Abstract
Background
We hypothesized that heart failure (HF) patients who recover left ventricular function (HF-Recovered) have a distinct clinical phenotype, biology and prognosis compared to HF with reduced ejection fraction (HF-REF) and HF with preserved ejection fraction (HF-PEF).
Methods and Results
The Penn Heart Failure Study (PHFS) is a prospective cohort of 1,821 chronic HF patients recruited from tertiary HF clinics. Participants were divided into three categories based on echocardiograms: HF-REF if EF<50%, HF-PEF if EF consistently ≥50%, and HF-Recovered if EF on enrollment to PHFS was ≥50% but prior EF<50%. A significant portion of HF-Recovered patients had an abnormal biomarker profile at baseline, including 44% with detectable Troponin I, although in comparison, median levels of BNP, sFlt-1, Troponin I and creatinine were greater in HF-REF and HF-PEF patients. In unadjusted Cox models over a maximum follow-up of 8.9 years, the hazard ratio (HR) for death, transplant or ventricular assist device in HF-REF was 4.1 (95%CI 2.4–6.8; p<0.001) and in HF-PEF was 2.3 (95%CI 1.2–4.5; p=0.013), as compared to HF-Recovered. The unadjusted HR for cardiac hospitalization in HF-REF was 2.0 (95%CI 1.5–2.7; p<0.001) and in HF-PEF was 1.3 (95%CI 0.90–2.0; p=0.15), compared to HF-Recovered. Results were similar in adjusted models.
Conclusions
HF-Recovered is associated with a better biomarker profile and event-free survival than HF-REF and HF-PEF. However, these patients still have abnormalities in biomarkers and experience a significant number of HF hospitalizations, suggesting persistent HF risk.
Keywords: heart failure, myocardial recovery, remodeling
Introduction
Recovery of left ventricular (LV) function is a primary goal of therapy in heart failure with reduced ejection fraction (HF-REF). Pharmacologic therapy, coronary revascularization, cardiac resynchronization, and ventricular-assist devices are all used to achieve this objective.1–6 However, little is known about the natural history, prognosis, and need for continued chronic therapies in patients when there is recovery of LV function (e.g. heart failure and recovered ejection fraction, or HF-Recovered). 7–10 In fact, many of these patients continue HF-REF treatment, while others may discontinue therapy or become misclassified as heart failure with preserved ejection fraction (HF-PEF).11
Increases in EF and/or improvements in LV geometry in response to therapy may result in a milder heart failure phenotype and a prognostic improvement. This favorable myocardial response has been referred to as “reverse remodeling” or “myocardial remission.”12 Understanding the clinical and biologic features of the HF-Recovered population is central to understanding what predicts reverse remodeling and, ultimately, complete recovery. This understanding is particularly relevant in the field of mechanical circulatory support, where using left ventricular assist devices as a bridge to recovery is an evolving clinical practice and focus of intense investigation.13
We sought to characterize the HF-Recovered population, which includes a spectrum of patients with reverse remodeling, and compare to HF-REF and HF-PEF. We hypothesized that the HF-Recovered population has a distinct clinical phenotype, biology, and prognosis. We explored this hypothesis by analyzing baseline clinical data, biomarker data representing several key biological pathways (neurohormonal activation, myocyte stress and injury, oxidative stress, inflammation, and vascular remodeling), and long-term clinical outcomes from a diverse, outpatient-based multicenter heart failure cohort.
Materials & Methods
Study Population
The Penn Heart Failure Study is a prospective cohort study of patients referred to three U.S. heart failure specialty centers: University of Pennsylvania (Philadelphia, PA), Case Western Reserve University (Cleveland, OH), and University of Wisconsin (Madison, WI), using a harmonized protocol. An institutional review committee at each participating center approved the study protocol; each participant gave informed written consent. The inclusion criteria were: referral to an outpatient heart failure specialty clinic at these centers for evaluation and treatment of heart failure; and absence of a non-cardiac condition likely leading to mortality within six months. All new referrals at each center were directly screened for eligibility by study coordinators in the clinics through direct review of individual medical records. Eligible patients were then approached at the time of their clinic visit and offered participation. Over the course of the study, approximately one third of eligible patients consented to participate. Participants were recruited from 2003 to 2012.14
At time of study entry, detailed clinical data were obtained using a standardized questionnaire administered to the patient and treating physician, with verification via medical records. The primary etiology of heart failure and the presence or absence of co-morbidities was defined by the heart failure cardiologist at each center based on available clinical data. Venous blood samples were obtained at enrollment, processed, and stored at −80°C until time of assay. Two-dimensional transthoracic echocardiography was performed in all participants at an Intersocietal Commission for the Accreditation of Echocardiography Laboratories accredited laboratory as part of routine clinical practice. The median time between echocardiogram measurement and study enrollment was 71 days. A level III-certified echocardiographer estimated left ventricular ejection fraction (EF) at each center. Dedicated research personnel obtained follow-up data on all participants every six months according to the study protocol, either through in-person encounters in the clinic or through telephone encounters. At each follow-up encounter, study endpoints (death, ventricular assist device (VAD), transplant, hospitalizations) were captured and verified through death certificates, medical records, or contract with patients’ family members. Hospitalizations were classified as cardiovascular or non-cardiovascular based on medical record adjudication of admission diagnoses by a heart failure expert. Participants were censored at time of last follow-up.
