Abstract
Animal studies suggest that hypothermia decreases seizure burden, while limited human data are inconclusive. This retrospective cohort study examines the relationship between therapeutic hypothermia and seizure in neonates with hypoxic-ischemic encephalopathy. Our center admitted 224 neonates from July 2004 to December 2011 who met institutional cooling criteria. Seventy-three neonates were born during the pre-cooling era, prior to November 2007, and 151 were born during the cooling era. Among neonates with moderate encephalopathy, the incidence of seizure in cooled infants was less than half the incidence in those not cooled (26% cooling versus 61% pre-cooling era; RR=0.43, 95% CI 0.30 to 0.61). Among neonates with severe encephalopathy, there was no difference in the incidence (83% versus 87%; RR=1.05, 95% CI 0.78 to 1.39). These results support animal data and suggest a mechanism by which neonates with moderate encephalopathy may benefit more from cooling than neonates with severe encephalopathy.
Keywords: Birth Asphyxia, Epilepsy, Hypoxic-ischemic Encephalopathy, Incidence, Neurocritical Care
INTRODUCTION
Neonatal seizures are common, affecting 1 to 4 neonates per 1000 live term births in the United States.1–4 Hypoxic-ischemic encephalopathy is the leading cause of neonatal seizures.2 In infants with hypoxic-ischemic encephalopathy, there is increasing evidence that the seizures predict worse outcomes, such as an increased risk of brain injury, cerebral palsy and epilepsy.5–12 Therapeutic hypothermia is the only effective treatment currently available for neonates with hypoxic-ischemic encephalopathy, and has become the standard of care.13–19
Although animal studies report anti-seizure effects of hypothermia,20–22 clinical evidence presents conflicting results. Meta-analyses of randomized controlled trials fail to show an effect of cooling on seizures in neonates,23,24 while more recent small, single center observational studies indicate that therapeutic hypothermia may decrease the incidence of seizure25 and seizure burden.26, 27 If cooling does in fact reduce seizure incidence, then the clinical implication would be that fewer patients with hypoxic-ischemic encephalopathy would receive potentially harmful28 antiepileptic drugs, whose efficacy is limited.29
The objective of this retrospective cohort study was to examine the relationship between therapeutic hypothermia and cumulative incidence of seizure in neonates with hypoxic-ischemic encephalopathy. We hypothesized that neonates treated with therapeutic hypothermia are less likely to have seizures than neonates who did not receive the intervention.
METHODS
Subjects
Inclusion Criteria
Neonates admitted to the University of California, San Francisco Intensive Care Nursery from July 2004 to December 2011, who met previously described institutional cooling criteria,30 were included in the study.
Exclusion Criteria
Subjects were excluded for the following: identification later than 6 hours of birth, birth weight less than 1800 grams, coagulopathy with active bleeding, requiring extracorporeal membrane oxygenation (ECMO) prior to 6 hours of life, severe congenital anomalies, syndromes or known metabolic disorders.
Selection and Group Assignment
The 2004 to 2011 University of California, San Francisco Intensive Care Nursery database, which contained information on all discharged neonates, was compiled prospectively in a systematic manner using a protocol and pre-determined variable definitions. The database was continuously sampled for a diagnosis of hypoxic ischemic encephalopathy. Since cooling therapy became available at the study institution on November 1, 2007, neonates born prior to this date were in the “pre-cooling era” group and neonates born on or after this date were in the “cooling era” group. Patients in the cooling era group who did not receive therapeutic hypothermia were excluded after sensitivity analysis showed omission produced similar results.
