Abstract
Background
While calcific aortic stenosis is common, calcification of the other three heart valves is not. The aortic valve interstitial cell (VIC) has been implicated in the pathogenesis of aortic stenosis. Pro-inflammatory stimulation of aortic VICs induces an osteogenic and inflammatory phenotypic change. We hypothesized that the VICs of the other heart valves do not undergo these changes. Using isolated human VICs from normal aortic, mitral, pulmonary and tricuspid valves, our purpose was to compare the osteogenic response to pro-inflammatory stimulation via TLR-4.
Materials And Methods
Aortic, pulmonic, mitral, and tricuspid (n=4 for each valve type) VICs were isolated from hearts valves explanted from patients undergoing cardiac transplantation. Cells were cultured and grown to confluence in passage 2-6 before treatment with LPS (100-200ng/mL) for 24 or 48 hours. Cells were characterized by immunofluorescent staining. TLR-4 expression was analyzed (immunoblotting, flow cytometry). BMP-2 and intercellular adhesion molecule-1 (ICAM-1) production were determined (immunoblotting). Monocyte chemoattractant protein-1 (MCP-1) levels were determined by ELISA. Statistics were by Mann-Whitney U test.
Results
TLR-4 stimulation induced BMP-2 production only in aortic VICs (p<0.05). ICAM-1 production and MCP-1 secretion increased in a similar fashion among TLR4-stimulated VICs from all four valves.
Conclusions
Pro-inflammatory stimulation induces an osteogenic phenotype in aortic VICs but not mitral, pulmonic, or tricuspid VICs. We conclude that this differential osteogenic response of aortic VICs contributes to the pathogenesis of calcific aortic stenosis.
Keywords: aortic stenosis, valve interstitial cell, TLR-4
Introduction
Calcific aortic stenosis is the third most prevalent cardiovascular disease in the United States, exceeded in prevalence only by hypertension and coronary artery disease1. It is the leading indication for heart valve replacement surgery. While calcification of the aortic valve is common, it is noteworthy that calcification of the other heart valves is not
The pathogenesis of calcific aortic stenosis is not well understood. It has traditionally been considered a degenerative process, one in which calcium passively accumulates on the aortic valve leaflets. However, evidence is accumulating that aortic stenosis is an active disease process - one in which mechanisms of inflammation play an important role2,3.
The valve interstitial cell (VIC) is the principle cell type found in cardiac valve leaflets4. In the aortic valve, the aortic VIC has been implicated in the pathogenesis of calcific aortic stenosis5. In response to pro-inflammatory stimulation via activation of Toll-like Receptor 4 (TLR-4), the aortic VIC has been shown to undergo a phenotypic change from that of a myofibroblast to that of an osteogenic and inflammatory phenotype6. In aortic VICs, such osteogenic phenotypic changes are characterized by the production of the important boneforming protein, bone morphogenetic protein-2 (BMP-2), and an inflammatory phenotype is characterized by the production of intracellular adhesion molecule-1 (ICAM-1)6. Previous studies have also demonstrated that these inflammatory responses are exaggerated in VICs isolated from stenotic aortic valves7,8, further emphasizing their importance to the disease process.
Little is known about the biological behavior of VICs from the mitral, tricuspid and pulmonary valves. It is interesting to note that these three valves rarely, if ever, calcify. But as the aortic VIC has been implicated in calcification of the aortic valve, we hypothesized that intrinsic differences may exist among the four cardiac valves that help to explain why aortic valves calcify but the mitral, tricuspid and pulmonary valves rarely, if ever, do. We specifically hypothesized that unlike aortic VICs, the VICs from mitral, tricuspid and pulmonary valves do not undergo an osteogenic phenotypic change in response to pro-inflammatory stimulation.
Using isolated human VICs from aortic, mitral, tricuspid and pulmonary valves, the purposes of this study were to determine the differences in TLR-4-induced expression of an osteogenic phenotype and an inflammatory phenotype. The results of this study demonstrate that TLR-4 stimulation induced an inflammatory response in all four types of VICs. However, TLR-4 stimulation induced an osteogenic phenotype only in aortic VICs.
Materials and Methods
This study was approved by the Colorado multiple institutional review board at the University of Colorado School of Medicine.