Classification of Heart Failure Subgroups
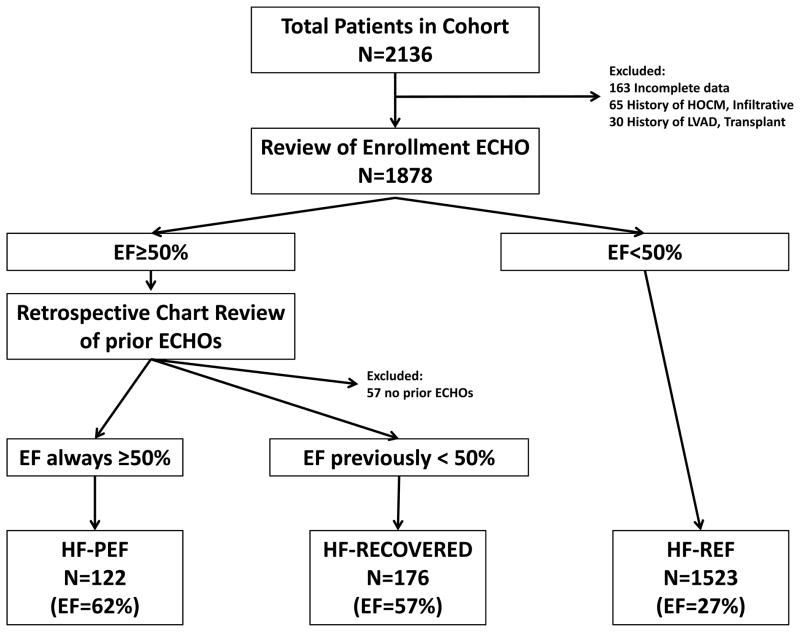
For this analysis, participants were classified into three categories of heart failure: Heart Failure with Reduced EF (HF-REF), Heart Failure with Preserved EF (HF-PEF), or Heart Failure with Recovered EF (HF-Recovered). Patients were classified as HF-REF if enrollment echocardiogram showed an EF <50%. Patients with normal left ventricular function as defined by EF ≥50%, were further classified into HF-PEF versus HF-Recovered based on additional retrospective chart review of prior echocardiograms. Patients who only had previously documented EF≥50% were considered HF-PEF, while those with a documented history of EF<50% were considered HF-Recovered. Patients with incomplete baseline information were excluded. Additionally, patients with hypertrophic cardiomyopathy and infiltrative cardiomyopathies were excluded. The natural history of these conditions is often distinct from HF-PEF and, in cases such as amyloidosis, particularly poor. As such, we felt that inclusion of these subjects would be inappropriate and could bias the results toward worse outcomes in HF-PEF. Patients with normal EF on enrollment, but no prior documentation of his/her EF were excluded from this study.
Biomarker Assays
All biomarkers were measured from banked plasma obtained at the time of study enrollment. Brain natriuretic peptide (BNP), troponin I (TnI), high-sensitivity C-reactive protein (hsCRP), uric acid, and creatinine were measured using standard ARCHITECT immunoassays (Abbott Laboratories, Abbott Park, IL). Placental growth factor (PlGF), soluble fms-like tyrosine kinase receptor-1 (sFlt-1) and myeloperoxidase (MPO) were measured using prototype ARCHITECT chemiluminescent microparticle-based immunoassays (Abbott Laboratories, Abbott Park, IL). Soluble toll-like receptor-2 (ST2) was measured via a high sensitivity sandwich monoclonal immunoassay (Presage ST2 Assay, Critical Diagnostics, San Diego, CA). 15
Statistical Methods
Characteristics of study participants at enrollment were summarized using standard descriptive statistics; ANOVA, Kruskal-Wallis tests, and Fisher’s exact tests were used to compare characteristics between heart failure categories. For certain covariates, such as biomarker levels, data may have only been available for a subset of patients. In these situations, the relevant size of the available subset was noted in the corresponding table. Imputation was not performed for missing data. Among participants with serum biomarkers collected at enrollment, biomarker levels were summarized using medians with 25th and 75th percentiles. Kruskal Wallis rank-sum tests were used to compare biomarker levels between heart failure categories. A Kaplan-Meier curve was used to summarize the survival distribution for the composite terminal event of all-cause death, cardiac transplantation, or VAD placement. Cox regression models were used to determine the relative hazard of the composite terminal event between heart failure categories. A non-parametric, recurrent event survival curve was used to summarize the survival distribution for cardiac hospitalization16. Recurrent event models were used to determine the relative hazard of cardiac hospitalization between heart failure categories. A joint frailty model was used to accommodate informative censoring by the composite terminal event17. Adjustment variables were selected a priori and included age, sex, race (Caucasian, African American, or other), heart failure etiology (ischemic or non-ischemic), history of chronic kidney disease, history of hypertension, and enrollment site. All analyses were completed using R 3.0.1 (R Development Core Team, Vienna, Austria), including the survival, survrec, and frailtypack extension packages.18
Results
Of the 2,136 patients in our cohort, 163 patients had insufficient baseline data to include in this analysis, 65 patients had a history of hypertrophic cardiomyopathy or infiltrative heart diseases, and 30 had a history of left ventricular assist device implantation or heart transplantation. 1,878 patients met study criteria, but 57 patients with an EF ≥50% were excluded due to lack of prior echocardiogram and EF data. The final 1,821 patients in our study cohort were categorized as shown in Figure 1: 1,523 patients had HF-REF (mean ejection fraction of 27%), 122 patients had HF-PEF (mean ejection fraction of 62%), and 176 patients had HF-Recovered EF (mean ejection fraction of 57%). The HF-Recovered EF group median EF nadir (25th, 75th percentile) was 28% (20, 35) prior to enrollment in our cohort. The median difference between enrollment EF and EF nadir (ie, the amount of recovery) was 28% (20, 35) during a median time period of 29 months (16, 53).
