Abstract
The nuclear retinoid X receptor (RXR) agonist, bexarotene, has been implicated in recovery of cognitive function in mouse models of Alzheimer’s disease. Since AD genetic mouse models also show abnormal neural hyperexcitability, which may play a destructive role in memory storage and retrieval, we studied whether bexarotene exerted dynamic network effects on EEG cortical spike discharge rate and spectral frequency in an AD (hAPP J20 model) and non-AD (Kv1.1 null) mouse models of epilepsy. We find that oral treatment with bexarotene over one week acutely reduced spike discharges in both models and seizures in the Kv1.1 null mouse model without major alterations in the background frequency of brain rhythms. The effect was reversible and exhibited a similar rapid onset in hippocampal slices. While the exact mechanisms are unknown, bexarotene counteracts both Aβ-induced and Aβ-independent increases in cortical network hyperexcitability.
1. Introduction
The RXR agonist, bexarotene increases ApoE expression and exerts a striking improvement in cognitive function in an AD mouse model (Cramer et al., 2012). Salient effects include reduction in amyloid-β plaque burden, clearance of soluble Aβ within 72 hours of treatment, and improvement in neural network activity and cognitive behaviors within one week. Recent studies confirmed the reduction in soluble forms of Aβ, including Aβ oligomers, in response to bexarotene treatment (Bachmeier et al., 2013; Fitz et al., 2013; Price et al., 2013; Tesseur et al., 2013; Ulrich et al., 2013; Veeraraghavalu et al., 2013) and improved cognition and memory (Fitz, et al., 2013; Tesseur, et al., 2013), however, plaque loss was not confirmed (Fitz, et al., 2013; Laclair et al., 2013; Price, et al., 2013; Tesseur, et al., 2013; Veeraraghavalu, et al., 2013). These data support an accumulating body of evidence linking soluble forms of Aβ, rather than plaque deposition, with memory decline.
The cellular mechanisms leading to the dynamic and potentially reversible component of dementia in Aβ overexpression models of AD and their amelioration by bexarotene remain poorly understood. There is ample evidence at the single cell level for Aβ-linked defects in synaptic plasticity, and these are accompanied by circuit level network discharges and seizures in most experimental genetic models of AD (Blanchard et al., 2002; Minkeviciene et al., 2009; Mucke and Selkoe, 2012). Network hyperexcitability may arise from changes in intrinsic membrane properties as well as synaptic and connectivity defects and all have been described in AD models (Palop and Mucke, 2010). Clearance of Aβ from the brain is facilitated by apolipoprotein E (ApoE), and ApoE expression is transcriptionally induced through the action of the nuclear peroxisome proliferator activated receptor (PPARγ) and liver X receptors (LXR) in coordination with RXRs. The primary mechanism of action of RXR receptors is to alter gene expression (Boehm et al., 1995; Hurst, 2000; Lalloyer et al., 2009), but the extent and time course of its effects on neuronal excitability mechanisms are unknown, particularly in the early stages of its exposure to CNS pathways.
In this pilot study, we sought evidence for early changes in brain excitability that might correlate with the improvement in cognitive function due to bexarotene, as well as to determine whether this effect was strictly dependent on Aβ pathology by comparing two distinct mutant mouse models of neural hyperexcitability, one AD and one non-AD model.
2. Methods
2.1 Animals
Mice were obtained from the Baylor Developmental Neurogenetics breeding colony. The hAPP J20 mice were a generous gift from Drs L. Mucke, E. Roberson. Kv1.1 null mice (background Black Swiss N:NIHS-BC) or J20 mice (background C57BL/6+DBA/2+Swiss-Webster) were treated with a single daily oral gavage for 7 days of either 100 mg/kg/day bexarotene (Targretin® capsule dissolved in 2 mL of water (Cramer, et al., 2012) or vehicle (PBS) for 5 days. Between recordings, mice were housed at 22° C with a 12 hour light/dark cycle and fed ad libitum. Mouse breeding and experiments were carried out under IACUC approved protocols at Baylor College of Medicine.
