Abstract
Research in humans has highlighted the importance of the amygdala for transient modulation of cortical areas for enhanced processing of emotional stimuli. However, non-human animal data has shown that amygdala dependent threat (fear) learning can also lead to long lasting changes in cortical sensitivity, persisting even after extinction of fear responses. The neural mechanisms of long-lasting traces of such conditioning in humans have not yet been explored. We used functional magnetic resonance imaging (fMRI) and assessed skin conductance responses (SCR) during threat acquisition, extinction learning and extinction retrieval. We provide evidence of lasting cortical plasticity in the human brain following threat extinction and show that enhanced blood oxygen level-dependent (BOLD) signal to the learned threat stimulus in the auditory association cortex is resistant to extinction. These findings point to a parallel avenue by which cortical processing of potentially dangerous stimuli can be long lasting, even when immediate threat and the associated amygdala modulation have subsided.
Keywords: Fear conditioning, Fear extinction, Auditory fear conditioning
1. Introduction
It is well known that emotion can influence perception through amplified processing of emotionally relevant stimuli, especially when danger is involved (Vuilleumier & Driver, 2007). We are just beginning to understand the brain mechanisms underlying such emotional modulation of perceptual processing. Recent investigations into the effects of emotion on perception have focused on a neural model by which the amygdala can alter perception through transient modulatory effects of emotional arousal on sensory processing regions in the cerebral cortex (Rotshtein, Malach, Hadar, Graif & Hendler, 2001; Sabatinelli, Bradley, Fitzsimmon, & Lang, 2005; Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004), however studies in non-human animals have shown that sensory cortical reactivity following threat learning can also be long lasting (e.g., Weinberger, 1998).
In Pavlovian threat (fear) conditioning, a conditioned stimulus (CS), such as a tone, is paired with an electric shock unconditioned stimulus (US). The CS then elicits a conditioned behavioral response, and increased neural activity in the amygdala (e.g. LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). Subsequently, repeated presentation of the CS without the US then results in extinction of the conditioned behavioral responses and diminished amygdala reactivity to the conditioned stimulus (Phelps, Delgado, Nearing, & LeDoux, 2004). Animal models have shown enhanced sensory cortex reactivity to the CS that, in some circumstances, persists through extinction (Armony, Quirk, & LeDoux, 1998; Quirk, Armony, & LeDoux, 1997; Weinberger, 1998). The possible capacity of threat conditioning to influence perceptual representation of predictive cues in human cortex has recently been demonstrated using an odor discrimination task, showing that Pavlovian aversive conditioning has the capacity to induce plastic changes in the piriform cortex, resulting in an updated perceptual representation (Li, Howard, Parrish, & Gottfried, 2008). In parallel, it has been shown that threat learning can lead to experience-dependent cholinergically modulated plasticity in the human auditory cortex (Thiel, Friston, & Dolan, 2002). However, it is not known if after successful extinction learning in humans, cortical plasticity is maintained as has been shown in non-human animals (Armony et al., 1998; Quirk et al., 1997; Weinberger, 1998). The spontaneous recurrence or relapse of anxiety disorders and the chronic course of Post Traumatic Stress Disorder (PTSD) could be clinical correlates of extinction resistant plastic neural changes.
Fear conditioning and subsequent extinction learning has been used as a model for the treatment of anxiety disorders, implicating the amygdala and ventromedial prefrontal cortex (vmPFC) as critical components of the underlying neural circuitry (Milad & Quirk, 2012). Morgan, Romanski, and LeDoux (1993) were the first to show that pretraining vmPFC lesions had no effect on threat conditioning but impaired extinction. Since then a large body of non-human animal work demonstrated that the infralimbic prefrontal cortex (IL) plays a critical role in the acquisition and retrieval of extinction (Bouton, 1993; Bush, Sotres-Bayon, and LeDoux, 2007; Milad & Quirk, 2002, 2012; Rescorla, 2004; Sotres-Bayon & Quirk, 2010). Building on this animal model, Phelps et al. (2004) showed that activation in the vmPFC during acquisition and retention of extinction, providing an analogue of the IL in humans and suggesting that the mechanism for extinction learning may be preserved across species. Furthermore it has been shown in humans that the magnitude of vmPFC activation to the extinguished stimulus is positively correlated with the magnitude of extinction retention (Milad, Wight et al., 2007a, Milad, Quirk, et al. 2007b) and that the thickness of the vmPFC is correlated with extinction retrieval (Hartley, Fischl, & Phelps, 2011; Milad, Orr, Pitman, & Rauch, 2005). Although it is known that conditioned responses to the CS can recover after extinction through passage of time (spontaneous recovery), changing contexts (renewal) or by presenting the US alone (reinstatement) (Bouton, 2004; Rescorla, 2004; Rescorla & Heth, 1975), possible cortical extinction-resistant changes in the human brain have received little attention.