Figure 1.
Flow diagram of patient classification. HOCM, hypertrophic obstructive cardiomyopathy; LVAD, left ventricular assist device; ECHO, echocardiogram; EF, ejection fraction; HF-PEF, heart failure with preserved ejection fraction; HF-RECOVERED, heart failure with recovered ejection fraction; HF-REF, heart failure with reduced ejection fraction.
Demographics and Medical History
Baseline clinical characteristics for each of these subgroups are shown in Table 1. HF-Recovered patients were younger than HF-PEF patients, and similar in age to patients with HF-REF. HF-Recovered patients also had a lower prevalence of coronary artery disease requiring revascularization and, consequently, fewer with an ischemic etiology than HF-REF. Additionally, the HF-Recovered population had less chronic kidney disease (CKD) than either the HF-REF or the HF-PEF populations. Hypertension prevalence in HF-Recovered was similar to HF-REF (59% in both groups), but lower than HF-PEF (78%).
Table 1.
Characteristics of study participants at enrollment; summaries presented as n (%) unless noted otherwise
| Reduced n = 1523 |
Preserved n = 122 |
Recovered n = 176 |
P* | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years, mean (SD) | 56 (14) | 63 (14) | 57 (13) | < 0.001 |
| Male | 1061 (70) | 56 (46) | 94 (53) | < 0.001 |
| Race | ||||
| Caucasian | 1132 (74) | 81 (66) | 145 (82) | 0.003 |
| African American | 334 (22) | 39 (32) | 30 (17) | |
| Other | 57 (4) | 2 (2) | 1 (1) | |
| Medical history and risk factors | ||||
| History of atrial fibrillation or flutter | 548 (39) | 40 (35) | 61 (37) | 0.73 |
| History of carotid artery disease | 56 (4) | 7 (6) | 6 (3) | 0.46 |
| History of chronic kidney disease | 236 (15) | 24 (20) | 16 (9) | 0.022 |
| History of diabetes | 463 (30) | 40 (33) | 47 (27) | 0.50 |
| History of hypercholesterolemia | 755 (50) | 76 (62) | 85 (48) | 0.022 |
| History of hypertension | 892 (59) | 95 (78) | 104 (59) | < 0.001 |
| History of obstructive sleep apnea | 325 (21) | 39 (32) | 51 (29) | 0.004 |
| History of ventricular tachycardia | 442 (32) | 8 (7) | 43 (27) | < 0.001 |
| Previous coronary artery bypass graft | 321 (21) | 17 (14) | 18 (10) | < 0.001 |
| Previous stent placement | 348 (23) | 24 (20) | 26 (15) | 0.033 |
| Tobacco use | 0.19 | |||
| Current | 154 (10) | 6 (5) | 19 (11) | |
| Former | 840 (55) | 70 (57) | 86 (49) | |
| Never | 529 (35) | 46 (38) | 71 (40) | |
| Heart failure characteristics | ||||
| NYHA functional classification | < 0.001 | |||
| I | 230 (15) | 26 (21) | 50 (28) | |
| II | 692 (45) | 57 (47) | 87 (49) | |
| III | 491 (32) | 33 (27) | 32 (18) | |
| IV | 110 (7) | 6 (5) | 7 (4) | |
| Ischemic etiology | 545 (36) | 21 (17) | 29 (16) | < 0.001 |
| Cardiac resynchronization therapy | 437 (29) | 0 (0) | 26 (15) | < 0.001 |
| Defibrillator | 751 (49) | 4 (3) | 39 (22) | < 0.001 |
| Time since onset, years, median (25th, 75th percentile)± | 6.4 (1.4, 14) | 5.3 (1.6, 13) | 6.5 (2.7, 11) | 0.41 |
| Medication use | ||||
| ACE inhibitors or ARBs | 1371 (90) | 85 (70) | 149 (85) | < 0.001 |
| Aldosterone antagonists | 580 (38) | 20 (16) | 35 (20) | < 0.001 |
| Aspirin | 879 (58) | 64 (52) | 86 (49) | 0.053 |
| Beta-blockers | 1399 (92) | 84 (69) | 154 (88) | < 0.001 |
| Digoxin | 659 (43) | 9 (7) | 41 (23) | < 0.001 |
| Diuretics | 1252 (82) | 87 (71) | 121 (69) | < 0.001 |
| HMG CoA reductase inhibitors | 811 (53) | 58 (48) | 94 (53) | 0.48 |
| Clinical measures, mean (SD) | ||||
| Body mass index, kg/m2 | 29.7 (7.0) | 33.3 (9.3) | 31.1 (7.9) | < 0.001 |
| Systolic blood pressure, mmHg | 113 (20) | 128 (22) | 120 (22) | < 0.001 |
| Diastolic blood pressure, mmHg | 70 (12) | 73 (13) | 71 (12) | 0.022 |
| eGFR, ml/min/1.73 m2 | 68 (30) | 62 (25) | 70 (22) | 0.074 |
| Sodium, mEq/L | 139.1 (3.3) | 139.4 (3.2) | 139.3 (3.0) | 0.50 |
| Ejection fraction, % | 27 (11) | 62 (8) | 57 (7) | < 0.001 |
P values obtained from ANOVA, Kruskal-Wallis tests, or Fisher’s exact tests
Time since onset of heart failure was based on participant recall of when they were first told they had a heart problem
SD, standard deviation; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; eGFR; estimated glomerular filtration rate (Modification of Diet in Renal Disease formula (MDRD))
Heart Failure Characteristics
HF-Recovered patients had less severe symptoms, with a greater prevalence of NYHA I or II patients than HF-REF or HF-PEF. HF-Recovered and HF-REF patients were prescribed beta-blockers and ACE inhibitors or Angiotensin receptor blockers more frequently than HF-PEF. Aldosterone antagonist use was greatest in the HF-REF group as were digoxin and diuretic use. There was no difference in aspirin or statin use across groups. ICD and CRT use were greatest in the HF-REF population, followed by HF-Recovered, with little or no use in the HF-PEF group.