Reagents
Medium 199 was purchased from Lonza (Walkersville, MD). Donkey serum and fetal bovine serum (FBS) were obtained from Aleken chemicals (Nash, TX). Protein assay reagents and chemiluminescent substrate (ECL) were obtained from Thermo Scientific (Rockford, IL). Two-times Laemmli sample buffer, nitrocellulose membranes, and 4-20% gradient polyacrylamide Mini-Protean TGX gels were purchased from Bio-Rad (Hercules, CA). The MCP-1 ELISA kit was purchased from R&D systems (Minneapolis, MN). Rabbit polyclonal antibody for antihuman ICAM-1 and rabbit polyclonal anti-human BMP-2 antibody were purchased from Prosci (Poway, CA). Rabbit-derived anti-human TLR-4 was obtained from Santa Cruz (Dallas, TX). Anti-human TLR-4-phycoerythrin conjugated antibody (TLR4-PE) and mouse IgG2a K isotype phycoerytherin conjugated antibody (IgG-PE) were purchased from eBioscience (San Diego, CA). Mouse-derived anti-human alpha-smooth muscle actin antibody and rabbit-derived antivimentin antibody were purchased from Abcam (Cambridge, MA). All other chemicals were purchased from Sigma Chemical (St. Louis, MO).
Cell isolation/culture
Normal valve leaflets were sterilely removed from the explanted hearts of four patients undergoing heart transplant at the University of Colorado Hospital. None of the patients were taking statins prior to transplantation. The valve leaflets were thin, pliable, and normal appearing without any calcification. Valve interstitial cells were isolated using collagenase via a previously described method9. Cell cultures were maintained in a filtered (0.2μm) mixture of medium 199, penicillin G, streptomycin, amphotericin B, and 10% fetal bovine serum. They were placed in an incubator which remained at a temperature of 37 degrees Celsius and 5% CO2. Cells were grown to passage 2-6 before treatment.
LPS stimulation
A solution of LPS was diluted to 100ng/mL or 200ng/mL, using phosphate-buffered saline (PBS). The cells were treated with this working concentration for 24 or 48 hours depending on the experiment. Cells were plated onto twelve or 6 well plates prior to treatments. Treatments were performed in ¼ strength (2.5%FBS) media.
Immunofluorescence
Cells were stained using a method described previously9. Cells were plated onto chamber slides. After washing twice with PBS, they were permeabilized using a solution composed of 30% acetone and 70% methanol for about 8 minutes. Following another PBS wash, cells were fixed with 4% paraformaldehyde (PFA) for 8 minutes. After another PBS wash, cells were blocked with donkey serum for 45 minutes at room temperature. Next, cells were incubated overnight at 4 degrees Celsius with diluted solutions containing the primary antibodies to smooth muscle actin (diluted 1:50 in 1% bovine serum albumin (BSA) in PBS) and vimentin (diluted 1:100 in 1% BSA in PBS). Slides were then washed with PBS again before placing secondary antibodies (indocarbocyanine Cy3-conjugated donkey anti-mouse antibody and Alexa-488 conjugated donkey anti-rabbit antibody) for 1hour in the dark. Cells were also counterstained with bisbenzimide to stain the cell nuclei. After another wash with PBS, cells were rinsed with deionized water and then covered with mounting medium and a cover glass which we sealed with nail polish. Slides were read using a Leica DMRXA confocal microscope (Leica Mikroskopie and Systeme, Wetzlar, Germany). We imaged the alpha-smooth muscle actin, vimentin, and nuclei on the red, green, and blue channels respectively. Slidebook software was used to obtain images (Intelligent Imaging Innovations, Denver, CO).
Immunoblotting
Immunoblotting was used to determine levels of BMP-2, ICAM-1, and TLR-4. Cell lysis was performed using 1× Laemmli sample buffer with β-mercaptoethanol. Lysates were then loaded into 15-well 4-20% gradient mini-Protean TGX gels (Bio-Rad, Hercules, CA) and run at 180V for 40 minutes. The gel was transferred to a nitrocellulose membrane using 100V for 70 minutes. The membranes were then cross-linked using a UV Stratalinker (Stratagene, La Jolla, CA) twice. To block the membrane, 5% dry milk in 0.1% Tween in phosphate buffered saline (T-PBS) solution was used. The membrane was washed three times with T-PBS and then incubated at 4 degrees Celsius overnight with primary antibody diluted in 5% BSA. After washing with TBS three times, membranes were incubated with the proper horseradish peroxidase-conjugated secondary antibody diluted in 5% dry milk with T-PBS for an hour. Then the membranes were washed again three times with T-PBS and treated with ECL for 2 minutes at room temperature. Exposures were read using a Bio-rad Chemi-Doc MP Imaging system (Hercules, CA). Analysis was performed with Image-J densitometry software provided by the National Institute of Health.
