Abstract
Objective
To assess the feasibility of using community health workers to administer short or ultra-short screening instruments during routine community-based prenatal outreach for detecting probable depression at 12 weeks postpartum.
Methods
During pregnancy and at 12 weeks postpartum, the 10-item Edinburgh Postnatal Depression Scale (EPDS-10) was administered to 249 Xhosa-speaking black African women living in Khayelitsha, South Africa. We compared the operating characteristics of the prenatal EPDS-10, as well as 4 short and ultra-short subscales, with the criterion standard of probable postpartum depression.
Results
Seventy-nine (31.7%) women were assessed as having probable postpartum depression. A prenatal EPDS-10 score of 13 or higher had 0.67 sensitivity and 0.67 specificity for detecting probable postpartum depression. Briefer subscales performed similarly.
Conclusion
Community health workers successfully conducted community-based screening for depression in a resource-limited setting using short or ultra-short screening instruments. However, overall feasibility was limited because prenatal screening failed to accurately predict probable depression during the postpartum period.
Keywords: Postpartum depression, Screening, Sub-Saharan Africa
1. Introduction
In South Africa, 35%–47% of women have been diagnosed with major depressive disorder during pregnancy and the postpartum period [1]. Major depressive disorder is accompanied by substantial morbidity and disability, and newborn children of mothers with depression have poorer health and socioemotional development, with adverse implications for their long-term psychosocial, cognitive, and economic wellbeing [2]. Yet, while many Sub-Saharan African countries have a greater prevalence of postpartum depression compared with high-income countries, many of their mental health systems are under-resourced [3].
Major depressive disorder is often underdiagnosed by providers throughout pregnancy and the postpartum period [4]. In many low- and middle-income countries, opportunities to screen for postpartum depression are limited by losses to follow-up from care during the first few weeks after delivery. Furthermore, postpartum care in these settings is focused primarily on the health of the newborn, with little attention paid to maternal wellbeing [5]. Prenatal screening has been proposed as a potentially important strategy to aid in the identification of women at risk for developing postpartum depression [6] but little research exists to inform programmatic work in this area. The role of prenatal screening is further complicated by the substantial changes in depressive symptomatology that occur throughout pregnancy and postpartum. In previously published studies from the USA and Europe, a large proportion of women were found to present with elevated symptoms of depression during pregnancy that subsequently declined after delivery [7,8]. Thus, it is very possible that prenatal screening may label large numbers of women “at risk” when, in fact, their symptoms may naturally resolve over time without psychological or psychopharmacologic intervention.
To address this gap in the literature, we conducted a study to determine the criterion-related validity (considered a part of the larger domain of construct validity [9]) of the 10-item Edinburgh Postnatal Depression Scale (EPDS-10) [10] when administered during pregnancy to screen for postpartum depression among high-risk women living in a South African township. We trained community health workers to implement the screening as part of their routine community-based outreach, for 2 reasons. First, many low- and middle-income countries have sought to explore task-shifting strategies to maximize human resources for health care [11]. Second, South Africa’s primary healthcare system is currently being restructured to better meet the healthcare needs of the population, and community health workers will occupy a central role in screening people in the community and referring potential patients for further diagnostic assessment to primary healthcare facilities [12].
2. Materials and methods
A prospective cohort study was conducted from May 1, 2010, to February 18, 2011, in Khayelitsha—a socioeconomically deprived peri-urban settlement with high rates of unemployment on the outskirts of Cape Town, South Africa. Residents of Khayelitsha are primarily Xhosa-speaking black Africans, most of whom live in informal housing (i.e. shacks) on unserviced land. Khayelitsha has the highest age-standardized mortality rates relative to other subdistricts of Cape Town, with the leading causes being HIV/AIDS, homicide, and tuberculosis [13]. The prevalence of postpartum depression in this population has been estimated to exceed 30%–40% [1,14,15].