Clinical Measures
Mean blood pressure in the HF-Recovered group was higher than in HF-REF, but lower than in the HF-PEF population. There were no significant differences between mean serum sodium levels across the groups.
Biochemical profiles
Table 2 describes baseline biomarker data in each of the HF categories. Serum creatinine, BNP, TnI, and sFlt-1 were lowest in the HF-Recovered group and highest in HF-REF. Uric acid was also lowest in the HF-Recovered group. There was no difference in PlGF, hs-CRP or MPO across HF groups. However, nearly a third of HF-Recovered patients (30%) still had a BNP above the 95th percentile (135 pg/ml) suggesting persistent neurohormonal activation. 19 Nearly half of the HF-Recovered group had evidence of oxidative stress with 47% of patients having uric acid levels above the 95th percentile (2.6–6 mg/dl for females and 3.5–7.2 mg/dl for males).20 Detectable TnI levels were seen in 44% of HF-Recovered patients. Additionally, sFlt-1 was lowest in HF-Recovered population, but 26% of that population was still above the 95th percentile (322.9 pg/ml). 21 ST2 was highest in the HF-PEF population, but lowest in HF-REF with 22% of the HF-Recovered population above the 95th percentile (37.9 ng/ml). 22 Overall, HF-Recovered patients were biochemically distinct from HF-PEF and HF-REF patients and had the lowest levels of risk markers.
Table 2.
Serum biomarkers collected at enrollment; summaries presented as median (25th, 75th percentile) unless noted otherwise
| Reduced n = 1187 |
Preserved n = 94 |
Recovered n = 142 |
P* | |
|---|---|---|---|---|
| BNP, pg/mL | 214 (65, 681) | 77 (39, 234) | 66 (25, 159) | < 0.001 |
| PlGF, pg/mL | 18.8 (15.2, 23.1) | 18.5 (15.6, 22.5) | 18.4 (15.2, 22.3) | 0.86 |
| sFlt-1, pg/mL | 311 (261, 384) | 310 (267, 363) | 281 (247, 326) | < 0.001 |
| hsCRP, mg/L | 0.37 (0.15, 0.92) | 0.34 (0.15, 0.98) | 0.26 (0.11, 0.70) | 0.11 |
| MPO, pmol/L | 136 (94, 224) | 165 (106, 262) | 130 (94, 207) | 0.20 |