Enzyme-Linked Immunosorbent Assay (ELISA)
Kits obtained from R&D Systems were utilized to perform ELISA for MCP-1. An amount of 100μL of cell culture media was added to two wells and the average was used for analysis. ELISA testing was performed according to R&D protocol. Plates were interpreted with a Synergy H1 Hybrid Reader produced by BioTek (Winooski, VT).
Flow Cytometry
Cells were mobilized using trypsin and trypsin-neutralizing solution. Cells were then centrifuged twice at 270rcf for 5 minutes using cold phosphate-buffered saline (PBS). Cells were blocked with 1% BSA in PBS for 30 minutes before centrifuging again. After removing the supernatant, cells were conjugated with IgG-PE or TLR-4-PE in PBS for 10 minutes. Diamidine-2-phenylindole (DAPI) was used to select live cells for analysis. Positively staining cells were compared with background fluorescence by utilizing the IgG-PE control antibody peak. Samples were run on a Beckman-Coulter Gallios flow cytometer (Brea, CA). Kaluza software was used to analyze data.
Statistical Analysis
Data are presented as mean ± the standard error of the mean (SEM). Statistical analysis was performed using the Mann-Whitney U test. p < 0.05 was significant.
Results
Patient Characteristics
Aortic, pulmonary, mitral, and tricuspid valves were obtained from four male patients who underwent cardiac transplantation for idiopathic dilated cardiomyopathy. Patient ages ranged from 20 to 49 years. Prior to transplantation, these patients all had echocardiograms showing no significant valvular disease. All patients also underwent coronary angiography prior to transplantation, and none had coronary artery disease. None of the patients used tobacco.
VICs from all four valves demonstrated a myofibroblast phenotype
Aortic, pulmonic, mitral, and tricuspid VICs were isolated from explanted hearts. Immunofluorescent staining was used to confirm that cells from all four valves demonstrated a myofibroblast phenotype at baseline. VICs from all four valves stained positively for alphasmooth muscle actin and vimentin (Figure 1A-D). All of the cells visualized expressed vimentin, while about 70% of VICs visualized expressed alpha-smooth muscle actin.
Figure 1.
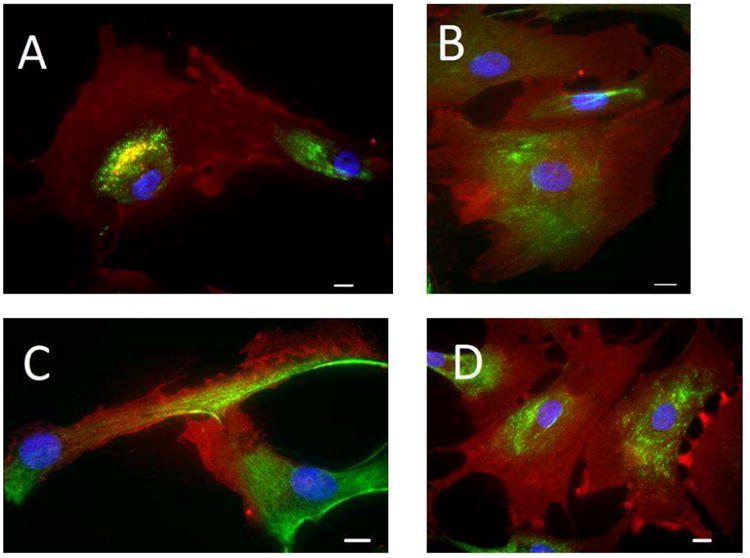
Characterization of VICs. Using explanted human hearts, VICs were isolated from the aortic (A), pulmonary (B), mitral (C), and tricuspid (D) valves. Cells are stained for alphasmooth muscle actin (red), vimentin (green), and nuclear counterstain (blue). All four VICs types exhibit myofibroblast phenotype. Bar = 30μm
VICs from all four valves contain similar amounts of TLR-4 regardless of TLR-4 stimulation
Immunoblotting was performed using VIC cell lysates from the aortic, pulmonic, mitral, and tricuspid valves to compare levels of TLR-4. As demonstrated in Figure 2A, TLR-4 protein levels were not significantly different among VICs from the four valves. Cells were stimulated for 24 hours with LPS at a concentration of 100ng/mL. Cell lysates were analyzed and compared to control using immunoblotting. Levels of TLR4 did not change after stimulation with LPS in the aortic valve (Figure 2B). The same was true of the three other valves (data not shown).
