention of conditioned fear during early extinction. By late extinction all groups successfully extinguished, as characterized by no main effect of CS type (F1,40 = .86, P = .36) and no CS x Group interaction (F2,40 = 1.44, P = .25). In all, these findings indicate similar patterns of fear-potentiated startle to the CS+ and CS− across groups on Day 1 during acquisition and extinction.
Figure 2.
Fear-potentiated startle results from Acquisition and Extinction (Day 1) and Spontaneous Recovery and Renewal (Day 2). (a) Subjects in all three groups exhibited greater responses to the CS+ (reinforced conditioned stimulus) versus the CS− (unreinforced conditioned stimulus) during fear conditioning, indicating successful acquisition of conditioned fear. (b) By late extinction, there were no differences in responses between the CS+ versus the CS− in any group, indicating successful immediate fear extinction on Day 1. (c) All groups exhibited spontaneous recovery when tested in the extinction context. Note that Group ABC was tested for spontaneous recovery in the acquisition context (A). (d) There was little evidence of fear renewal following the recovery test. ** denotes P < .001, * denotes P < .05. ITI = probes presented during intertrial interval.
3.2. Spontaneous recovery (Day 2)
Spontaneous recovery was evident in the extinction context 24 hrs following extinction training, as revealed main effect of CS type (F1,40 = 12.20, P = .001), with larger responses to the CS+ than CS− (Figure 2c). These effects did not interact with group (F2,40 = .73, P = .88), suggesting that, as predicted, the multiple context extinction manipulation did not differentially affect spontaneous recovery in the extinction context.
3.3. Renewal (Day 2)
The renewal test immediately followed the spontaneous recovery test and was conducted in a novel context that varied both textural and color cues across the three main contextual elements. We observed a marginally significant effect of CS type (F1,40 = 2.90, P = .1), with larger responses to the CS+ than the CS− (Figure 2d). These effects did not significantly interact with group (F2,40 = 2.70, P = .08), in contrast to predictions (see Discussion).
3.4. Reinstatement (Day 2)
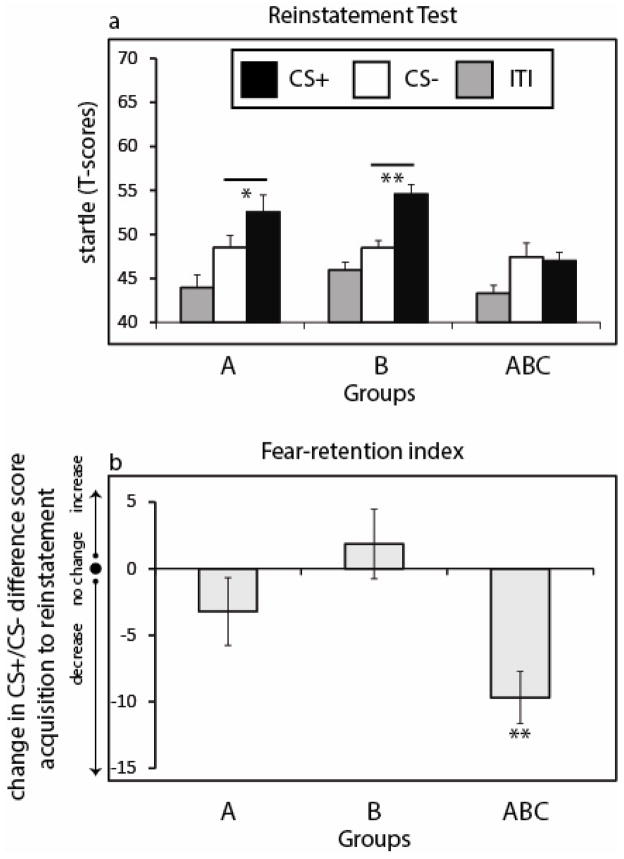
Reinstatement generated a main effect of CS type (F1,40 = 11.46, P = .002). Importantly, the CS+ versus CS− contrast did interact with group (F2,40 = 4.10, P = .02) (Figure 3a). Post-hoc t-tests showed greater responses to the CS+ than the CS− in Group A (t13= 2.58, P = .02) and Group B (t13= 3.38, P = .005) but not in Group ABC (t14= .26, P = .80). Thus, as predicted, extinction in multiple contexts reduced fear reinstatement relative to extinction in single contexts.