| ST2, ng/mL | 24.7 (19.8, 41.3) | 27.9 (21.5, 40.0) | 25.1 (18.2, 34.7) | 0.037 |
| TnI | < 0.001 | |||
| Detectable, n (%) | 810 (68) | 45 (48) | 63 (44) | |
| TnI, ng/mL, median (IQR)** | 0.020 (0.010, 0.040) | 0.010 (0.010, 0.030) | 0.010 (0.010, 0.020) | |
| Creatinine, mg/dL | 0.93 (0.77, 1.27) | 0.90 (0.77, 1.39) | 0.82 (0.72, 1.12) | 0.003 |
| Uric acid, mg/dL | 7.0 (5.7, 8.9) | 7.2 (5.5, 8.6) | 6.5 (5.1, 8.6) | 0.035 |
P values obtained from Kruskal-Wallis rank-sum tests
BNP, B-type natriuretic peptide; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase receptor-1; hsCRP, high-sensitivity C-reactive protein; MPO, myeloperoxidase; ST2, soluble toll-like receptor-2; TnI, troponin I
Adverse Cardiovascular Outcomes
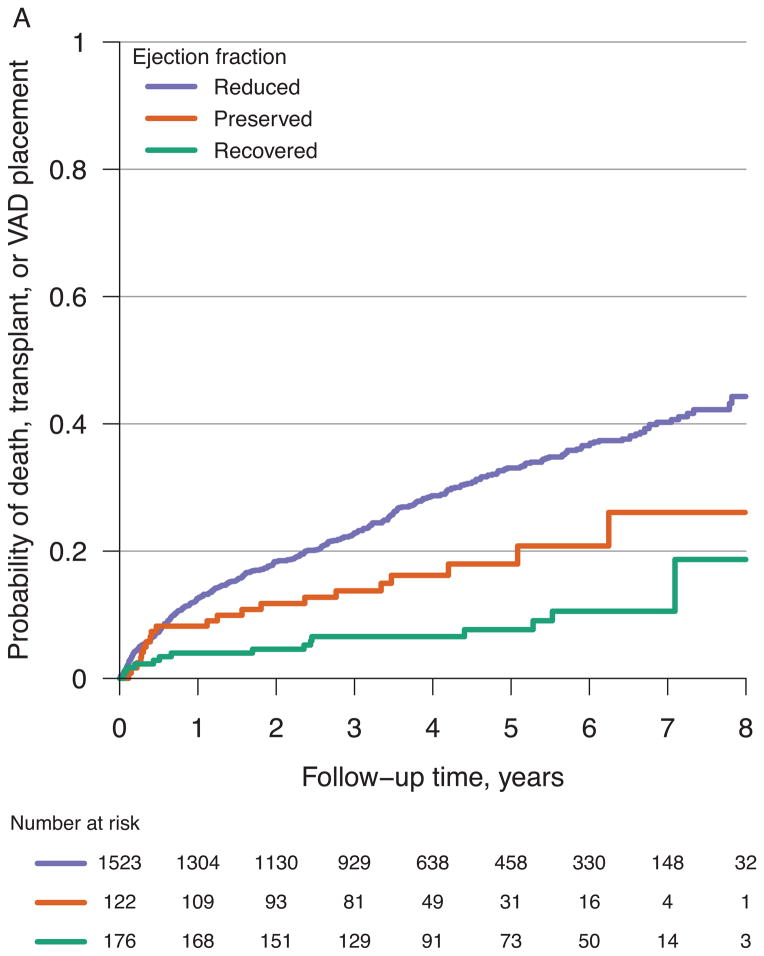
The 1821 participants were followed for a maximum of 8.9 years and a median of 3.6 years. 507 of these participants experienced a terminal event, including 335 deaths, 129 transplants, and 43 VAD placements. Figure 2A compares the probability of reaching these discrete events over time across HF categories. In unadjusted Cox models (Table 3), the hazard ratio for all-cause death, cardiac transplantation or VAD placement in HF-REF versus HF-Recovered was 4.1 (95% CI 2.4–6.8; p<0.001). The unadjusted hazard ratio for HF-PEF versus HF-Recovered was 2.3 (95% CI 1.2–4.5; p=0.013). In multivariable adjusted models (age, gender, race, heart failure etiology, history of chronic kidney disease, history of hypertension, and site), these associations remained statistically significant (Table 3). A sensitivity analysis restricted to all-cause mortality provided similar results (data not shown). Overall, both the HF-REF and HF-PEF subgroups had a substantially increased risk of death, transplantation, or VAD placement compared to HF-Recovered. However, it is important to note that nearly 20% of the HF-Recovered population suffered from death, cardiac transplantation, or VAD by 8 years of follow-up.
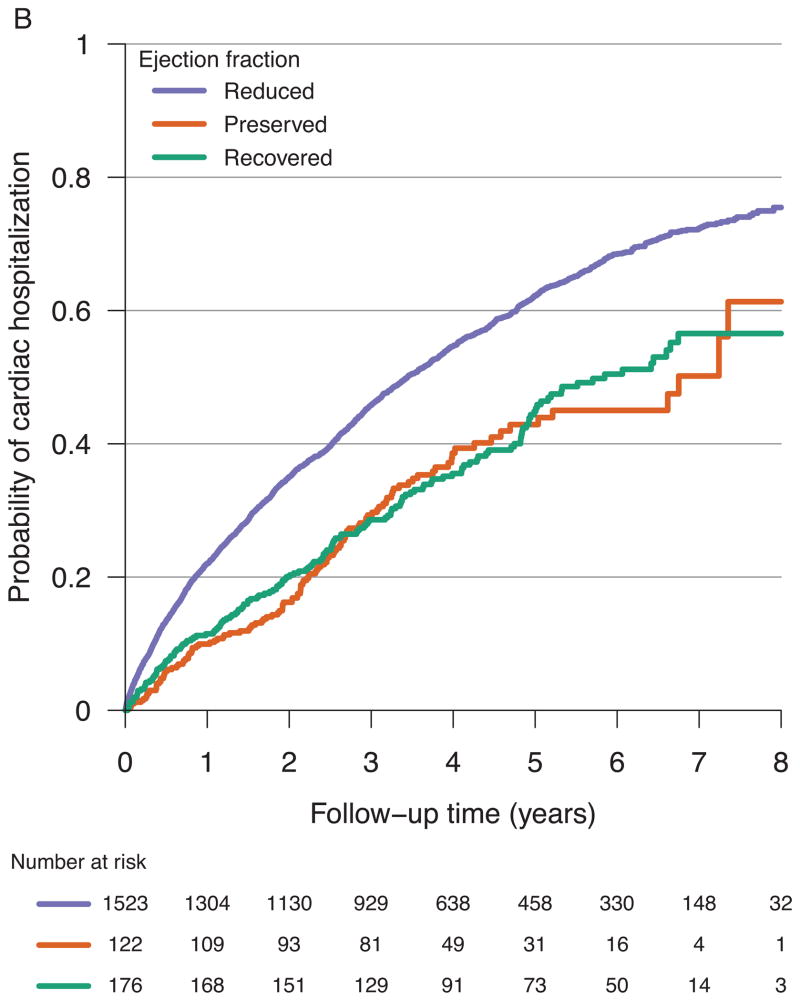
Figure 2.