Figure 2.
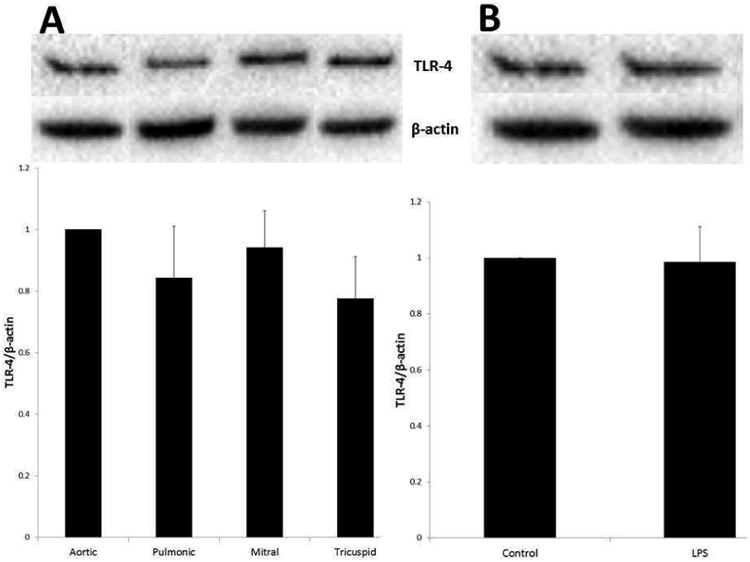
Receptor levels of TLR are similar between VICs from the four heart valves. Untreated cell lysates were analyzed for TLR-4 using immunoblotting (A). TLR-4 levels did not differ significantly between VICs from different valves. Cells were treated with LPS (100ng/mL) for 24 hours and lysates were analyzed for TLR-4 levels. Shown above is the aortic valve data normalized to control (B). There was no significant change between control and LPS-stimulated levels of the TLR-4. This applied to the other three valves as well (data not shown).
Surface levels of TLR-4 are the similar among VICs from all four valves
Flow cytometry was utilized to compare the surface receptor density expression among the four VIC types. VICs were labeled with a fluorescent anti-TLR4-PE antibody or IgG-PE. The median fluorescence intensity was compared to the median fluorescence intensity of the control isotype (mouse IgG-PE). Sharp peaks were generated for the TLR-4 PE antibody and the control isotype antibody for VICs from the aortic (Figure 3A), pulmonic (Figure 3B), mitral (Figure 3C), and tricuspid (Figure 3D) valves. The ratio of median fluorescence intensity of the TLR-4-PE antibody to the median control IgG conjugated cells was compared between the valves, and similar results were obtained for left and right sided heart valves (Figure 3E). Utilization of DAPI allowed us to analyze data for live cells only, which represented on average about 60-80% of each sample. Approximately 5-10,000 events were recorded for each sample.
Figure 3.