We recruited a consecutive sample of study participants from among women newly enrolled into a maternal/child health and nutrition program operated by the Philani Maternal Child Health and Nutrition Project, which is a community-based nongovernmental organization that has been based in Khayelitsha for more than 3 decades. As part of Philani’s standard operating procedures, its community health workers conduct routine community-based outreach, identifying all pregnant women and inviting them to take part in their ongoing maternal/child health and nutrition program. At the time of enrollment, we invited Philani participants to also participate in our postpartum depression screening study. Throughout the study, participants who were thought to be at acutely elevated risk for suicide were referred to a local non-governmental organization that provides community-based mental health services throughout the Western Cape region. All participants provided written informed consent. Ethical approval for all study procedures was granted by the Health Research Ethics Committee, Faculty of Health Sciences, Stellenbosch University; the Committee on Human Research, University of California at San Francisco; and the Office of Human Research Administration, Harvard School of Public Health.
At the baseline prenatal visit, Philani outreach workers used survey software programmed into a mobile phone [16–18] to administer the Xhosa version of the EPDS-10. We used the EPDS-10 because it has been extensively used and validated for both prenatal and postpartum administration. Several studies have supported its construct validity, criterion-related validity, and internal structure among Xhosa-speaking women in South Africa when administered in the prenatal period, and its criterion-related validity and internal structure when administered during the postpartum period [19]. Similar to Kabir et al. [20] and Rochat et al. [21], we defined 4 short and ultra-short subscales of the EPDS-10 to investigate their potential as screening instruments: the 2-item analog of the Patient Health Questionnaire (EPDS-2); the 3-item anxiety symptoms subscale (EPDS-3); an abbreviated 5-item version of the depressive symptoms subscale (EPDS-5) [21]; and the 7-item depressive symptoms subscale (EPDS-7). Additional data were gathered on demographics, socioeconomic status, health behaviors, and self-reported HIV serostatus.
Although the postpartum-onset specifier in the Diagnostic and Statistical Manual of Mental Disorders describes a 4-week onset [22], the assessment of postpartum depression in clinical practice, as well as in many research studies, extends well beyond this period. Consequently, we scheduled our research assistants to visit participants at their homes 3 months after birth (±2 weeks to allow for scheduling difficulties) for the postpartum interview assessment. Following previous studies in this population, we used an EPDS score of 13 or higher as the cutoff to define the criterion standard of probable postpartum depression [11,14,21].
We used the nonparametric equality-of-medians test to determine whether women lost to follow-up versus those not lost to follow-up were drawn from populations with the same median level of depression symptom severity. The Cronbach α coefficient was used to estimate the internal consistency of the EPDS-10 and each of its shortened subscales, and the Pearson correlation coefficient was used to estimate the correlations between them. We compared the operating characteristics of the prenatal EPDS-2, EPDS-3, EPDS-5, EPDS-7, and EPDS-10 with the criterion standard of probable postpartum depression, calculating sensitivity, specificity, and likelihood ratios using standard formulas. We then generated receiver operating characteristic (ROC) curves, calculating the areas under the curves using the trapezoidal rule and comparing the areas under the curves using the algorithm suggested by DeLong et al. [23]. The α level used to determine statistical significance was 0.05. All analyses were conducted using Stata version 12.1 (StataCorp, College Station, TX, USA).
3. Results
Outreach workers initially recruited and obtained consent from a consecutive sample of 361 women at various stages of pregnancy, typically during the second or third trimester. Twenty-nine (8.0%) women were lost to follow-up: 22 women’s records in the Philani program were lost or closed for unknown reasons, and the whereabouts of 7 were unknown. The women who were lost to follow-up had a greater median EPDS-10 compared with those not lost to follow-up, but the difference was not significant (14 vs 10; P=0.44). In addition to those lost to follow-up, another 83 women (23.0%) were not successfully interviewed at the 3-month postpartum visit: 22 completed the interview outside the pre-specified time window; 47 relocated to the Eastern Cape shortly after giving birth (a common cultural phenomenon); 8 had dropped out of the Philani program; and 6 rescinded consent.