Probability of all-cause death, cardiac transplantation, or VAD placement for all participants (A) and probability of cardiac hospitalization for all participants (B) from time of referral to an outpatient HF specialty care center.
Table 3.
Hazard ratios for all-cause death, cardiac transplantation, or VAD placement
| Preserved vs. recovered | Reduced vs. recovered | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Model 1 | 2.3 (1.2, 4.5) | 0.013 | 4.1 (2.4, 6.8) | < 0.001 |
| Model 2 | 2.1 (1.1, 4.0) | 0.031 | 3.5 (2.1, 5.8) | < 0.001 |
| Model 3 | 2.0 (1.0, 4.0) | 0.037 | 3.4 (2.0, 5.7) | < 0.001 |
Model 1: Unadjusted
Model 2: Adjusted for age, gender, race (Caucasian, African America, or other), heart failure etiology (ischemic or non-ischemic), and site
Model 3: Adjusted for age, gender, race (Caucasian, African America, or other), heart failure etiology (ischemic or non-ischemic), history of chronic kidney disease, history of hypertension, and site
Among the 964 subjects who experienced one or more hospitalizations in the cohort, there were 2,453 total hospitalizations: 406 participants had 1; 224 had 2; 118 had 3; 87 had 4; and 129 had 5 or more. Surprisingly, the HF-Recovered group had a substantial number of hospitalizations that was similar to the incidence in the HF-Preserved group (Figure 2B). Utilizing recurrent event models (Table 4), the unadjusted hazard ratio for cardiac hospitalization in HF-PEF versus HF-Recovered was 1.3 (95% CI 0.90–2.0; p=0.15) and did not prove significant after adjusting for multiple confounders. The unadjusted hazard ratio for cardiac hospitalization in HF-REF versus HF-Recovered was 2.0 (95% CI 1.5–2.7; p<0.001) and remained significant after adjustment for multiple potential confounders (Table 4). Although the baseline clinical and biochemical profile as well as event free survival was better compared to HF-REF, HF-Recovered patients still remain at risk for adverse events. This is evidenced by abnormal biomarker levels in a substantial number of patients and by hospitalization rates similar to HF-PEF.
Table 4.
Hazard ratios for cardiac hospitalization
| Preserved vs. recovered | Reduced vs. recovered | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Model 1 | 1.3 (0.90, 2.0) | 0.15 | 2.0 (1.5, 2.7) | < 0.001 |
| Model 2 | 1.3 (0.86, 2.0) | 0.21 | 1.8 (1.3, 2.5) | < 0.001 |
| Model 3 | 1.3 (0.90, 1.9) | 0.16 | 1.8 (1.4, 2.4) | < 0.001 |
Model 1: Unadjusted
Model 2: Adjusted for age, gender, race (Caucasian, African America, or other), heart failure etiology (ischemic or non-ischemic), and site
Model 3: Adjusted for age, gender, race (Caucasian, African America, or other), heart failure etiology (ischemic or non-ischemic), history of chronic kidney disease, history of hypertension
Discussion
The current heart failure paradigm is to classify patients into one of two categories: HF-REF and HF-PEF. However, it is possible that a number of HF-Recovered patients are being erroneously classified as HF-PEF. Punnoose et al. reported nearly 70% of patients with normal EF on echocardiography in their tertiary care referral center registry had had a reduced EF previously and suggested that this group of patients represents a separate clinical entity.11 The HF-Recovered patient, we also believe, represents a third, distinct category along the continuum of heart failure. Our data suggest that the HF-Recovered population has different demographics, comorbidities, and symptom severity from the HF-REF and HF-PEF populations. In this study, we compared key biological pathways across these populations by utilizing both traditional biomarkers (BNP, uric acid, MPO, CRP, troponin), as well as novel biomarkers such as ST2, PlGF, and sFlt-1. ST2, a receptor involved in a cardioprotective signaling system in response to myocyte stress, has been shown to be an independent predictor of HF outcomes.15 Additionally, we compared evidence of vascular remodeling across HF groups by measuring plasma levels of the angiogenic ligand PlGF and the circulating form of its target receptor, soluble fms-like tyrosine kinase 1 (sFlt-1). Higher levels of sFlt-1, but not PlGF, have previously been shown to independently predict adverse HF outcomes. 23 We determined that a substantial number of HF-recovered patients had abnormal BNP, uric acid, st2, and sFlt-1, as defined as being > 95th percentile, and nearly half had detectable TnI. These findings suggest that there is persistent neurohormonal activation; increased oxidative stress; and cardiomyocyte injury and stress despite apparent recovery of ejection fraction. Although HF-Recovered had the best prognosis in terms of death, cardiac transplantation, or ventricular assist device placement, we note that the HF-Recovered patients did not, by any means, have a normal prognosis. Despite normalization of ejection fraction, these patients continued to experience substantial heart failure symptoms and clinical events, and the number of cardiac hospitalizations and symptomatology was similar to that of the HF-PEF population.