2.2 EEG recordings
Silver wire electrodes (0.005″ diameter) soldered to a microminiature connector were implanted bilaterally into the subdural space over frontal and parietal cortex of mice under Avertin anesthesia (250 mg/kg) several days prior to recording. Simultaneous EEG and behavioral video EEG monitoring was performed using a digital electroencephalograph (Harmonie 6.1, Stellate Systems, Montreal, Canada) from 11 homozygous Kv1.1 null mice (5 vehicle, 6 bexarotene treated), aged 5–10 weeks, moving freely in the test cage. J20 mice were allowed to acclimate to the recording chamber for one day prior to acquiring baseline EEG data. A total of 23 hAPP J20 mice were recorded (aged 4–18 months, 14 received bexarotene, 9 received vehicle, ages between the groups were similar). Mice were monitored by video EEG over a 4 hour period each day. After a baseline recording period, mice were administered drug once daily and recorded at same time each day for 7 days, drug was discontinued, and mice recorded for an additional 1–7 day washout monitoring period. Vehicle-treated (PBS) control mice were treated identically and recorded at the same time for 5 days.
2.3 In vitro slice recordings
Transverse hippocampal slices were prepared from wildtype J20 mice (aged 5–8 weeks) as described in (Holth et al., 2013). There was no spontaneous network bursting activity in normal (2.5mM KCl) solutions, and after obtaining a baseline, ACSF containing 7.5 mM KCl was washed on the slices. After 15–25 minutes, CA3 neurons began synchronously discharging and the burst frequency was determined using Clampfit and Origin software. Following a 20 minute period for stable acquisition of burst frequency, 10 μM bexarotene was washed on, allowed to equilibrate, and the burst frequency was measured 20 minutes after drug introduction.
2.4 Spectral analysis
Standardized samples of EEG activity (the final 30 minutes of the first hour of each recording session) were selected to ensure uniformity. Files were processed using the time frequency (Morlet wavelets) and frequency power spectrum density (Welch) transforms in Matlab (R2011b, Mathworks, Inc, Natick, MA). Examples are shown (Fig 1D, 2F) of the power spectra of Morlet wavelets for frequency ranges up to the low gamma range (40 Hz) over the 30 minute time period. Power is expressed as μV2/Hz. For Welch transforms, three second epochs were analyzed with 20% overlap for grouping into major frequency bands (α,β,γ1,γ2,δ,θ) with parietal and temporal leads averaged. Data was compiled from 6 of the Kv1.1 null mice and 5 of the hAPP J20 mice.
Figure 1.
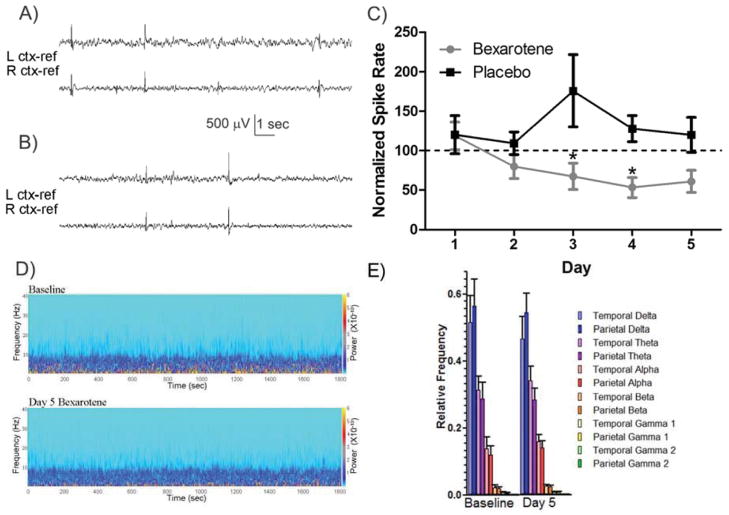
Bexarotene reduces interictal spike rate in hAPP J20 mice. A) Representative 15 seconds of baseline cortical spiking in hAPP J20 mouse. B) Representative 15 seconds of cortical EEG after 4 days of bexarotene treatment. Scale bar is 500μV, 1 second. C) Normalized spike rate plotted as a function of days of treatment with either placebo or bexarotene. Bexarotene treatment significantly decreased spike rate from pre-drug baseline (p<0.05), and days 3 and 4 were significantly lower than placebo values. D) Examples of time-frequency (Morlet wavelets) power spectra from representative hAPP J20 mouse baseline and day 5 of bexarotene. Power scale (right y axis) refers to μV2/Hz. E) Summarized relative frequency analysis (Welch power spectrum density) of major frequency bands (α,β,γ1,γ2,δ,θ) for hAPP J20 mice (n=5) on baseline and day 5.