The goal of the present study was to determine whether long lasting effects of emotional learning, reflected in persistent sensitivity changes in sensory cortex, can be observed in the human brain. This would offer a secondary mechanism alongside temporary online modulation of emotional processing, in which lasting cortical changes allow enhanced processing of relevant emotional stimuli.
We developed an auditory threat conditioning paradigm adapted for use during functional magnetic resonance imaging (fMRI) to examine long lasting CS elicited changes in the human auditory cortex during threat learning and extinction. We used fMRI to measure blood-oxygenation-dependent (BOLD) responses as an index of differential brain activation. Our physiological measure of conditioning was skin conductance responses (SCR).
2. Materials and methods
2.1. Participants
Twenty-four healthy right-handed subjects, between 18 and 40 years of age, were recruited through posted advertisements. Eight of these subjects were eliminated after day 1 due to a lack of SCR (nonresponders, N = 3) or a failure to show acquisition of the conditioned response (n = 5). The remaining sixteen subjects (8 male, 8 female, mean age of 27 years) completed the study. All subjects gave informed consent and were paid for their participation.
2.2. Design and procedure
Sixteen subjects were scanned during our auditory conditioning and extinction paradigm. The CSs were two easily distinguishable pure tones (800 and 170 Hz) that were outside the range of the scanner noise. The unconditioned stimulus (US) was a mild electric shock to the wrist. All CSs were presented for 4 s, with a 12 s fixed inter-trial-interval (ITI). One of the tones was designated as the CS+ (paired with the shock on 42% of the trials) and the other as the CS− (never paired with the shock). Subjects were instructed of these contingencies prior to the start of the conditioning paradigm. There were three phases to the study. The first phase was acquisition, consisting of randomized unreinforced presentations of the CS+ and CS− (15 repetitions each), intermixed with an additional 8 reinforced presentations of the CS+ that coterminated with the US. The extinction phase immediately followed acquisition and consisted of randomized unreinforced presentations of the CS+ and CS− (19 repetitions each). Approximately 24 h after the first session, subjects participated in the third phase, re-extinction, which was similar to extinction and consisted of 19 randomized unreinforced presentations of the CS+ and CS− each. Prior to reextinction, subjects were told that the procedure would be similar to day 1, but shorter. The order of the trials and the designation of tones to CS+ and CS− were counterbalanced across subjects using two pseudorandom orders.
Mild electric shocks were delivered through a stimulating bar electrode attached with a Velcro strap to the right wrist. A Grass Instruments stimulator was used with cable leads that were magnetically shielded and grounded through an RF filter. The subjects were asked to set the level of shock for themselves using a work up procedure prior to scanning on day 1. In this procedure, the subject was first given a mild shock (200 ms duration, 50 pulses/s) which was gradually increased to the maximum level the subject indicated was “uncomfortable, but not painful” (the maximum shock possibly given will be 50 V). Skin conductance was assessed with shielded Ag–AgCl electrodes attached to the middle phalanges of the second and third fingers of the left hand using BIOPAC systems skin conductance module. The electrode cables were grounded through an RF filter panel. Offline data analysis of SCR waveforms was conducted using AcqKnowledge software. The level of SCR was assessed as the base to peak difference for the largest deflection in the 0.5–4.5 s window following stimulus onset (see LaBar, LeDoux, Spencer & Phelps, 1995). The SCR analysis included only trials that did not coterminate with a presentation of the US.