From a patient care perspective, these findings provide a rationale to continue background medical and/or device therapy for HF-Recovered patients. This recommendation is consistent with prior reports suggesting cessation of medical therapy was associated with a recurrence of LV dysfunction in patients who had previously improved or “recovered” their EF.8, 24 In fact, we note that the majority of patients in our HF-recovered group remained on medical therapy despite normalization of LV function. This is consistent with the idea that a recovery of ejection fraction does not necessarily constitute recovery from heart failure. For example, clinicians may have been reluctant to discontinue diuretics due to either previous failed attempts or persistent signs of volume overload, such as lower extremity edema or jugular venous distension. Our observations are important to the interpretation of myocardial recovery versus remission or reverse remodeling. The HF-Recovered cohort, defined by echocardiographic measurement of EF, consists of patients who have a milder, but persistent HF phenotype. However, we believe that this population represents a spectrum of patients exhibiting reverse remodeling (ie, improved ventricular function and milder clinical HF phenotype), a minority of whom have complete myocardial recovery as defined by normal chamber size, myocyte function, and neurohormonal profile, with freedom from future HF events. Distinguishing patients with complete myocardial recovery from those “in remission” represents an important clinical challenge, as routine assessment of myocyte function at a cellular level is impractical in most patients.12 This challenge underscores a need to identify biologic characteristics and predictors of complete myocardial recovery within the reverse remodeling or “in remission” population indicative of the underlying mechanisms. Such an approach might include comprehensive biomarker panels, sophisticated imaging techniques (e.g. MRI, PET scanning), and metabolomics. Additionally, insights from gene expression and micro RNA analyses performed in LVAD patients may provide additional information to characterize true recovery in non-VAD populations who are earlier in the disease course. 25
Our study has limitations. To begin our analysis, we chose to emulate a practical situation faced in clinical practice: what to do when the EF on echocardiography has “normalized” (e.g. EF>50%) in a patient with HF-REF. Although EF is commonly used in clinical practice, it is at best a crude marker of cardiac function and remodeling, as it does not properly capture other features such as absolute ventricular volumes, geometry, and ventricular-vascular coupling. In addition, there is no consensus on the appropriate definition or ejection fraction cut-off value for classification of patients as HF-PEF or HF-Recovered. 10, 26, 27 Current AHA guidelines use an EF ≥50% for HF-PEF noting the 41–49% category as intermediary with unclear pathophysiology. Sweitzer et al. demonstrated patients in the EF 40–55% group are more similar to patients with an EF <40% than those with a higher ejection fraction.28 As such, we chose an EF ≥50% for our HF-PEF and HF-Recovered groups. The determination of which “normal EF” patients to include in the HF-Recovery group (as opposed to HF-PEF) was based on retrospective review of echocardiograms done prior to enrollment of the patient in this study. To guard against misclassification of patients as HF-PEF, subjects were excluded if we could not identify previous echocardiograms to confirm a stable, preserved EF.
We note that the referral nature of this population provides both strengths and limitations. The strength is that the participants span a full spectrum of disease, including mild, moderate, and advanced heart failure. This allows us to compare findings across such subgroups using data from three US centers in order to make inferences about differences in disease mechanisms, pathophysiology, and outcome. We believe these inferences are likely to be generalizable to similar populations of mild, moderate, and advanced heart failure, but may not be extrapolated to the general population for the purpose of risk prediction. Additionally, the proportion of HF-PEF patients may be lower than what may be experienced in a general population as HF-REF patients are more likely to be referred to tertiary specialty centers. Despite these limitations, review of our table 1 findings shows typical features of HF-PEF in our HF-PEF subgroup, including a female bias, higher BMI, and high prevalence of hypertension and atrial arrhythmias.29, 30 Finally, the effect of medication dosages and the effect of cardiac resynchronization therapy (CRT) were not analyzed as part of this study. This is in part due to lack of clear consensus on the appropriate medication regiment for true HF-PEF as well as the HF-Recovered population. Nevertheless, the vast majority of patients across groups were on neurohormonal antagonists and diuretic therapy. Due to the relatively small size of our HF-Recovered cohort treated with CRT (n=26), however, we have insufficient data to analyze predictors of CRT response or to determine the specific prognosis of CRT “responders.”
In summary, our data suggest that the HF-Recovered EF population represents a distinct HF phenotype with biochemical properties and natural history that differs from the traditional HF-REF and HF-PEF populations. Furthermore, these patients continue to experience heart failure events, suggesting that this is not true myocardial recovery. Our study underscores the need to further investigate pathophysiological differences in these patient populations in an effort to better tailor therapy. It also highlights the need to identify characteristics and predictors of both reverse remodeling and myocardial recovery in the pre-LVAD cardiomyopathy population.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by NIH HL088577 (Dr. Cappola). Support for biochemical assays was provided by Abbott and Critical Diagnostics.