The analytic sample consisted of the 249 women successfully interviewed at the 3-month postpartum visit. Summary statistics are displayed in Table 1. The median age was 26 years (interquartile range [IQR], 23–29 years) and most were unmarried (153 [61.4%]). In addition, few mothers were employed (34 [13.7%]) and most lived in informal housing (144 [57.8%]). Seventy (28.1%) mothers reported being HIV seropositive. At baseline, the median EPDS-10 score was 10 (IQR, 5–17). The estimated Cronbach α coefficients for the EPDS-2, EPDS-3, EPDS-5, EPDS-7, and EPDS-10 were 0.54, 0.78, 0.79, 0.83, and 0.88, respectively. The Pearson correlation coefficients between the EPDS-10 and the shortened subscales ranged from 0.84 to 0.97.
Table 1.
Summary statistics a
| Characteristic | Value |
|---|---|
| Age, y b | 26 (23–29) |
| Married b | 90 (36.1) |
| Self-reported HIV serostatus c | |
| HIV positive | 70 (28.1) |
| HIV negative | 160 (64.3) |
| Never tested | 8 (3.2) |
| Employed d | 34 (13.7) |
| Receives child support grant e | 142 (57.0) |
| Amount of grant, Rand c | 250 (250–500) |
| Informal housing f | 144 (57.8) |
| House has flush toilet f | 137 (55.0) |
| Household experienced ≥1 day with no food in past 12 months g | 24 (9.6) |
| Smokes cigarettes h | 14 (5.6) |
| Takes alcohol b | 23 (9.2) |
Values are given as median (interquartile range) or No. (%).
Six missing values.
Eleven missing values.
Eight missing values.
Twenty-five missing values.
Seven missing values.
Three missing values.
Four missing values.
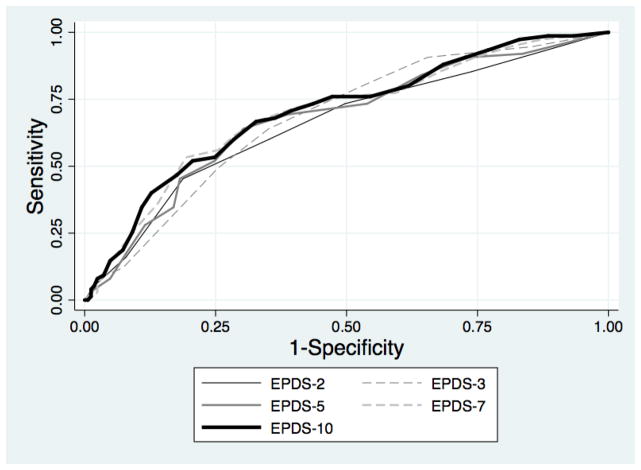
At the 3-month postpartum visit, 79 (31.7%) women were assessed as having probable postpartum depression. The areas under the ROC curves of the prenatal EPDS-10 and its briefer subsets ranged from 0.66 to 0.70 (Table 2). A prenatal EPDS-10 score of 13 or higher maximized the Youden Index, with 0.67 sensitivity and 0.67 specificity for detecting probable postpartum depression. The ROC curves for the EPDS-3 and EPDS-7 were similar to that of the EPDS-10 (Figure 1). A prenatal EPDS-2 score of 4 or higher had 0.44 sensitivity and 0.82 specificity for detecting probable postpartum depression.
Table 2.
Sensitivity and specificity of prenatal screening for detecting probable postpartum depression
| EPDS-2 | EPDS-3 | EPDS-5 | EPDS-7 | EPDS-10 | |
|---|---|---|---|---|---|
| Area under ROC curve | 0.66 (0.58–0.73) | 0.67 (0.60–0.74) | 0.67 (0.60–0.75) | 0.69 (0.62–0.77) | 0.70 (0.63–0.77) |
| χ2 (P value) for comparison with area under ROC curve for EPDS-10 | 4.10 (0.04) | 1.56 (0.21) | 4.63 (0.03) | 0.26 (0.61) | |
| Threshold to maximize Youden Index | ≥4 | ≥16 | ≥10 | ≥11 | ≥13 |
| Sensitivity | 0.44 | 0.64 | 0.68 | 0.65 | 0.67 |
| Specificity | 0.82 | 0.64 | 0.61 | 0.67 | 0.67 |
| Positive likelihood ratio | 2.41 | 1.79 | 1.75 | 1.98 | 2.04 |
Abbreviation: EPDS, Edinburgh Postnatal Depression Scale; ROC, receiver operating characteristic.