2.3. Neuroimaging acquisition and analysis
The study was conducted at the NYU Center for Brain Imaging using a 3T Siemens Allegra scanner and a Siemens head coil. The scanning session began with MPRAGE anatomical scans to obtain a 3D volume for slice selection. This scan was followed by acquisition of 3 mm thick axial slices to obtain anatomical slices in the same plane as the functional data acquisition. Functional scans used a gradient echo sequence, TR = 2 s, TE = 20, flip ANGLE = 90, FOV = 192, 3 mm slice thickness. A total of 39 axial slices were sampled for whole brain coverage. The in-plane resolution was 3mm × 3 mm. Functional image acquisition was divided into three runs on day 1, such that the second break between sessions occurred 8 trials into the Extinction phase, and could not indicate a transition between the phases. There were two runs on day 2, both for extinction. Between runs there was a break of approximately 15–30 s.
Imaging data were analyzed using Brain Voyager software (version QX). The data were temporally and spatially smoothed (4 mm FWHM) and motion corrected. We set a threshold of 2 mm of movement in any direction to eliminate subjects with excessive head movement. None of the subjects exceeded this threshold. Structural and functional data of each participant were transformed to standard Talairach stereotaxic space (Talairach & Tournoux, 1998).
Based on the models of the auditory threat learning circuitry in animals, we selected anatomical regions of interest (ROIs) in the bilateral amygdala, auditory thalamus, auditory cortex and ventral medial prefrontal cortex (vmPFC), which was selected as an analog to the infralimbic region known to play a role in extinction learning in non-human animals. Within these anatomical ROIs we then identified functional clusters using a contrast of both CS+ and CS− tones vs. background noise during ITI (defined as voxels within 10 mm diameter sphere of the peak coordinates that showed significance of p < 0.01 in the statistical group map, Bonferroni corrected).
Consistent with previous studies, a lower threshold was used for the amygdala due to the a priori hypotheses for response (P < 0.005, uncorrected) and was selected using a contrast of CS+ > CS− during early acquisition. For all ROIs we performed paired two-way t-tests on the percent BOLD signal change evoked by the CS+ compared to the CS− during each stage (early and late phases of acquisition, day 1 extinction, day 2 extinction) of the experiment.
3. Results
3.1. Psychophysiological measures of threat learning and extinction
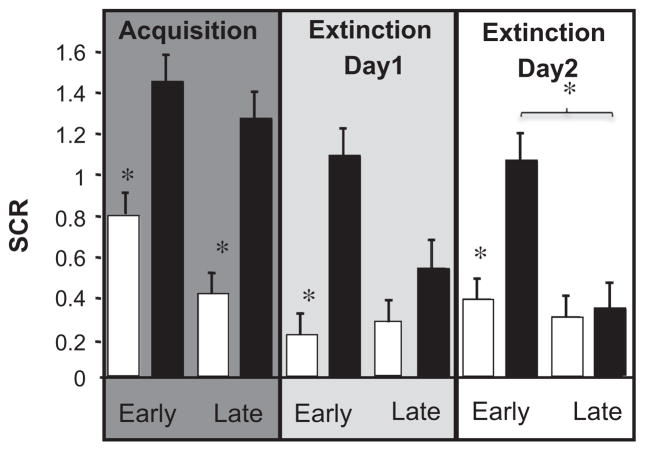
During acquisition, the participants showed increased conditioned responses, as indicated by a greater SCR to the CS+ as compared to the CS− in early and late acquisition (paired two-way t tests, t(15) = 3.02; t(15) = 3.44, respectively, both P < 0.01). This increase of SCR to the CS+ persisted during early extinction on both days (t(15) = 3.21; t(15) = 2.93, respectively, both P < 0.01), which diminished, as compared to the CS− in late phases of the extinction sessions (t(15) = 1.04; t(15) = 0.83, respectively, both NS) (see Fig. 1).
Fig. 1.