Figure 3.
Fear-potentiated startle results from the Reinstatement test (Day 2). (a) Reinstatement evoked enhanced responses to the CS+ (reinforced conditioned stimulus) versus the CS− (unreinforced conditioned stimulus) in Group A and Group B, but not in Group ABC. (b) Difference scores (CS+ minus CS−) were calculated for each subject for acquisition and reinstatement. Difference scores from acquisition were subtracted from reinstatement to yield a fear-retention index, where positive values indicate an increase in differential fear from acquisition to reinstatement and negative values indicate a decrease in differential fear. While Group A and Group B exhibited similar levels of differential responding between acquisition and reinstatement test, subjects in Group ABC showed a significant decline in startle. ** denotes P < .001, * denotes P < .05. ITI = baseline startle probes presented during the intertrial interval.
To ensure that the group difference in reinstatement also applied at the individual subject level, we derived a difference score that computed, for each subject, the amount of reinstatement as a function of the amount of fear responding at initial acquisition on Day 1 (Figure 3b). The differential response at acquisition (CS+ minus CS− difference score) for each subject was subtracted from the corresponding differential response at reinstatement, yielding an index of how much fear was retained on Day 2 during the reinstatement test. There was a main effect of group on the change in difference scores as determined by a one-way ANOVA (F2,40 = 6.03, P = .005). Post-hoc t-tests showed that, whereas Groups A and B showed no change in the differential response to the CS+ versus CS−, Group ABC showed a significant decline in differential responding, t14= −4.95, P < .001.
4. Discussion
We found that performing extinction in multiple contexts, as compared to extinction in a single novel context or in the fear acquisition context, diminishes reinstatement of conditioned fear when tested 24 hrs later in a novel environment. Prior human studies examining the effects of extinction in multiple contexts have focused on fear renewal, and have tested return of fear on the same day as acquisition. We show here that this procedure attenuates another important return of fear phenomenon -- reinstatement -- and that the effect of extinction under multiple contexts extends to a 24-hr follow-up test of the extinction memory. Notably, extinction in multiple contexts did not affect spontaneous recovery, a return of fear phenomenon that occurs following a delay but is not considered spatially context-dependent in the same manner as renewal and reinstatement. Ultimately, these results suggest that virtual reality may be an effective tool that could be adapted to prevent context-dependent relapse following psychological treatment, especially in cases for which relapse is induced by exposure to an aversive event, as shown here.
Because the multiple-context extinction manipulation had selective effects, it is difficult to ascribe these findings to a single mechanism. However, we can exclude certain mechanisms proposed as potentially mediating generalization of extinction following multiple-context extinction (Bouton et al., 2006). For one, diminished reinstatement following multiple-context extinction cannot be attributed simply to a shared feature between one of the multiple contexts (A, B, and C) and the novel context (D), as there were no shared perceptual features (color and texture) other than the overall global scaffolding of the environment. A second proposal was that switching contexts during extinction would sustain fear responses via renewal, leading to more effective extinction. However, this explanation does not fit with the present results, since responding by late extinction was similar across the three groups. We note that this conclusion is somewhat constrained by the low number of startle probes delivered during extinction training. Future studies on multiple-context extinction in humans should consider delivering more startle probes to enhance the power to detect differences across the session (e.g. Norrholm et al., 2008).
Another prediction of multiple-context extinction is that switching contexts helps to release the CS from protection from extinction (Rescorla, 2003). In other words, if the extinction context is acquiring inhibitory associations with the US, thereby allowing the CS to maintain its associative value, then removing or alternating these features over the course of extinction should provide a chance for the CS to lose its associative value. This explanation of multiple-context extinction would predict more effective extinction overall that should be reflected in reduced recovery, renewal, and reinstatement. However, the present study only found a clear effect during reinstatement, complicating this explanation. Thus, these findings suggest that rather than promoting extinction learning itself, encountering the CS in multiple contexts affords a more specific benefit to future encounters with the CS in a novel environment.