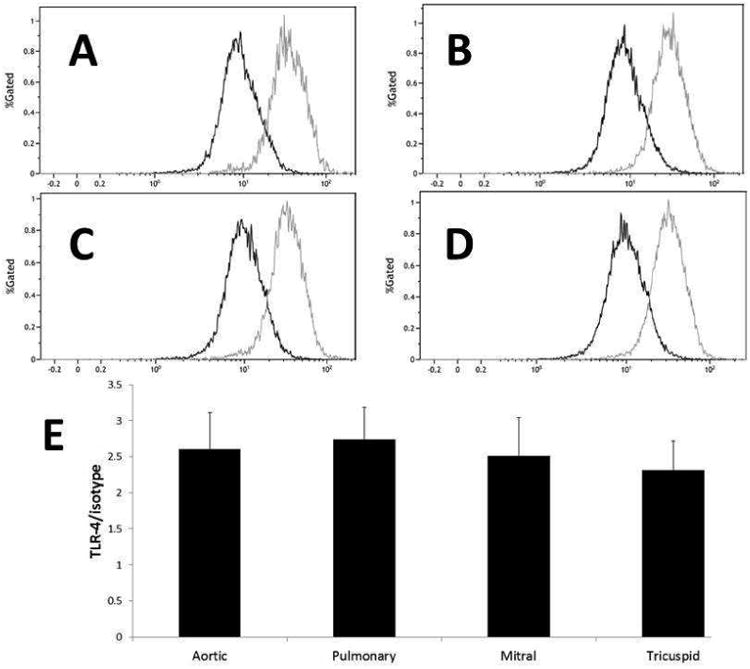
VICs express similar surface levels of TLR-4. VICs were digested with trypsin/TNS and conjugated with antibodies to TLR-4 PE or Isotype control. The gray line in each flow cytometry plot represents TLR-4 antibody results compared to the isotype (darker line). Data are presented for the aortic (A), pulmonary (B), mitral (C), and tricuspid (D) VICs. The graph represents the ratio of the median of the mean fluorescence intensity normalized to the isotype control (E). Ratios did not differ significantly among VICs for each valve, indicating a similar surface concentration of the TLR-4 receptor.
Only Aortic VICs express an osteogenic phenotype after TLR-4 stimulation
VICs from all four valves were treated with LPS at a concentration of 200ng/mL for 48 hours. Lysates were analyzed for levels of BMP-2 using immunoblotting (Figure 4). BMP-2 levels increased significantly from control only for the aortic VICs (p<0.05). After TLR stimulation, aortic VIC production of BMP-2 was greater than the pulmonary, mitral, or tricuspid VICs (p<0.05).
Figure 4.
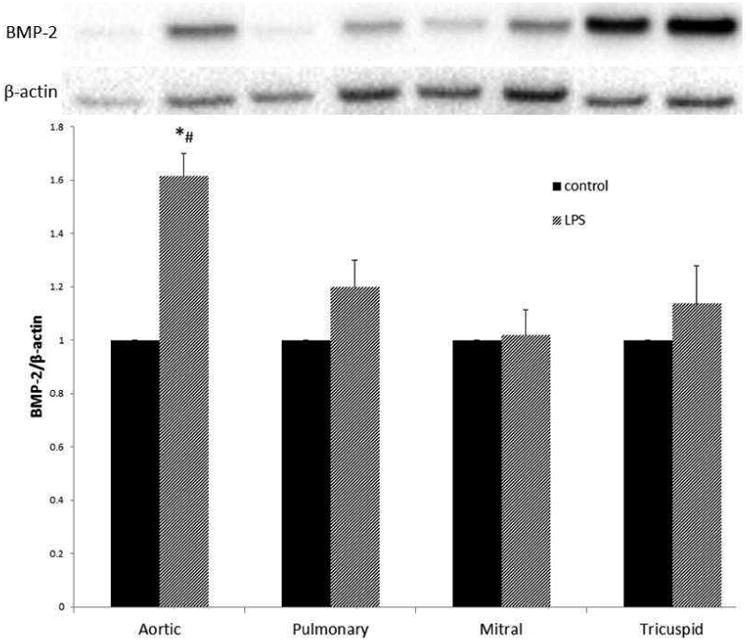
Aortic VICs adopt an osteogenic phenotype after TLR stimulation, while the pulmonic, mitral, and tricuspid VICs do not. VICs were lysed after stimulation with LPS (200ng/mL) for 48 hours. Lysates were analyzed using immunoblotting. Levels of BMP-2 increased significantly (*p<0.05) compared to control in the aortic valve, but not the other three heart valves after LPS stimulation. Also, BMP-2 levels after LPS stimulation in Aortic VICs were greater compared to LPS-stimulated BMP-2 levels in the other three heart valves (#p<0.05). Data are normalized to control for each valve.
VICs from all four valve lines adopt an inflammatory phenotype after TLR4 stimulation
Aortic, pulmonic, mitral and tricuspid VICs were treated for 24 hours with LPS at a concentration of 100ng/mL. Lysates were analyzed for levels of ICAM-1 using immunoblotting (Figure 5A). All four cell lines showed increases in ICAM-1 levels compared to control (p<0.05), but the TLR-stimulated levels of ICAM-1 production were the same for all four VICs. Cell media were analyzed for levels of MCP-1 using ELISA (Figure 5B). VICs from all four valves had an increase in MCP-1 after TLR4 stimulation compared to control (p<0.05), but this increase was similar across valve types.