Skin conductance response (SCR) during auditory threat learning. Square root transformed mean SCR to the CS+ (black bar) and CS− (white bar) averaged for all subjects (n = 16) across acquisition, day 1 and day 2 extinction. Data is divided into an early (19 trials of each stimulus) and late phase (19 trials of each stimulus) for each session. Asterisks represent a significant difference (P < 0.01) of CS+ vs. CS−, and during extinction between CS+ in early vs. late extinction.
3.2. fMRI results
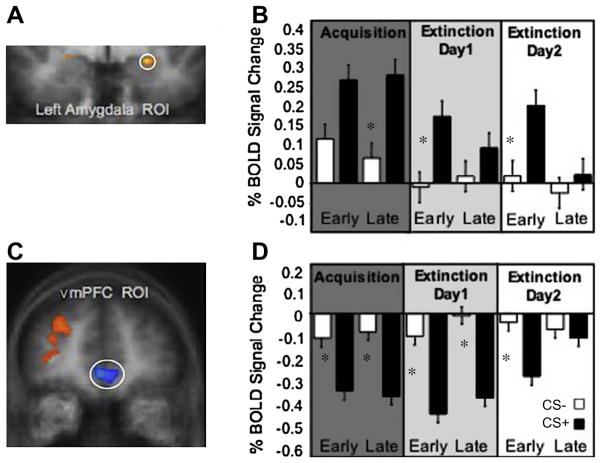
As expected, the left amygdala (x, y, z = −22, −3, −9; Fig. 2a), ROI defined as CS+ > CS− during early acquisition, showed a pattern of activation that mimicked the SCR, showing increased responses to the CS+ compared to the CS− during late acquisition and early extinction on both days (t(15) = 2.15; t(15) = 1.61; t(15) = 1.54, respectively, all P < 0.01) and no significant difference between CS+ and CS− activation during late extinction on both days (t(15) = 1.17; t(15) = 0.98, respectively, both NS; Fig. 2b).
Fig. 2.
Amygdala and vmPFC BOLD signal during threat acquisition and extinction. (A) Left Amygdala activation. (B) Amygdala mean% BOLD signal changes to CS− and CS+ during acquisition, extinction day 1, extinction day 2. (C) vmPFC activation. (D) vmPFC mean% BOLD signal changes to CS− and CS+ during acquisition, extinction day 1 and extinction day 2. Asterisks represent significantly greater BOLD responses to the CS+ than the CS− for each phase, P < 0.01.
The vmPFC (x, y, z = −1, 41, −2; Fig. 2c), ROI defined as all tones (CS+ and CS− > background noise), showed a similar pattern of differential responding but in the opposite direction prior to full extinction on day 2. That is, there was a significant decrease in CS+ evoked BOLD response in relation to the CS− during early and late acquisition and extinction on day 1 (t(15) = −2.74; t(15) = −2.98; t(15) = −4.02, t(15) = 2.15; respectively, all P < 0.01; Fig. 2d). On day 2 BOLD vmPFC responses to the CS+ t(15) started increasing with still a significant difference CS+ vs. CS− difference during early extinction (t(15) = −2.34; P < 0.01) but no difference during late extinction (t(15) = −1.30; NS).
We also conducted a post hoc, exploratory ROI analysis on the dorsal anterior cingulate (dACC x, y, z = −3, 24, 22) using a contrast of all tones vs. background. The dACC has been implicated in human threat learning analogous to conditioned threat responses found in the rodent prelimbic cortex (PL) (Knight, Smith, Cheng, Stein, & Helmstetter, 2004; Phelps et al., 2004; Milad, Wight et al., 2007a, Milad,. Quirk, et al. 2007b). It has been suggested that the rodent PL is critical for maintaining conditioned freezing and preventing the course of extinction learning (Milad & Quirk, 2012). Replicating previous findings, we found significantly greater dACC activation for CS+ vs. CS− during all of acquisition (t(15) = 2.42; t(15) = 2.74; respectively, both P < 0.01) and extinction on day 1 (t(15) = 2.27; t(15) = 2.32; respectively, both P < 0.01), including early extinction on day 2 (t(15) = 2.09, P < 0.01), but no difference by the end of extinction training for CS+ vs. CS− (t(15) = 0.92; NS). These results further support the idea that the dACC is involved in maintaining the expression of fear, but that its activity subsides when the conditioned responses have successfully been extinguished.