It is important to also consider whether the reinstatement reduction in Group ABC was due merely to exposure to multiple contexts. Multi-context exposure would increase the overall novelty of the background cues during extinction and reduce the novelty of moving to a new context for the reinstatement test. However, we note that Group B switched contexts from acquisition to extinction and thus had exposure to two different contexts overall, yet reinstatement effects were robust in this group. Nonetheless future studies should consider exposing all groups to all contexts in the extinction phase (with CS delivery patterns across contexts similar to the present study) to further determine the potential role of mere context exposure.
If time is considered a context, then it is possible that a different experimental manipulation could reduce spontaneous recovery. Namely, conducting extinction across multiple temporal delays could serve as an experimental analogue to the multiple-spatial context extinction training in the present study. Such studies would help to further differentiate the various forms of fear recovery, which behaviorally appear similar but are related to different underlying mechanisms.
Some limitations of the present study should be mentioned, specifically in reference to the fear renewal test. In contrast to predictions, fear renewal was weak (only marginally significant as a main effect), and we did not find the proposed group interaction effect. First, renewal was tested in a novel context, which prior studies have shown to be less robust than renewal tested in the acquisition context (Vervliet et al., 2013). Second, the 24-hr retention interval may weaken renewal effects. Most prior studies of fear renewal in humans have taken place within the same testing day (but see Huff et al., 2009, who showed both spontaneous recovery and renewal following a 24-hr delay using physical context shifts). Altogether, the use of a novel context and a 24-hr retention interval may contribute to a floor effect that obscured a true group difference.
Another methodological factor is that renewal was tested immediately after the spontaneous recovery test on Day 2. Thus, some subjects may have habituated to the startle probes prior to the renewal test, while some subjects may have shown carryover effects from extinction recall to renewal. The spontaneous recovery test also provided an opportunity for subjects to learn that the US was still being omitted, which may have created the expectancy that the US would continue to be omitted during the renewal test. Reinstatement testing may have been successful at uncovering group differences due to dishabituation and recovered shock expectancy created by reexposure to the electric shocks. These limitations combined to render the results from the fear renewal test inconclusive.
On a methodological note, these issues point to the challenges inherent to testing multiple return of fear phenomena within the same experimental paradigm. In light of these limitations, we propose that future studies examine renewal and recovery between subjects, or counterbalance the sequence of these tests to rule out the potential contribution of order effects. It will also be important to include more startle probes, and/or other psychophysiological measures (e.g. skin conductance responses) in order to provide more power to detect trial-by-trial changes within and across experimental phases.
Finally, this study extended our prior work (Huff et al., 2011; Huff, Zeilinski, Fecteau, Brady, and LaBar, 2010) to reveal the value of immersive virtual reality platforms to experimentally manipulate spatial contexts during fear conditioning. The current study required that four distinct contexts be presented, which can be challenging using physical context shifts. Although the present study only varied two key visual features of the environmental display (both texture and color of the 3 primary scene elements, yielding 6 configurations in total), other sensory features, such as environmental sounds, could be incorporated to further enhance the multisensory aspects of the manipulation. Immersive virtual environments are an emerging clinical tool for the treatment of anxiety (Parsons and Rizzo, 2008). This technology provides a valuable opportunity for patients to confront fear evoking stimuli or situations in order to reduce anxiety symptoms and develop efficient coping strategies.
In summary, whereas extinction in multiple virtual reality contexts did not affect 24-hr spontaneous recovery, it effectively diminished reinstatement in healthy humans relative to extinction in a single context. We conclude that this procedure holds promise as a means to reduce fear recovery following an aversive event, which is a leading cause of fear relapse. Future investigations should construct immersive virtual environments that contain greater spatiotemporal detail (or are tailored to each patient’s contextual triggers) to heighten the sense that feared stimuli are being encountered across a range of different environments. These VR procedures may prove beneficial for studying other forms of conditioned learning in humans, including active avoidance and conditioned place preferences. In turn, these techniques may uncover potential avenues for the treatment of anxiety conditions and may provide important outcome measures to compare the relative success of different treatments.