Figure 1.
Receiver operating characteristic curves for the Edinburgh Postnatal Depression Scale (EPDS)-2, EPDS-3, EPDS-5, EPDS 7, and EPDS-10 administered during pregnancy.
4. Discussion
In a prospective cohort study of 249 women, we sought to demonstrate the feasibility of using community health workers to conduct community-based screening for depression among high-risk women living in a peri-urban settlement near Cape Town. Community health workers were able to successfully incorporate the screening activities into their routine workflow. However, overall feasibility was limited because prenatal screening with the EPDS-10, at a threshold of 13 or higher, had only 0.67 sensitivity and 0.67 specificity for detecting probable postpartum depression. Shortened screening instruments derived from the EPDS-10 performed similarly. The present findings have important implications for the development of programs to support maternal and child wellbeing in resource-limited settings.
The study demonstrated the feasibility of incorporating depression screening into community health workers’ routine workflow. Although task shifting to non-specialist, lay health workers has been increasingly emphasized in the global mental health agenda [24], interventions must be designed with their heavy workloads in mind. It is possible that the use and administration of short or ultra-short screening instruments, potentially facilitated with mobile technologies [18,25], could fill this gap. In the present study, the community health workers were able to screen for depression among study participants during the routine course of their community-based outreach work. Furthermore, the estimated rates of prenatal depression obtained were comparable to those derived in similar populations using trained research assistants [1,15,19].
Overall feasibility, however, was limited. The 3-, 5-, and 7-item subscales administered during pregnancy had comparable performance to the EPDS-10 and to each other but, in contrast to prior work, they had relatively low sensitivities and specificities for detecting probable postpartum depression. The EPDS-2 performed even more poorly. Although the present study had a low rate of loss to follow-up, nearly one-third of participants originally enrolled were not successfully interviewed at the postpartum visit. An assessment of the overall feasibility of prenatal screening for postpartum depression would, therefore, need to address other health system and structural barriers, such as retention in care from pregnancy to the postpartum period, and migration and appropriate transfers of care.
The screening properties we estimated for the EPDS-10 compared similarly to estimates derived from studies conducted in high-income countries. Gaynes et al. [26] reviewed 9 studies of postpartum depression screening in the USA and high-income Commonwealth countries and found that the sensitivity of the EPDS varied from 0.75 to 1.0 and its specificity varied from 0.70 to 0.99. In a meta-analysis focused on studies conducted in African settings [19], the pooled sensitivity and specificity of the EPDS for detecting postpartum depression were 0.94 and 0.77, respectively. By contrast, studies of prenatal screening for postpartum depression conducted in high-income countries have estimated the sensitivities of various screening instruments to be much lower (ranging from 0.23 to 0.82) but with comparable specificity (0.43–0.96) [6]. Using the Xhosa version of the EPDS-10 validated in the target population for both prenatal and postpartum use, we extended the findings of Austin and Lumley [6] to a resource-limited setting in South Africa. However, we found that the EPDS-10 administered during pregnancy was neither sensitive nor specific for detecting probable postpartum depression; for a hypothetical sample of 1000 women who undergo screening during pregnancy, we estimate that there would be 101 women likely to develop postpartum depression who would screen negative and 237 women unlikely to develop postpartum depression who would screen positive. The findings indicate that wider implementation of such screening programs would have relatively limited benefits in terms of detection accuracy.