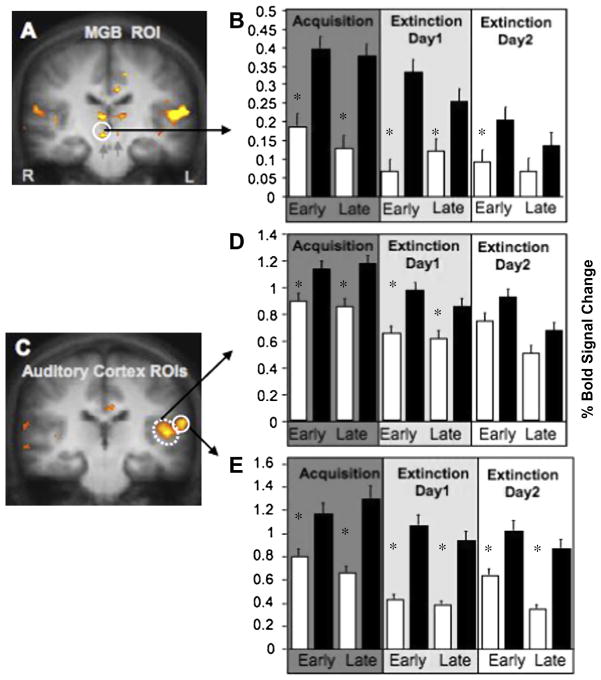
To explore the effects of threat learning on sensory processing regions, we examined BOLD responses in the auditory thalamus (x, y, z, = 4, −2, −12; Fig. 3a), as well as the primary (x, y, z = −46, −21, 9; Fig. 3c) and auditory association cortices (x, y, z = −60, −21, 13; Fig. 3c), all a priori ROIs defined as regions responsive to all tones (all CS+ and CS− > background noise). Thalamic BOLD signals increased in response to the CS+ in relation to the CS− during early and late acquisition (t(15) = 2.33; t(15) = 2.15; respectively, both P < 0.01) and decreased gradually during subsequent extinction phases (t(15) = 2.11; t(15) = 1.90; t(15) = 1.73; respectively, all P < 0.01; Fig. 3b), such that the CS+ was not significantly different from the CS− during the final extinction phase on day 2 (t(15) = 0.96; NS).
Fig. 3.
Auditory thalamus (MGB) and Auditory Cortex BOLD signal during threat acquisition and extinction. (A) Auditory thalamus activation (circle). (B) Auditory thalamus mean% BOLD signal changes to CS− and CS+ during acquisition, extinction day 1, extinction day 2. (C) Primary auditory cortex (dashed circle) and auditory association (solid circle) cortex activation revealed by the CS+ and CS− against background noise contrast. (D) Primary auditory cortex mean% BOLD signal changes to CS+ and CS− during acquisition, extinction day 1, extinction day 2. (E) Auditory association cortex mean% BOLD signal changes to CS− and CS+ during acquisition, extinction day 1, extinction day 2. Asterisks represent significantly greater BOLD responses to the CS+ than the CS− for each phase, P < 0.01.
The primary auditory cortex also showed a significant increase in BOLD response to the CS+ during early and late acquisition and extinction on day 1 (t(15) = 2.23; t(15) = 2.45; t(15) = 3.04; t(15) = 2.14; respectively, all P < 0.01; Fig. 3d), after which the CS+ no longer statistically differed from the CS− on day 2 (t(15) = 1.67; t(15) = 0.97; NS). We observed a different pattern in the auditory association cortex. During early and late acquisition, CS+ evoked BOLD responses significantly increased compared to the CS− (t(15) = 3.15; t(15) = 4.10; respectively, both P < 0.001,), consistent with the pattern observed in the amygdala, auditory thalamus and primary auditory cortex. However, in contrast to these regions, the pattern in the auditory association cortex was maintained throughout both extinction sessions (t(15) = 3.58; t(15) = 3.82; t(15) = 4.10; t(15) = 4.38; respectively, all P < 0.001; Fig. 3e) including the last phase of extinction on day 2 (Fig. 4).