Figure 5.
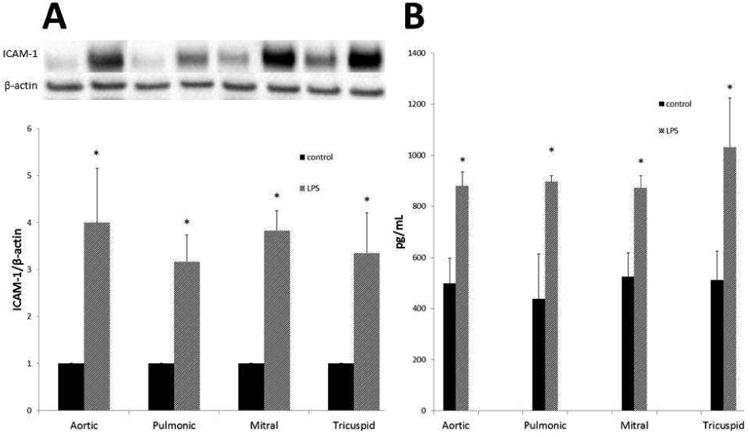
VICs from all four heart valves adopt an inflammatory phenotype after TLR stimulation. VICs were stimulated with LPS (100ng/mL) for 24 hours. Cell lysates were analyzed by immunoblotting to detect levels of ICAM-1 (A). VIC production of ICAM-1 increased significantly from control for all four valve lines (*p<0.05). However, levels of LPS-stimulated ICAM-1 were not significantly different between VICs from the four valves. Data are normalized to control for each valve. Cell media was analyzed for MCP-1 levels using ELISA (B). Levels of MCP-1 increased significantly compared to control for VICs isolated from all four heart valves (*p<0.05), yet LPS-stimulated secreted MCP-1 levels did not differ significantly between the aortic, pulmonary, mitral, or tricuspid valves.
Discussion
The results of the present study demonstrate an important difference in the response to TLR-4 stimulation among the VICs from the four types of heart valves. TLR-4 expression was not different among the different types of VICs. In addition, TLR-4-induced production of ICAM-1 and MCP-1 increased in all four types of VICs. However, TLR-4 stimulation induced an osteogenic phenotype as characterized by BMP-2 production in aortic VICs but not VICs from mitral, tricuspid or pulmonary valves. These data suggest that a differential response to pro-inflammatory stimulation may help explain the clinical observation that calcification of the aortic valve is common, but calcification of the mitral, tricuspid and aortic valves is not.
In this study, human VICs were isolated, grown and studied in vitro. With any in vitro study, there can be concern that the cells may behave differently than those in vivo. Our group has previously shown that human aortic VICs isolated from explanted valves and grown in culture maintain cellular activity similar to freshly isolated cells6. Additionally, human AVICs in vitro lack the input from the other cell types found in the aortic valve, and thus limit our ability to understand the effects of these interactions. The results of the present study highlight important differences in the responses of VICs from different valves after TLR-4 stimulation.
To our knowledge, the present study is the first to compare the inflammatory and osteogenic responses to pro-inflammatory stimulation via TLR-4 in VICs from all four heart valves. Other data comparing VICs from the four heart valves are sparse. A review of the literature by Sherif and colleagues9 suggested that even when human right-sided heart valves were subjected to the higher pressures and forceful hemodynamics typically experienced by left-sided valves, the incidence of valve calcification did not increase. Comparing left- and right- sided porcine valve interstitial cells, Bouchard-Martel and colleagues10 found higher alkaline phosphatase activity in VICs from the aortic and mitral valves compared to the tricuspid and pulmonary VICs. Yang and colleagues7 demonstrated that human aortic VICs express higher levels of BMP-2 and Runt-related transcription factor-2 (Runx-2) after TLR stimulation compared to pulmonary VICs.