Figure 2.
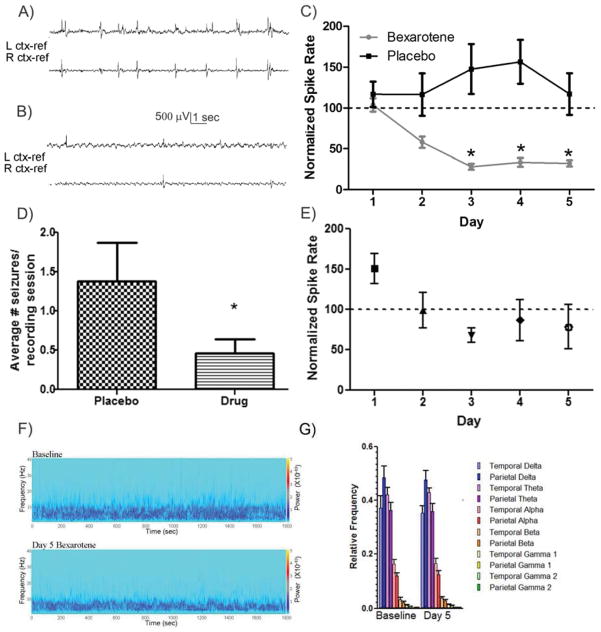
Bexarotene reduces interictal spike rate in Kv1.1 null mice. A) Representative 15 second interval of baseline cortical spiking, and B) after bexarotene treatment for 3 days. Scale bar is 500μV, 1 second. C) Normalized spike rate plotted as a function of days of treatment with either placebo or baseline. The overall trend of bexarotene treatment was significantly reduced (p<0.05) and days 3, 4, and 5 were significantly lower than placebo values (p<0.05). D) Seizures were significantly reduced in bexarotene treated (0. 458 ± 0.18) versus placebo treated (1.38 ± 0.49) as normalized by recording sessions (p<0.05). E) Kv1.1 null mice were treated once (day 1) with bexarotene and recorded from over 5 subsequent days. No significant effect was found with only a single treatment. F) Examples of time frequency (Morlet wavelets) power spectra from a representative Kv1.1 null mouse baseline and day 5 of bexarotene. Power scale (right y axis) refers to μV2/Hz. G) Summarized relative frequency analysis (Welch power spectrum density) of major frequency bands (α,β,γ1,γ2,δ,θ) for Kv1.1 null mice (n=6) on baseline and day 5.
2.5 Statistical analysis
All data are expressed as mean ± S.E.M unless otherwise stated. Graphpad PRISM version 5.04 (San Diego, CA) was used to test for statistical significance. The p-values for bexarotene versus placebo treated interictal spike rate were generated by two-way ANOVA (time and drug) with repeated measures and Bonferroni post-hoc analysis. P-values for the one time treatment with bexarotene were generated by one-way ANOVA (time) with repeated measures and Bonferroni post-hoc analysis. P-values for differences in Kv1.1 null seizure rate were determined by t-test. Data was tested for normality and passed unless noted and, in case of non-normality, a Mann-Whitney test was used. A paired t-test was used for the before and after drug application in the slice electrophysiology experiments. For both hAPP J20 and Kv1.1 null mice, two-way ANOVA analysis revealed the interaction between time and drug was significant (p<0.05) as well as drug treatment (p<0.05).