There are several potential explanations for the present findings. First, prenatal screening for postpartum depression is likely to be most predictive in a population comprising women whose baseline symptoms of depression (whether elevated or not) remain stable throughout the period of follow-up. However, it is well known that a large proportion of women present with elevated symptoms of depression during pregnancy but experience substantial reductions in symptoms after delivery. This phenomenon has been demonstrated in several studies in the USA and Europe [7,8]. Moreover, study participants were enrolled in an ongoing maternal/child health and nutrition program, and the routine social support provided by the community health workers could also have contributed to decreases in depression symptom severity. Such changes in symptomatology would have biased our estimates toward the null. Second, 29 women (8.0% of the study sample) who were initially interviewed at baseline were lost to follow-up. Although this was a relatively low rate of loss to follow-up, this subgroup of women had a higher baseline EPDS-10 score. If the subgroup of women lost to follow-up had chronically elevated symptoms throughout the follow-up period, their exclusion from the analysis would have biased our estimates toward the null. Third, because we designed the study to fit into the routine procedures of a community-based nutrition and wellbeing program implemented by community health workers, we did not have reliable data on gestational ages. Through their community-based outreach work, the Philani outreach workers identify expectant mothers at various stages of pregnancy, typically during the second or third trimester, but the estimated dates of delivery can be overestimated or underestimated by several weeks.
Although the present study was based on a relatively small sample, the participants had a nearly identical demographic and socioeconomic profile to that of a much larger, nearly universal sample of pregnant women recruited to participate in a cluster-randomized controlled trial from a different area of Khayelitsha [18]. Moreover, the prevalence of probable prenatal depression estimated in the randomized trial was similar to the present estimate [14]. Although neither of these studies used structured clinical interviews, in a study conducted among Zulu-speaking women presenting to a prenatal clinic in KwaZulu-Natal, South Africa, the estimated prevalence of major depressive disorder based on structured clinical interviews (47%) was comparable to (higher than) the estimated prevalence of probable depression based on the EPDS (41%–44%) [21]. The present estimate of the prevalence of probable postpartum depression (31.7%) is also comparable to (slightly higher than) the prevalence of postpartum major depressive disorder estimated in this same population using structured clinical interviews [1]. Thus, although the present findings do not support the use of prenatal screening for postpartum depression, they do underscore the fact that maternal mental health is an important public health issue. Further research on early detection and intervention for depressed mood in this vulnerable population is needed.
Synopsis.
Community-based prenatal screening by community health workers was feasible but failed to accurately predict postpartum depression in a South African township.
Acknowledgments
The study was funded by the Medical Research Council of South Africa and the US National Institutes of Health (NIH; R25MH060482). M.T. received financial support from the National Research Foundation (South Africa) and the Department for International Development (UK). A.C.T. received financial support from NIH (K23MH096620) and the Robert Wood Johnson Health and Society Scholars Program.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai AC, Tomlinson M. Mental health spillovers and the Millennium Development Goals: The case of perinatal depression in Khayelitsha, South Africa. J Glob Health. 2012;2(1):010302. doi: 10.7189/jogh.02.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heckman JJ. The economics, technology, and neuroscience of human capability formation. Proc Natl Acad Sci U S A. 2007;104(33):13250–5. doi: 10.1073/pnas.0701362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson M, Lund C. Why does mental health not get the attention it deserves? An application of the Shiffman and Smith framework. PLoS Med. 2012;9(2):e1001178. doi: 10.1371/journal.pmed.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fergerson SS, Jamieson DJ, Lindsay M. Diagnosing postpartum depression: can we do better? Am J Obstet Gynecol. 2002;186(5):899–902. doi: 10.1067/mob.2002.123404. [DOI] [PubMed] [Google Scholar]
- 5.Ijumba P, Padarath A. South African Health Review. 2006 http://www.hst.org.za/uploads/files/begining_06.pdf. Published October 2006.
