Abstract
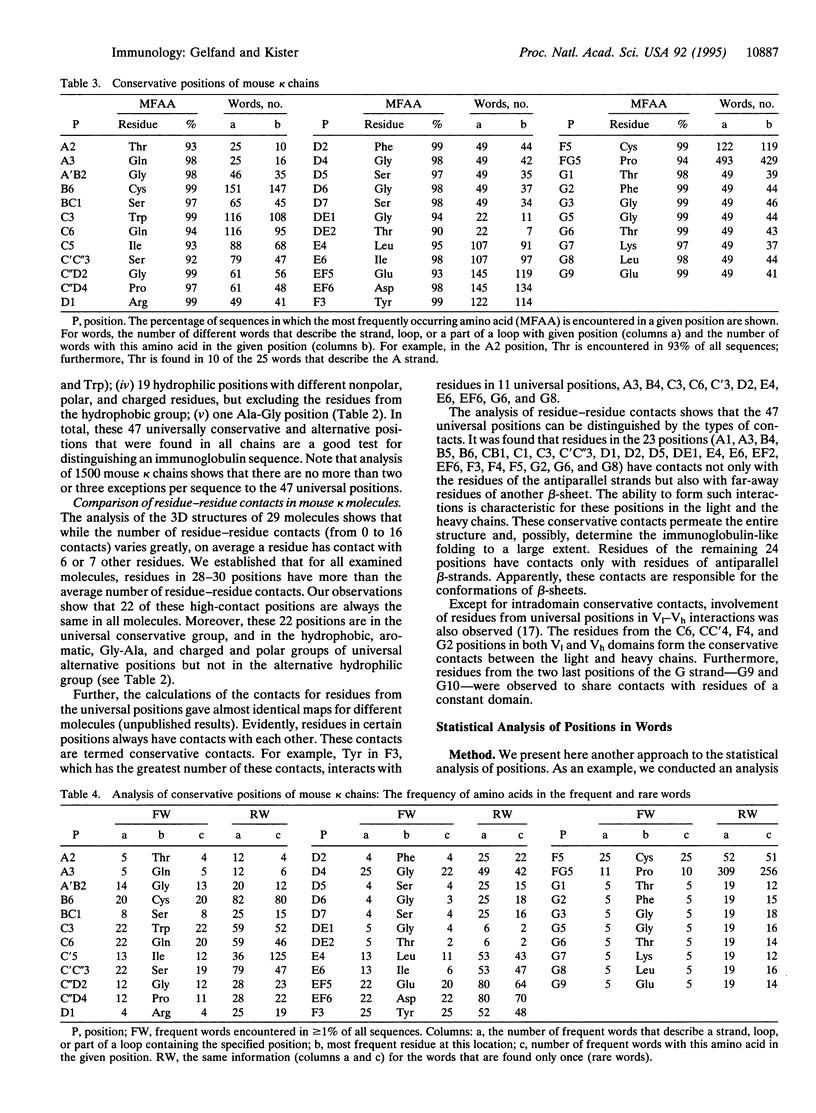
Methods of structural and statistical analysis of the relation between the sequence and secondary and three-dimensional structures are developed. About 5000 secondary structures of immunoglobulin molecules from the Kabat data base were predicted. Two statistical analyses of amino acids reveal 47 universal positions in strands and loops. Eight universally conservative positions out of the 47 are singled out because they contain the same amino acid in > 90% of all chains. The remaining 39 positions, which we term universally alternative positions, were divided into five groups: hydrophobic, charged and polar, aromatic, hydrophilic, and Gly-Ala, corresponding to the residues that occupied them in almost all chains. The analysis of residue-residue contacts shows that the 47 universal positions can be distinguished by the number and types of contacts. The calculations of contact maps in the 29 antibody structures revealed that residues in 24 of these 47 positions have contacts only with residues of antiparallel beta-strands in the same beta-sheet and residues in the remaining 23 positions always have far-away contacts with residues from other beta-sheets as well. In addition, residues in 6 of the 47 universal positions are also involved in interactions with residues of the other variable or constant domains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Levitt M., Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. The predicted structure of immunoglobulin D1.3 and its comparison with the crystal structure. Science. 1986 Aug 15;233(4765):755–758. doi: 10.1126/science.3090684. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Turn prediction in proteins using a pattern-matching approach. Biochemistry. 1986 Jan 14;25(1):266–275. doi: 10.1021/bi00349a037. [DOI] [PubMed] [Google Scholar]
- Harpaz Y., Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J Mol Biol. 1994 May 13;238(4):528–539. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- Havel T. F., Snow M. E. A new method for building protein conformations from sequence alignments with homologues of known structure. J Mol Biol. 1991 Jan 5;217(1):1–7. doi: 10.1016/0022-2836(91)90603-4. [DOI] [PubMed] [Google Scholar]
- Hazes B., Hol W. G. Comparison of the hemocyanin beta-barrel with other Greek key beta-barrels: possible importance of the "beta-zipper" in protein structure and folding. Proteins. 1992 Mar;12(3):278–298. doi: 10.1002/prot.340120306. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. The structural basis of antibody complementarity. Adv Protein Chem. 1978;32:1–75. [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Nauchitel V. V., Somorjai R. L. Gaussian neighborhood: a new measure of accessibility for residues of protein molecules. Proteins. 1993 Jan;15(1):50–61. doi: 10.1002/prot.340150107. [DOI] [PubMed] [Google Scholar]
- Padlan E. A. Anatomy of the antibody molecule. Mol Immunol. 1994 Feb;31(3):169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Padlan E. A. Evaluation of the structural variation among light chain variable domains. Mol Immunol. 1979 May;16(5):287–296. doi: 10.1016/0161-5890(79)90128-7. [DOI] [PubMed] [Google Scholar]
- Rooman M. J., Wodak S. J. Weak correlation between predictive power of individual sequence patterns and overall prediction accuracy in proteins. Proteins. 1991;9(1):69–78. doi: 10.1002/prot.340090108. [DOI] [PubMed] [Google Scholar]
- Rost B., Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993 Jul 20;232(2):584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Taylor W. R., Orengo C. A. A holistic approach to protein structure alignment. Protein Eng. 1989 May;2(7):505–519. doi: 10.1093/protein/2.7.505. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Chothia C., Lesk A. M. Structural determinants of the conformations of medium-sized loops in proteins. Proteins. 1989;6(4):382–394. doi: 10.1002/prot.340060405. [DOI] [PubMed] [Google Scholar]
- Zhang X., Mesirov J. P., Waltz D. L. Hybrid system for protein secondary structure prediction. J Mol Biol. 1992 Jun 20;225(4):1049–1063. doi: 10.1016/0022-2836(92)90104-r. [DOI] [PubMed] [Google Scholar]