3. Results
3.1 Effect of Bexarotene on in vivo cortical epileptiform spiking in hAPP J20 model of AD
Abnormal spontaneous epileptiform spiking is a prominent feature of the cortical and hippocampal EEG in hAPP J20 mice (Palop and Mucke, 2010; Roberson et al., 2011). We first examined the short term effect of a daily bexarotene dose (100 mg/kg/day as used in (Cramer, et al., 2012) on the spontaneous discharge rate in adult hAPP J20 transgenic mice over a one week period (n=14). Within the first 72 hours following administration, bexarotene reduced the spike discharge rate to 67.25 ± 16.74% of the baseline spike rate, which was significantly different from vehicle control spike rates (n=9, p<0.05, Bonferroni post-hoc, Fig 1C). The spike suppression reached a low of 53.16 ± 12.47% of the normalized spike rate after 4 days of bexarotene treatment, which was also significantly different from vehicle treated hAPP J20 mice (p<0.05, Bonferroni post-hoc, Fig 1C). We next examined the background frequency spectrum of the rhythmic EEG activity before and after bexarotene treatment and found no significant difference between days of bexarotene treatment (Fig 1D, E). This indicates that bexarotene reduces aberrant network discharge activity without major alterations in the time spent in high frequency or slow wave activity EEG patterns. Additionally, after the completion of bexarotene treatment, the spike rate in J20 mice returned to 105.47 ± 26.4 % of the baseline level within 24 hours (Mann-Whitney, data not shown), indicating that the antiepileptic effects of bexarotene may be quickly reversible. While abnormal spike discharge is a hallmark of J20 EEG patterns, electrographic seizures did not occur frequently enough in this model to allow comparative analysis during the one week test interval.
3.2 Effect of Bexarotene on in vivo cortical epileptiform spiking in Kv1.1 null model of epilepsy
We next sought to determine whether the beneficial effects of bexarotene on cortical hyperexcitability were dependent on the presence of Aβ-induced pathology in the J20 model, or whether it might be effective in other hyperexcitability models. The Kv1.1 null mouse model has been well studied due to its pronounced epileptic phenotype (Glasscock et al., 2010; Holth, et al., 2013; Simeone et al., 2013; Smart et al., 1998). Somewhat surprisingly, in the absence of known Aβ pathology, bexarotene significantly reduced the spike discharge rate to 28.09 ± 3.7% (n=6) of baseline within 3 days of daily treatment and differed significantly from the spike rate in vehicle treated (n=5) Kv1.1 null mice (Fig 2C, Bonferroni post-hoc, p<0.05). In addition to the depressed spike rate, the number of seizures in mice treated with bexarotene per recording session was significantly reduced from 1.38 ± 0.49 seizures for vehicle to 0.458 ± 0.18 seizures for bexarotene (p<0.05, t-test, Fig 2D). Following discontinuation of bexarotene treatment, interictal spike rate returned to 130.84 ± 50.2% of baseline within 24 hours indicating that the reduction in hyperexcitability, as also seen in J20 mice, is rapidly reversible following drug removal (data not shown). We also performed spectral analysis on EEG from the Kv1.1 null mice treated with bexarotene, and found no significant difference in the power among the 5 major frequency bandwidths (α,β,γ,δ,θ) between the pre-drug baseline activity and any day of bexarotene treatment (Fig 2F, G). This again suggested that bexarotene reduces aberrant network discharge activity without major alterations in the time spent in high frequency or slow wave activity EEG patterns. In order to determine whether the effects of bexarotene could outlast a single exposure, we treated Kv1.1 null mice for only one day with bexarotene and followed the interictal spike rate over 5 days (n=5). We found no significant difference from baseline spike rate throughout the 5 day period, indicating that the beneficial excitability effect of bexarotene is contingent upon continuous drug exposure. (One-way ANOVA, Fig 2E, p>0.05).
3.3 Bexarotene has acute effects on isolated hippocampal network excitability
To examine the rate of change in network excitability due to bexarotene treatment, hippocampal slices from non-transgenic (wildtype) J20 mice were exposed to high extracellular levels of KCl (7.5mM) in the bathing solution to induce network bursting. Application of 10 μM bexarotene to these slices lowered the spike rate from 0.128 ± 0.013 Hz to 0.112 ± 0.012 Hz within 20 minutes of exposure which was significantly reduced by paired t-test analysis (data not shown). Taken together, the early and reversible effects of bexarotene in vivo, and its smaller, but significant, rapid effect on circuit excitability in vitro were unexpected, given the known transcriptional impact of RXR activation, and raise the possibility of alternate mechanisms of action for bexarotene-induced decreases in neuronal hyperexcitability.