Measurements
Patient demographic characteristics were extracted from the University of California, San Francisco Intensive Care Nursery database and chart review. Encephalopathy severity was assigned based on chart documentation of the worst mental status observed during the seven days following birth. Neonates who were not responsive to arousal maneuvers were designated as having severe encephalopathy and neonates who were hyperalert and/or lethargic were designated as having moderate encephalopathy. Neonates with unclear encephalopathy severity (n=16) due to poor documentation or use of paralytic medication were categorized as having moderate encephalopathy after sensitivity analysis showed that excluding neonates with unclear encephalopathy severity did not qualitatively alter the results. To test the reliability of encephalopathy severity categorization, a second researcher adjudicated this variable for a subset of 20 patients, which resulted in nearly perfect inter-rater reliability (unweighted kappa=0.83). Neonates, who were treated with therapeutic hypothermia received whole-body cooling via a cooling unit and blanket (CSZ Blanketrol III™, CITY). Rectal temperature remained at 33.5 degrees Celsius for 72 consecutive hours. Morphine was given throughout cooling to provide adequate sedation to avoid shivering.
Physician, nursing, transport, and referring hospital notes were reviewed for the presence of seizure prior to hospital discharge. Clinical seizure diagnosis was based on Mizrahi and Kellaway characterizations,31 and electrographic seizure diagnosis was based on electroencephalogram (EEG) reports written by neurophysiologists blinded to the study hypothesis. Pre-cooling era seizure monitoring was at the discretion of the treating physician, typically video-EEG for 2 hours or less when clinical suspicion of seizure arose. In contrast, cooling era seizure monitoring involved continuous monitoring with both amplitude-integrated electroencephalogram (aEEG) and conventional video-EEG applied according to the international 10–20 system, modified for neonates, from the time of admission until the completion of rewarming.10,32 Antiepileptic drug treatment was initiated after a suspected seizure, not prophylactically.
Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at University of California, San Francisco.33 Data were collected with the approval of the University of California, San Francisco Committee on Human Research.
Analysis
To assess for baseline differences between the groups, the chi-squared and Fisher’s exact tests were used for comparisons of categorical variables and the t- and rank-sum tests were used for continuous variables with parametric and non-parametric distributions, respectively. The risk of having a seizure was compared between the pre-cooling era group and the cooling-era group, expressed as a risk ratio (RR). Since encephalopathy severity was a priori identified as a confounder, repeat analysis was stratified by encephalopathy severity. The statistical significances of these effects were determined using the chi-squared test. Proportions of seizure diagnoses supported by EEG evidence were compared using the chi-squared test.
For all analyses, p-values <0.05 were considered significant and all tests were two sided. Analyses were performed using Stata 12 (StataCorp, College Station, Texas).