- 6.Austin MP, Lumley J. Antenatal screening for postnatal depression: a systematic review. Acta Psychiatr Scand. 2003;107(1):10–7. doi: 10.1034/j.1600-0447.2003.02024.x. [DOI] [PubMed] [Google Scholar]
- 7.Mora PA, Bennett IM, Elo IT, Mathew L, Coyne JC, Culhane JF. Distinct trajectories of perinatal depressive symptomatology: evidence from growth mixture modeling. Am J Epidemiol. 2009;169(1):24–32. doi: 10.1093/aje/kwn283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter-Dallay AL, Cosnefroy O, Glatigny-Dallay E, Verdoux H, Rascle N. Evolution of perinatal depressive symptoms from pregnancy to two years postpartum in a low-risk sample: the MATQUID cohort. J Affect Disord. 2012;139(1):23–9. doi: 10.1016/j.jad.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 9.American Educational Research Association, American Psychological Association, National Council on Measurement in Education. Standards for Educational and Psychological Testing. Washington, D.C: American Psychological Association; 1999. [Google Scholar]
- 10.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression. Scale Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 11.Honikman S, van Heyningen T, Field S, Baron E, Tomlinson M. Stepped care for maternal mental health: a case study of the perinatal mental health project in South Africa. PLoS Med. 2012;9(5):e1001222. doi: 10.1371/journal.pmed.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padarath A, English R. South African Health Review. 2011 http://www.hst.org.za/sites/default/files/sahr_2011.pdf. Published December 2011.
- 13.Groenewald P, Bradshaw D, Daniels J, Zinyakatira N, Matzopoulos R, Bourne D, et al. Local-level mortality surveillance in resource-limited settings: a case study of Cape Town highlights disparities in health. Bull World Health Organ. 2010;88(6):444–51. doi: 10.2471/BLT.09.069435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson M, O’Connor MJ, le Roux IM, Stewart J, Mbewu N, Harwood J, et al. Multiple risk factors during pregnancy in South Africa: the need for a horizontal approach to perinatal care. Prev Sci. doi: 10.1007/s11121-013-0376-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewing S, Tomlinson M, le Roux IM, Chopra M, Tsai AC. Food insecurity and its association with co-occurring postnatal depression, hazardous drinking, and suicidality among women in peri-urban South Africa. J Affect Disord. 2013;150(2):460–5. doi: 10.1016/j.jad.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomlinson M, Solomon W, Singh Y, Doherty T, Chopra M, Ijumba P, et al. The use of mobile phones as a data collection tool: a report from a household survey in South Africa. BMC Med Inform Decis Mak. 2009;9:51. doi: 10.1186/1472-6947-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlinson M, Rotheram-Borus MJ, Doherty T, Swendeman D, Tsai AC, Ijumba P, et al. Value of a mobile information system to improve quality of care by community health workers. S Afr J Inform Manag. 2013;15(1):Article 528. doi: 10.4102/sajim.v15i1.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai AC, Tomlinson M, Dewing S, le Roux IM, Harwood JM, Chopra M, et al. Antenatal depression case finding by community health workers in South Africa: feasibility of a mobile phone application. Arch Womens Ment Health. doi: 10.1007/s00737-014-0426-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai AC, Scott JA, Hung KJ, Zhu JQ, Matthews LT, Psaros C, et al. Reliability and validity of instruments for assessing perinatal depression in African settings: systematic review and meta-analysis. PLoS One. 2013;8(12):e82521. doi: 10.1371/journal.pone.0082521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabir K, Sheeder J, Kelly LS. Identifying postpartum depression: are 3 questions as good as 10? Pediatrics. 2008;122(3):e696–702. doi: 10.1542/peds.2007-1759. [DOI] [PubMed] [Google Scholar]
- 21.Rochat TJ, Tomlinson M, Newell ML, Stein A. Detection of antenatal depression in rural HIV-affected populations with short and ultrashort versions of the Edinburgh Postnatal Depression Scale (EPDS) Arch Womens Ment Health. 2013;16(5):401–10. doi: 10.1007/s00737-013-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. DSM-IV. [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 24.Kagee A, Tsai AC, Lund C, Tomlinson M. Screening for common mental disorders in low resource settings: reasons for caution and a way forward. Int Health. 2013;5(1):11–4. doi: 10.1093/inthealth/ihs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlinson M, Rotheram-Borus MJ, Swartz L, Tsai AC. Scaling up mHealth: where is the evidence? PLoS Med. 2013;10(2):e1001382. doi: 10.1371/journal.pmed.1001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. Evidence Report/Technology Assessment. doi: 10.1037/e439372005-001. Number 119. http://archive.ahrq.gov/downloads/pub/evidence/pdf/peridepr/peridep.pdf. Published Fenruary 2005. [DOI] [PMC free article] [PubMed]