4. Discussion
We found early and pronounced effects of a once daily treatment of bexarotene on neural network excitability in two epilepsy models, the hAPP J20 and Kv1.1 null mice. These effects depended on continued exposure of the drug during the one week exposure, and cortical network discharges returned to baseline levels or above within 24 hours of the final dose. The mechanism of hyperexcitability in the J20 model is still under active investigation; however, recent evidence has pinpointed a critical role for impaired cortical interneurons (Verret et al., 2012). Therefore, one possible action of bexarotene might be to reduce the Aβ-induced disinhibition in this model within the rapid time frame identified in our study. While it is known that bexarotene has a 7 hour half-life, further experiments will be required to dissect the many possible actions of RXR activation on the neurobiology of the brain developing in the context of excess Aβ production (Howell et al., 2001).
On the other hand, the comparable effects of bexarotene on the Kv1.1 null model of cortical and hippocampal hyperexcitability indicate that its antiepileptic action may not depend upon Aβ-induced alterations. The effect on this model is particularly impressive because the hyperexcitability phenotype is robust compared to many genetic models of epilepsy. Interestingly, loss of Kv1.1 channels potentiates both excitatory and inhibitory neurons, hence bexarotene may stabilize neural network excitability in entirely separate ways. It will, therefore be of great interest to determine any potential direct membrane effects of bexarotene on intrinsic neuronal firing properties and synaptic plasticity in cortical and hippocampal pathways, it’s effectiveness in other AD and epilepsy models, and whether prolonged use of this drug might also produce sustained changes through downstream transcriptional remodeling of neural pathways critical for cognition.
Acknowledgments
We thank Rocco Lucero for blinded, independent spike rate verification. Supported by NIH T32NS043124-09 (VB), Epilepsy Foundation (JH) and Extendicare Foundation, George Mitchell Foundation, NIH P01 AG022074 (JLN, PI: L. Mucke) and NS 29709 (JLN)
Footnotes
Disclosures
VB, JH, JR, JN have no conflicts of interest. GL is an officer of ReXceptor, Inc which is a company engaged in commercializing bexarotene for treatment of AD. PC is a shareholder in ReXceptor, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmeier C, Beaulieu-Abdelahad D, Crawford F, Mullan M, Paris D. Stimulation of the retinoid X receptor facilitates beta-amyloid clearance across the blood-brain barrier. J Mol Neurosci. 2013;49:270–6. doi: 10.1007/s12031-012-9866-6. [DOI] [PubMed] [Google Scholar]
- Blanchard BJ, Thomas VL, Ingram VM. Mechanism of membrane depolarization caused by the Alzheimer Abeta1–42 peptide. Biochem Biophys Res Commun. 2002;293:1197–203. doi: 10.1016/S0006-291X(02)00346-7. [DOI] [PubMed] [Google Scholar]
- Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA, et al. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–55. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–6. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924-c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–75. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holth JK, Bomben VC, Reed JG, Inoue T, Younkin L, Younkin SG, Pautler RG, Botas J, Noebels JL. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J Neurosci. 2013;33:1651–9. doi: 10.1523/JNEUROSCI.3191-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SR, Shirley MA, Grese TA, Neel DA, Wells KE, Ulm EH. Bexarotene metabolism in rat, dog, and human, synthesis of oxidative metabolites, and in vitro activity at retinoid receptors. Drug Metab Dispos. 2001;29:990–8. [PubMed] [Google Scholar]
- Hurst RE. Bexarotene ligand pharmaceuticals. Curr Opin Investig Drugs. 2000;1:514–23. [PubMed] [Google Scholar]
- Laclair KD, Manaye KF, Lee DL, Allard JS, Savonenko AV, Troncoso JC, Wong PC. Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol Neurodegener. 2013;8:18. doi: 10.1186/1750-1326-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalloyer F, Pedersen TA, Gross B, Lestavel S, Yous S, Vallez E, Gustafsson JA, Mandrup S, Fievet C, Staels B, Tailleux A. Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler Thromb Vasc Biol. 2009;29:1488–95. doi: 10.1161/ATVBAHA.109.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–62. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–8. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924-d. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–11. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Simeone KA, Samson KK, Kim do Y, Rho JM. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol Dis. 2013;54:68–81. doi: 10.1016/j.nbd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D’Hooge R, De Strooper B. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924-e. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM, Jiang H, Cirrito JR, Holtzman DM. In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener. 2013;8:13. doi: 10.1186/1750-1326-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM, Tanzi RE, Vassar R, Sisodia SS. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924-f. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–21. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]