RESULTS
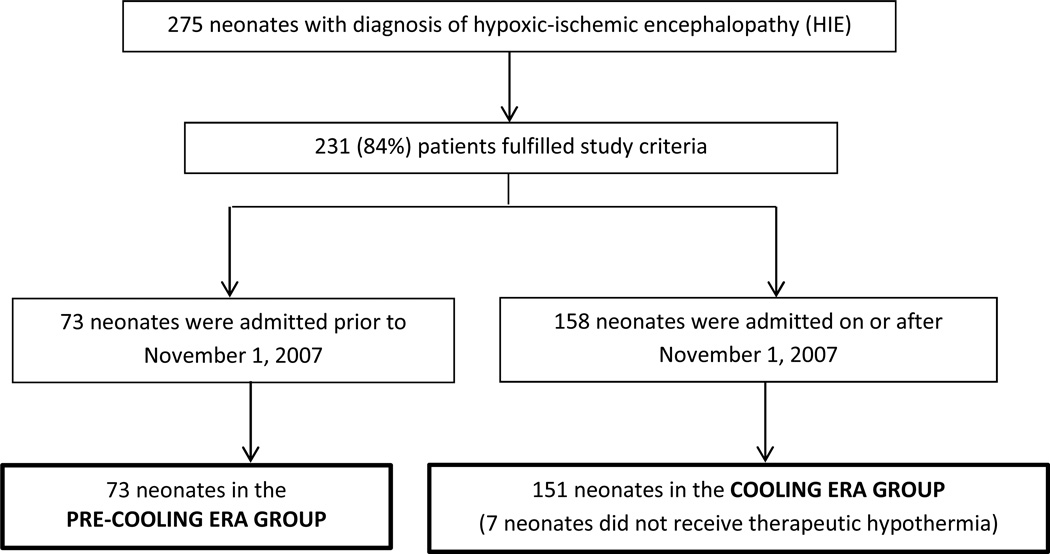
Of 275 neonates diagnosed with hypoxic-ischemic encephalopathy, 224 (81%) met study criteria (figure 1). Fifty-one patients were excluded for the following reasons: six had an hypoxic-ischemic encephalopathy event secondary to post-natal cardiopulmonary arrest, 23 had no documented encephalopathy during the first six hours of life, six did not meet criteria for perinatal asphyxia, eight had severe congenital anomalies, syndromes or known metabolic disorders, one had coagulopathy with active bleeding, and seven did not receive therapeutic hypothermia during the cooling era due to late referral or late recognition. Seventy-three neonates were born during the pre-cooling era and 151 were born during the cooling era. Within the cooling era group, 129 (85%) completed the therapeutic hypothermia protocol and 22 (15%) partially completed the therapeutic hypothermia protocol. Therapeutic hypothermia was stopped for severe cardiopulmonary instability or transition to comfort care. All baseline characteristics, except for sex, were similar between pre-cooling era and cooling era groups. Significantly fewer neonates were treated with phenobarbital in the cooling era compared to the pre-cooling era (table 1). Of note, among neonates with seizure, 93% (54/58) of cooling era and 96% (45/47) of pre-cooling era neonates were treated with phenobarbital (p=0.6).
Figure 1.
Flow diagram of study selection and group assignments.
Table 1.
Characteristics of 224 neonates with hypoxic-ischemic encephalopathy. Pre-cooling era neonates did not receive therapeutic hypothermia and cooling era neonates received therapeutic hypothermia.
| Cooling era (n=151) |
Pre-cooling era (n=73) |
p-value | |
|---|---|---|---|
| Male sex | 48% | 67% | 0.006 |
| Gestational age, weeks | 39.5 (1.5) | 39.6 (1.5) | 0.8 |
| Birth weight, grams | 3308 (547) | 3424 (618) | 0.2 |
| Cesarean section | 59% | 56% | 0.9 |
| 10-minute Apgar score | 5 (3 to 7) | 6 (4 to 7) | 0.06 |
| Base excess* | −18 (6) | −17 (7) | 0.5 |
| Encephalopathy severity | 0.5 | ||
| Moderate | 79% | 84% | |
| Severe | 21% | 16% | |
| Paralytic medication | 4% | 8% | 0.2 |
| Phenobarbital** | 38% | 64% | <0.001 |
| ICN Length of stay, days | 9 (7 to 12) | 11 (6 to 18) | 0.4 |
| Death before hospital discharge | 16% | 15% | 0.9 |
ICN, Intensive Care Nursery
Values are given in terms of %, mean (standard deviation), or median (25th to 75th percentile).
Based on worst base excess value from cord gas or first blood gas.
Antiepileptic drug treatment was given following a suspected seizure, not prophylactically.
Neonates during the cooling era were less likely to have seizures, diagnosed either clinically or by EEG, than those admitted during the pre-cooling era (RR=0.60, 95% CI 0.46 to 0.78) (table 2). Among neonates with moderate encephalopathy, those admitted during the cooling era were about half as likely to have seizures as those admitted during the pre-cooling era (RR=0.43, 95% CI 0.30 to 0.61). Meanwhile, no difference in seizure risk occurred among neonates with severe encephalopathy (RR=1.05, 95% CI 0.78 to 1.39).
Table 2.
The proportion of neonates with seizures in the pre-cooling and cooling era groups stratified by encephalopathy severity.