The results of the present study suggest that intrinsic differences exist in the responses of human VICs to TLR-4 stimulation. Toll-like receptor-4 is found on the cell surface as well as within a given cell. The results of the present study demonstrate that neither the total expression of TLR-4 nor the cell surface expression were different among the VICs from the 4 heart valves. However, the responses of the VICs to TLR-4 stimulation were different. TLR-4 stimulation produced significant increases in the inflammatory mediators, ICAM-1 and MCP-1, in all four types of VICs. However, TLR-4 stimulation only produced an increase in BMP-2 expression in aortic VICs; there was no change in BMP-2 production in the mitral, tricuspid or pulmonary VICs. Thus, the results of the present study demonstrated that pro-inflammatory stimulation via TLR-4 did induce an inflammatory phenotype in the VICs from all four heart valves, but it only induced an osteogenic phenotype in aortic VICs. Investigations into the intracellular mechanisms responsible for this differential response in aortic VICs were beyond the scope of the present study and will require further study. In aortic VICs, our laboratory has previously demonstrated that the intra-cellular signaling pathways responsible for inflammatory mechanisms differ from those responsible for osteogenic mechanisms11,12. It is therefore tempting to speculate that the intracellular signaling activated in aortic VICs by TLR-4 stimulation may be unique among the four types of VICS, culminating in the induction of an osteogenic phenotype in aortic but not other VICs. While evidence that shear stress and other mechanical factors may play a role in calcific aortic stenosis13 must be acknowledged, further research efforts may need to focus on both cellular and mechanical pathways of inflammation, as both are critical to disease progression.
In summary, the results of the present study demonstrated that while TLR-4 expression was not different among VICs from all four heart valves, the response to TLR-4 stimulation was different; TLR-4 stimulation induced an osteogenic phenotype in aortic VICs but not in VICs from mitral, tricuspid or pulmonary valves. Such a differential response to pro-inflammatory stimulation in human aortic VICs offers mechanistic insight into the observation that the aortic valve commonly calcifies and the other three valves do not.
Acknowledgments
Funded by grants from the American Heart Association (AHA: 11GRNT7900016) and the National Institutes of Health (NIH RO1 HL106582-01).
Footnotes
Presented at the 9th Annual Meeting of the Academic Surgical Congress, San Diego, California, February 4-7, 2013.
Disclosure Statement: The authors have nothing to disclose.
Contributions: Neil Venardos (conception, design, analysis, interpretation, data collection, writing the article), Nicole A Nadlonek (conception, design, data collection), Qiong Zhan (data collection), Michael J Weyant (conception, design, analysis, interpretation), T. Brett Reece (conception, design, analysis), Xianzhong Meng (design, analysis, interpretation, critical revision of article, obtaining funding), David A Fullerton (design, analysis, interpretation, critical revision of article, writing article, obtaining funding)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21(5):1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Xu S, Gotlieb AI. The progression of calcific aortic valve disease through injury, cell dysfunction, and disruptive biologic and physical force feedback loops. Cardiovasc Pathol. 2013;22(1):1–8. doi: 10.1016/j.carpath.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Leopold Ja. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv. 2012;5(4):605–14. doi: 10.1161/CIRCINTERVENTIONS.112.971028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chester AH, Taylor PM. Molecular and functional characteristics of heart-valve interstitial cells. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1437–43. doi: 10.1098/rstb.2007.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171(5):1407–18. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng X, Ao L, Song Y, et al. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294(1):C29–35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Fullerton Da, Su X, Ao L, Cleveland JC, Meng X. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of Toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J Am Coll Cardiol. 2009;53(6):491–500. doi: 10.1016/j.jacc.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 8.López J, Fernández-Pisonero I, Dueñas AI, et al. Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol. 2012;158(1):18–25. doi: 10.1016/j.ijcard.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 9.Sherif HMF. Calcification of left-sided valvular structures: evidence of a pro-inflammatory milieu. J Heart Valve Dis. 2009;18(1):52–60. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19301553. [PubMed] [Google Scholar]
- 10.Bouchard-Martel J, Roussel E, Drolet MC, Arsenault M, Couet J. Interstitial cells from left-sided heart valves display more calcification potential than from right-sided valves: an in-vitro study of porcine valves. J Heart Valve Dis. 2009;18(4):421–8. [PubMed] [Google Scholar]
- 11.Nadlonek N, Lee JH, Reece TB, et al. Interleukin-1 Beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa Beta. Ann Thorac Surg. 2013;96(1):155–62. doi: 10.1016/j.athoracsur.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Meng X, Su X, et al. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138(4):1008–15. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Arjunon S, Rathan S, Jo H, Yoganathan AP. Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng. 2013;41(7):1331–46. doi: 10.1007/s10439-013-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