Fig. 4.
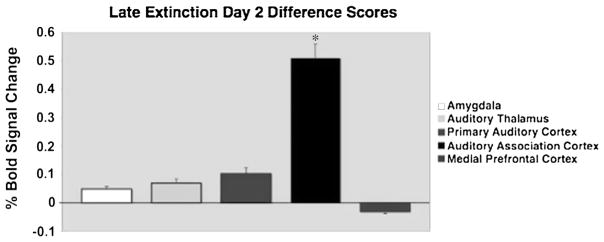
Difference scores (CS+ minus CS−) for the final stage of extinction on day 2, showing sustained activation to the conditioned tone only in the Auditory Association Cortex, P < 0.01.
4. Discussion
The present study provides novel evidence for a persisting representation of threat memories in the human auditory association cortex following extinction learning. We found that the representation of the threat memory persists in this region, even after physiological conditioned reactions have disappeared in the course of extinction, and differential BOLD responses to the CS+ are no longer present in other brain regions involved in threat learning. These findings point to an important avenue by which processing of potentially dangerous stimuli can be enhanced, namely through lasting rather than transient changes in human sensory cortex following threat learning.
Subjects in this study underwent successful differential threat conditioning and extinction learning as measured by SCR on day 1. The return of the conditioned response during early extinction on day 2 is potentially due to spontaneous recovery, a basic phenomenon of Pavlovian conditioning (Bouton, 1993). Nevertheless, the facilitated extinction from early to late phases on day 2 compared to day 1, as shown by a significant difference between differential SCR in late extinction across days, indicates that subjects recalled extinction learning on day 2. Neural models of extinction, built on both animal work and previous human visual conditioning studies, suggest that the vmPFC is involved in the acquisition and long-term expression of extinction learning (Milad & Quirk, 2012). Our results are in accordance with this notion and further indicate the involvement of the vmPFC in the recall of extinction memory for auditory stimuli in humans.
The pattern of amygdala activation mirrored the expression of threat learning and extinction as measured by SCR. Similar changes in amygdala activation patterns have been found in non-human animals in studies examining single unit responses during threat acquisition and extinction (Repa et al., 2001), and in humans during fMRI of conditioning with visual CSs (LaBar et al., 1998; Phelps et al., 2004; Milad, Wight et al., 2007a, Milad, Quirk, et al. 2007b; Schiller et al. (2008), Schiller, Levy, Niv, LeDoux, Phelps (2008)). In line with studies in rodents, conditioned thalamic responses to auditory stimuli increased during acquisition and decreased during subsequent extinction (McEchron et al., 1996).
In our study extinction resistant responses of the auditory association cortex, although not sufficient to drive defensive behavior, are nevertheless maintained and represented. This might be advantageous in allowing a quicker responding to a stimulus that has been predictive of danger in the past. It has been suggested that a network of extinction resistant neurons within the sensory cortex (Armony et al., 1998; Li et al., 2008) and lateral nucleus of the amygdala (Repa et al., 2001) act together to reengage the amygdala when threat reappears. This is based on evidence that, following extinction, the memory of the emotional event is persistent in the amygdala, as evident by neurons in the lateral nucleus that remain responsive to the CS+ even after extinction (Repa et al., 2001). In addition it has been shown that an intact amygdala is necessary for the maintenance of extinction resistant responses in auditory cortex (Chavez, McGaugh, & Weinberger, 2009). However, in the current study we could not demonstrate comparable, extinction resistant, localized BOLD responses within the human amygdala. This may be due to the relatively low spatial resolution of the conventional fMRI technique compared to single unit recordings of subnuclei that have been shown to mediate extinction resistant plasticity in the rat amygdala. It is possible that the amygdala BOLD responses we observed were primarily driven by the central nucleus, a subdivision of the amygdala known to decrease its responses during extinction (Milad & Quirk, 2012). Another region, namely, the primary auditory cortex, has also been shown to exhibit lasting changes following threat conditioning and other associative auditory learning paradigms in non-human animals (Fritz, Shamma, Elhilali, & Klein, 2003; Weinberger, 1998). In our study we found significant long-lasting changes to CS+ only in the auditory association cortex, which is the main auditory cortico-amygdala projection in rats (Doron & LeDoux, 1999). This would suggest that in humans a long lasting association is maintained by the auditory association cortex, rather than by the primary auditory cortex. In non-human animals associative representational plasticity in the auditory cortex has been shown to selectively facilitate responses to the