| Cooling era | Pre-cooling era | Risk Ratio (95% CI) |
p-value | |
|---|---|---|---|---|
| All Neonates | n=151 | n=73 | ||
| Any Seizure (EEG or Clinical) | 38% | 64% | 0.60 (0.46–0.78) | <0.001 |
| Clinical Seizure† | 27% | 64% | 0.42 (0.31–0.58) | <0.001 |
| EEG-confirmed Seizure | 27% | 19% | 1.42 (0.83–2.43) | 0.2 |
| Moderate Encephalopathy | n=120 | n=61 | ||
| Any Seizure (EEG or Clinical) | 26% | 61% | 0.43 (0.30–0.61) | <0.001 |
| Clinical Seizure† | 17% | 61% | 0.27 (0.18–0.43) | <0.001 |
| EEG-confirmed Seizure | 18% | 20% | 0.93 (0.50–1.75) | 0.8 |
| Severe Encephalopathy | n=31 | n=12 | ||
| Any Seizure (EEG or Clinical) | 87% | 83% | 1.05 (0.78–1.39) | 0.8 |
| Clinical Seizure† | 68% | 83% | 0.81 (0.57–1.15) | 0.3 |
| EEG-confirmed Seizure | 61% | 17% | 3.68 (1.01–13.44) | 0.02 |
EEG, electroencephalogram
Any clinical seizure with or without EEG-confirmation.
When excluding subclinical seizures, neonates in the cooling era were about half as likely to be diagnosed with clinical seizures (RR=0.42, 95% CI 0.31 to 0.58). Among neonates with moderate encephalopathy, those in the cooling era were approximately one-third as likely to be diagnosed with clinical seizures (RR=0.27, 95% CI 0.18 to 0.43). Neonates with severe encephalopathy, however, did not show a difference in clinical seizure frequency among pre-cooling and cooling era groups (RR=0.81, 95% CI 0.57 to 1.15).
Nearly half (45%) of neonates in the pre-cooling era had at least one EEG report available describing a minimum 30 minute duration during the first four days of life, while all (100%) neonates in the cooling era had at least one EEG report available describing continuous EEG monitoring during the period of hypothermia and rewarming. When analysis was restricted to seizures with an EEG-supported diagnosis, there was no difference in seizure risk among hypothermia treated neonates (RR=1.42, 95% CI 0.83 to 2.43), nor among the subgroup of neonates with moderate encephalopathy (RR=0.93, 95% CI 0.50 to 1.75). Among neonates with severe encephalopathy, those born in the cooling era were more likely to be diagnosed with electrographic seizures (RR=3.68, 95% CI 1.01 to 13.44). The higher incidence of electrographic seizures in this subgroup was associated with an increased likelihood of detection of subclinical seizures. Among neonates with seizures, not one (0/47) patient in the pre-cooling era was diagnosed with only subclinical seizures, while 29% (17/58) of patients in the cooling era were diagnosed with only subclinical seizures (p<0.001).
DISCUSSION
Therapeutic hypothermia was associated with lower incidence of seizure among neonates with hypoxic-ischemic encephalopathy. In spite of increased sensitivity of seizure detection in the cooling era, cooled neonates were about half as likely to be diagnosed with seizures compared to non-cooled neonates with moderate encephalopathy, while there was no association among neonates with severe encephalopathy. These results support pre-clinical and clinical studies that suggest that hypothermia has an anti-seizure effect in neonates.
Alternatively, these findings may reflect more accurate seizure diagnosis with the advent of continuous EEG monitoring in the cooling era. During the cooling era, fewer clinical spells were diagnosed as seizures, and there was an increased likelihood of diagnosing EEG-confirmed seizures, particularly subclinical seizures. Fewer than half of the subjects in the pre-cooling era had EEG monitoring of any duration, so it is almost certain that the incidence of EEG-confirmed seizures in the pre-cooling era was an underestimate. As a result, our ability to find an association between hypothermia and EEG-confirmed seizures was biased in the direction of showing no association or even a positive association as the data suggest.
Animal studies support that cooling therapy is associated with less epileptiform activity and fewer seizures. According to a fetal sheep model of severe hypoxia-ischemia, cooling was associated with a reduction in the number of epileptiform transients in the first 6 hours after asphyxia insult and a reduction in both early and late seizure amplitude.20 A piglet model showed a significant reduction in the number and duration of electrographic seizures associated with therapeutic hypothermia.21 Hypothermia also had anticonvulsant properties in an epilepsy model of rats treated with kainic acid.22 Cooling therapy is believed to protect against neural injury through multiple mechanisms that likely attenuate the excitatory environment in the brain.34 For this reason, these mechanisms may also decrease seizure activity.