Supplementary Material
Acknowledgments
We thank Caroline Schanche-Perret Gentil for help with data collection. This study was supported by NIH grants R01 DA027802 and F31 MH090682, as well as a Swedish Research Council Postdoctoral Fellowship award and a Sweden-America Foundation Postdoctoral Fellowship award to FA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armony JL, Dolan RJ. Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport. 2001;12:3407–3411. doi: 10.1097/00001756-200110290-00051. [DOI] [PubMed] [Google Scholar]
- Balooch SB, Neumann DL. Effects of multiple contexts and context similarity on the renewal of extinguished conditioned behaviour in an ABA design with humans. Learning and Motivation. 2011;42:53–63. [Google Scholar]
- Balooch SB, Neumann DL, Boschen MJ. Extinction treatment in multiple contexts attenuates ABC renewal in humans. Behav Res Ther. 2012;50:604–609. doi: 10.1016/j.brat.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behav Res Ther. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear - tests for the associative value of the context. Journal of Experimental Psychology-Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear - exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Huff NC, Hernandez JA, Blanding NQ, LaBar KS. Delayed Extinction Attenuates Conditioned Fear Renewal and Spontaneous Recovery in Humans. Behavioral Neuroscience. 2009;123:834–843. doi: 10.1037/a0016511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Hernandez JA, Fecteau ME, Zielinski DJ, Brady R, LaBar KS. Revealing context-specific conditioned fear memories with full immersion virtual reality. Front Behav Neurosci. 2011;5:75. doi: 10.3389/fnbeh.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Zeilinski DJ, Fecteau ME, Brady R, LaBar KS. Human fear conditioning conducted in full immersion 3-dimensional virtual reality. J Vis Exp. 2010 doi: 10.3791/1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Laborda MA, Miller RR. Preventing Return of Fear in an Animal Model of Anxiety: Additive Effects of Massive Extinction and Extinction in Multiple Contexts. Behavior Therapy. 2013;44:249–261. doi: 10.1016/j.beth.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AJ, Craske MG. Manipulations of exposure-based therapy to reduce return of fear: a replication. Behav Res Ther. 2000;38:1–12. doi: 10.1016/s0005-7967(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: A state-of-the-science review. Journal of Psychiatric Research. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Lipp OV, Cory SE. Conducting extinction in multiple contexts does not necessarily attenuate the renewal of shock expectancy in a fear-conditioning procedure with humans. Behav Res Ther. 2007;45:385–394. doi: 10.1016/j.brat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, Rothbaum B, Duncan EJ. Timing of Extinction Relative to Acquisition: A Parametric Analysis of Fear Extinction in Humans. Behavioral Neuroscience. 2008;122:1016–1030. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep Promotes Generalization of Extinction of Conditioned Fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Rizzo AA. Affective outcomes of virtual reality exposure therapy for anxiety and specific phobias: A meta-analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:250–261. doi: 10.1016/j.jbtep.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Rescorla RA. Protection from extinction. Learning & Behavior. 2003;31:124–132. doi: 10.3758/bf03195975. [DOI] [PubMed] [Google Scholar]
- Rodriguez BI, Craske MG, Mineka S, Hladek D. Context-specificity of relapse: effects of therapist and environmental context on return of fear. Behav Res Ther. 1999;37:845–862. doi: 10.1016/s0005-7967(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Slater M. From presence to consciousness through virtual reality. Nature Reviews Neuroscience. 2005;6:332–339. doi: 10.1038/nrn1651. [DOI] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, LeDoux JE, Phelps EA. Evidence for recovery of fear following immediate extinction in rats and humans. Learning & Memory. 2008;15:394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiban Y, Pauli P, Muhlberger A. Effect of multiple context exposure on renewal in spider phobia. Behav Res Ther. 2013;51:68–74. doi: 10.1016/j.brat.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Vurbic D, Novak C. Extensive extinction in multiple contexts eliminates the renewal of conditioned fear in rats. Learning and Motivation. 2009;40:147–159. [Google Scholar]
- Vansteenwegen D, Vervliet B, Iberico C, Baeyens F, Van den Bergh O, Hermans D. The repeated confrontation with videotapes of spiders in multiple contexts attenuates renewal of fear in spider-anxious students. Behav Res Ther. 2007;45:1169–1179. doi: 10.1016/j.brat.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annu Rev Clin Psychol. 2013;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, Iordanova M, McNally G, Richardson R, Harris JA. Reinstatement of fear to an extinguished conditioned stimulus: Two roles for context. Journal of Experimental Psychology Animal Behavior Processes. 2002;28:97–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.