tonal CSs with the characteristics of being highly specific, discriminative, rapidly acquired and very long lasting (Weinberger, 2007). In addition, it has been shown that when threat memories become long lasting they become to rely on sensory cortices (Sacco and Sacchetti, 2010). Furthermore, tones that acquire behavioral importance have been shown to have a larger area of representation in the auditory cortex and can be induced by stimulating the amygdala (Chavez et al., 2009). It is plausible that the amygdala is involved in initial “training” of the auditory cortex, to mark the significance of a certain sound, and that the association for this learned significance leads to a persistent memory trace in some cortical and amygdala neurons.
It would be of interest for future work to examine the behavioral significance of extinction resistant cortical responses. We hypothesize that when a previously conditioned stimulus is extinguished, and therefore does not elicit any bodily threat responses, a neural signature of the previously conditioned stimulus can still be preserved. Maintaining elevated cortical responding to a previously threatening stimulus could be a way to keep the possible threat of this stimulus highlighted. Future studies could test this hypothesis by using a previously extinguished CS+ as a CS− combined with a second CS− (novel) and novel CS+ to compare differential BOLD responses.
In summary, our results reveal that threat-related changes in the human auditory association cortex are long lasting and persist even when physiological conditioned responses (indicated by SCR) are no longer expressed. This suggests that emotion not only can lead to transient modulation of sensory regions by the amygdala, but that threat learning can leave a persistent mark on the sensitivity of human sensory cortex, which should affect future perception and actions. These persistent cortical changes following threat learning can have important clinical implications relating to the return of threat-elicited conditioned responses after initial successful extinction learning.
Acknowledgments
This work has been funded by NIH grants MH080756 and MH097085 to EAP, as well as by the James S. McDonnell Foundation and a Ruth Kirschstein National Research Service Award to AMAS.
References
- Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. Journal of Neuroscience. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. Review. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. Review. [DOI] [PubMed] [Google Scholar]
- Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: Isolating fear reactivity and fear recovery phenotypes. Journal of Traumatic Stress. 2007;20:413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiology of Learning and Memory. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron NN, LeDoux JE. Organization of projections to the lateral amygdala from auditory and visual areas of the thalamus in the rat. The Journal of Comparative Neurology. 1999;412:383–409. [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nature Neuroscience. 2003;11:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Green EJ, Winters RW, Nolen TG, Schneiderman N, McCabe PM. Changes of synaptic efficacy in the medial geniculate nucleus as a result of auditory classical conditioning. Journal of Neuroscience. 1996;16:1273–1283. doi: 10.1523/JNEUROSCI.16-03-01273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007b;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007a;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learning & Memory. 2004;11(5):501–509. doi: 10.1101/lm.77504. Review. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rotshtein P, Malach R, Hadar U, Graif M, Hendler T. Feeling or features: Different sensitivity to emotion in high-order visual cortex and amygdala. Neuron. 2001;32:747–757. doi: 10.1016/s0896-6273(01)00513-x. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmon JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, LeDoux JE, et al. Evidence for recovery of fear following immediate extinction in rats and humans. Learning & Memory. 2008a;15:394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: Reversal of fear in the human brain. Journal of Neuroscience. 2008b;45:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: More than just extinction. Current Opinion in Neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. New York: Thieme; 1998. [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experiencedependent plasticity in human auditory cortex. Neuron. 2002;3:567–574. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transaction of the Royal Society of London B. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: Characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning & Memory. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]