Clinical evidence of seizure reduction related to hypothermia is inconclusive. Two meta-analyses of clinical trials, which measured clinical seizures, failed to show an effect of hypothermia.23, 24 Meanwhile, in a small, observational study from our center, not one of 5 neonates with focal stroke who had been treated with hypothermia had seizures, while 7/10 (70%) non-cooled neonates with focal stroke had seizures.25 In a second single-center observational study, Low et al showed that the electrographic seizure burden among 15 cooled neonates was significantly lower than among 16 non-cooled neonates. In keeping with our findings, stratification by encephalopathy severity demonstrated that only cooled neonates with moderate encephalopathy had a significant reduction in electrographic seizure burden (49(26 to 89) versus 162(97 to 262) minutes).26 Using gold-standard electrographic seizure monitoring in both the cooled and non-cooled groups, Low et al provide evidence for anti-seizure benefits of cooling specific to neonates with moderate encephalopathy. Yet their study was not large enough to detect a difference in incidence of seizure, nor were they able to detect a difference in antiepileptic drug treatment. Most recently, a third study replicated these findings (n=69), showing reduced electrographic seizure burden (2.2 ± 0.6 versus 7.0± 1.0 log units) among cooled neonates with moderate encephalopathy.27 Of note, the lower incidence of electrographic seizure in the moderate encephalopathy groups supports our findings (28% cooled versus 87% non-cooled neonates). However, again, the authors did not observe a difference in antiepileptic drug treatment.
Clinical trial data suggest that neonates with moderate encephalopathy benefit the most from cooling. One meta-analysis concluded that death or disability at 18 months was significantly lower among cooled neonates with moderate encephalopathy but not in those with severe encephalopathy,35 while another, more recent meta-analysis suggested neonates with severe encephalopathy also benefit but perhaps to a lesser extent.36 If, in fact, hypothermia reduces seizures among neonates with moderate encephalopathy, then two potential mechanisms may explain why this subgroup benefits most: less harm due to the seizures, themselves, and less exposure to potentially toxic anticonvulsant medications.
While our results align well with current animal and clinical evidence, we recognize that this study has limitations related to its retrospective design. First, we note an increase in subjects during the cooling era. If this increase were due to better recognition of subjects with milder injury, the cooling population may have been enriched with subjects that were less likely to have seizures. However, we note that there were similar levels of measurable indicators of severity (10-minute Apgar, base excess, and encephalopathy) in the pre-cooling and cooling eras, and so it is unlikely that selection bias was a factor. Second, as discussed above, improved EEG monitoring and nursing and provider education during the cooling era may have changed the likelihood that bedside nurses and physicians documented and diagnosed clinical events in the absence of EEG correlates as seizures. Third, high dose anticonvulsant treatment may have led to inappropriate categorization of severe, rather than moderate, encephalopathy in few neonates. This misclassification would result in a less substantial relative risk among neonates with moderate encephalopathy but would not alter the observed effect among all neonates. Lastly, measurements were based on chart review and, although data extraction was standardized, variation in chart documentation limited our ability to account for all potential confounders. For example, we were unable to account for unmeasured changes in medical management between the pre-cooling and cooling eras, which may mean that the effect we observed between the two eras may be associated with secular changes in medical management in addition to or instead of therapeutic hypothermia. Despite these limitations, the central conclusion of the study is upheld by a robust sample size of 224 patients and corresponds with current animal and clinical data.
CONCLUSION
The results of this study show that cooled neonates with moderate encephalopathy were at lower risk of seizure than non-cooled neonates, and continuous EEG monitoring appeared to enhance recognition and diagnosis of seizures. Hypothermia and improved monitoring may contribute to improved neurological outcomes among cooled neonates with moderate encephalopathy by reducing seizure burden and leading to a decrease in the use of unnecessary anticonvulsant medications. Future studies are necessary to clarify the role of EEG monitoring in this population, as well as the mechanisms underlying reduced seizure risk associated with therapeutic hypothermia and related long-term neurologic outcomes.