Footnotes
Conflict of Interest Disclosures: Drs. Ky and Cappola are co-inventors on a pending patent for sFlt-1 as a cardiac biomarker. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86:431–8. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Colucci WS, Sackner-Bernstein JD, Liang CS, Goldscher DA, Freeman I, Kukin ML, Kinhal V, Udelson JE, Klapholz M, Gottlieb SS, Pearle D, Cody RJ, Gregory JJ, Kantrowitz NE, LeJemtel TH, Young ST, Lukas MA, Shusterman NH for the PRECISE Study Group. Double-blind placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation. 1996;94:2793–9. doi: 10.1161/01.cir.94.11.2793. [DOI] [PubMed] [Google Scholar]
- 3.Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–90. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 4.Bax JJ, Poldermans D, Elhendy A, Cornel JH, Boersma E, Rambaldi R, Roelandt JR, Fioretti PM. Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol. 1999;34:163–169. doi: 10.1016/s0735-1097(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 5.Boehmer JP, Starling RC, Cooper LT, Torre-Amione G, Wittstein I, Dec GW, Markham DW, Zucker MJ, Gorcsan J, 3rd, McTiernan C, Kip K, McNamara DM IMAC Investigators. Left ventricular assist device support and myocardial recovery in recent onset cardiomyopathy. J Card Fail. 2012;18:755–61. doi: 10.1016/j.cardfail.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Drakos SG, Wever-Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, Movsesian M, Li DY, Stehlik J, Kfoury AG UCAR (Utah Cardiac Recovery Program) Investigators. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol. 2013;61:1985–94. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steimle AE, Stevenson LW, Fonarow GC, Hamilton MA, Moriguchi JD. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol. 1994;23:553–9. doi: 10.1016/0735-1097(94)90735-8. [DOI] [PubMed] [Google Scholar]
- 8.Moon J, Ko YG, Chung N, Ha JW, Kang SM, Choi EY, Rim SJ. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Card. 2009;25:147–50. doi: 10.1016/s0828-282x(09)70497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teeter WA, Thibodeau JT, Rao K, Brickner ME, Toto KH, Nelson LL, Mishkin JD, Ayers CR, Miller JG, Mammen PP, Patel PC, Markham DW, Drazner MH. The natural history of new-onset heart failure with a severely depressed left ventricular ejection fraction: implications for timing of implantable cardioverter-defibrillator implantation. Am Heart J. 2012;164:358–64. doi: 10.1016/j.ahj.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J, 3rd, Kip KE, Dec GW IMAC Investigators. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58:1112–8. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17:527–532. doi: 10.1016/j.cardfail.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012;60:2465–72. doi: 10.1016/j.jacc.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drakos SG, Kfoury AG, Stehlik J, Selzman CH, Reid BB, Terrovitis JV, Nanas JN, Li DY. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126:230–41. doi: 10.1161/CIRCULATIONAHA.111.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, Goldberg LR, Jessup M, Cappola TP. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–90. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries D, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy W, Goldberg L, Jessup M, Cappola TP. High-Sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peña EA, Strawderman R, Hollander M. Nonparametric estimation with recurrent event data. J Am Stat Assoc Association. 2001;96:1299–1315. [Google Scholar]
- 17.Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, Brouste V, Soubeyran P. Joint frailty models for recurring events and death using maximum penalized likelihood estimation: Application on cancer events. Biostatistics. 2007;8:708–21. doi: 10.1093/biostatistics/kxl043. [DOI] [PubMed] [Google Scholar]
- 18.Rondeau V, Mazroui Y, Gonzalez JR. Frailtypack: An R package for the analysis of correlated survival data with frailty models using penalized likelihood estimation or parametrical estimation. J Stat Softw. 2012;47:1–28. [Google Scholar]
- 19.Architect B-Type Natriuretic Peptide [package insert] Abbott Park, IL: Abbott Laboratories; 2008. [Google Scholar]
- 20.Architect Aeroset Uric Acid [package insert] Abbott Park, IL: Abbott Laboratories; 2007. [Google Scholar]
- 21.Architect sFlt-1 Assay [package insert] Abbott Park, IL: Abbott Laboratories; 2012. [Google Scholar]
- 22.Presage ST2 Assay [package insert] San Diego, CA: Critical Diagnostics; 2013. [Google Scholar]
- 23.Ky B, French B, Ruparel K, Sweitzer NK, Fang JC, Levy WC, Sawyer DB, Cappola TP. The vascular marker soluble fms-like tyrosine kinase 1 is associated with disease severity and adverse outcomes in chronic heart failure. J Am Coll Cardiol. 2011;58:386–94. doi: 10.1016/j.jacc.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waagstein F, Caidahl K, Wallentin I, Bergh CH, Hjalmarson A. Long term beta blockade in dilated cardiomyopathy. Effects of short- and long-term metoprolol treatment followed by withdrawal and readministration of metoprolol. Circulation. 1989;80:551–63. doi: 10.1161/01.cir.80.3.551. [DOI] [PubMed] [Google Scholar]
- 25.Ramani R, Vela D, Segura A, McNamara D, Lemster B, Samarendra V, Kormos R, Toyoda Y, Bermudez C, Frazier OH, Moravec CS, Gorcsan J, 3rd, Taegtmeyer H, McTiernan CF. A micro-ribonucleic acid signature associated with recovery from assist device support in 2 groups of patients with severe heart failure. J Am Coll Cardiol. 2011;58:2270–8. doi: 10.1016/j.jacc.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–76. doi: 10.1016/j.jacc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–6. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg K, Swedberg K, McMurray J. Heart Failure with Preserved Left Ventricular Systolic Function: Epidemiology, Clinical Characteristics, and Prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 30.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.