Acknowledgements
We would like to thank Dr. Donna Ferriero for her thoughtful input and Jessica Kan for her contributions to data collection.
Funding: This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Numbers TL1TR000144 (SAO), K23NS066137 (HCG), KL2TR000143 (SLB), and Neonatal Brain Research Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thank you also to the CHIH Foundation Award in Neuroscience (SAO).
Footnotes
This study was performed at University of California, San Francisco and material was presented at the 2013 Pediatric Academic Societies Annual Meeting in Washington, D.C. and the 2013 National Clinical and Translational Sciences Predoctoral Programs Meeting in Rochester, MN.
Author Contributions:
Sharon A Orbach: Ms. Orbach contributed to study design, managed the collected data, conducted the initial analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
Michael Kuzniewicz: Dr. Kuzniewicz designed and managed the University of California, San Francisco database, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Sonia L Bonifacio: Dr. Bonifacio conceptualized the study, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Hannah C Glass: Dr. Glass conceptualized the study, contributed to study design and analysis, critically reviewed the manuscript, and approved the final manuscript as submitted.
Declaration of Conflicting Interests: There are no conflicts.
Ethical Approval: Data were collected with the approval of the University of California, San Francisco Committee on Human Research (approval numbers 10-03702 & 10-02694).
References
- 1.Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ. A population-based study of neonatal seizures in fayette county, kentucky. Neurology. 1995;45(4):724–732. doi: 10.1212/wnl.45.4.724. [DOI] [PubMed] [Google Scholar]
- 2.Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in newfoundland: A population-based study. J Pediatr. 1999;134(1):71–75. doi: 10.1016/s0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- 3.Saliba RM, Annegers JF, Waller DK, Tyson JE, Mizrahi EM. Incidence of neonatal seizures in harris county, texas, 1992–1994. Am J Epidemiol. 1999;150(7):763–769. doi: 10.1093/oxfordjournals.aje.a010079. [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: A population-based study, california 1998–2002. J Pediatr. 2009;154(1):24–28. e1. doi: 10.1016/j.jpeds.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwer MJ, de Vries LS, Pistorius L, Rademaker KJ, Groenendaal F, Benders MJ. Ultrasound measurements of the lateral ventricles in neonates: Why, how and when? A systematic review. Acta Paediatr. 2010;99(9):1298–1306. doi: 10.1111/j.1651-2227.2010.01830.x. [DOI] [PubMed] [Google Scholar]
- 6.Jensen FE. Neonatal seizures: An update on mechanisms and management. Clin Perinatol. 2009;36(4):881–900. vii. doi: 10.1016/j.clp.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: Randomized, controlled trial. Pediatrics. 2010;125(2):e358–e366. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 8.Garfinkle J, Shevell MI. Cerebral palsy, developmental delay, and epilepsy after neonatal seizures. Pediatr Neurol. 2011;44(2):88–96. doi: 10.1016/j.pediatrneurol.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Glass HC, Hong KJ, Rogers EE, et al. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res. 2011;70(5):535–540. doi: 10.1203/PDR.0b013e31822f24c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159(5):731–735. e1. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5(12):1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 12.Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36(4):901–14. vii–viii. doi: 10.1016/j.clp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Hoehn T, Hansmann G, Buhrer C, et al. Therapeutic hypothermia in neonates. review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation. 2008;78(1):7–12. doi: 10.1016/j.resuscitation.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Inder TE, Hunt RW, Morley CJ, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145(6):835–837. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: Efficacy outcomes. Pediatr Neurol. 2005;32(1):11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 17.Lin ZL, Yu HM, Lin J, Chen SQ, Liang ZQ, Zhang ZY. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: An experience from a single neonatal intensive care unit. J Perinatol. 2006;26(3):180–184. doi: 10.1038/sj.jp.7211412. [DOI] [PubMed] [Google Scholar]
- 18.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennet L, Dean JM, Wassink G, Gunn AJ. Differential effects of hypothermia on early and late epileptiform events after severe hypoxia in preterm fetal sheep. J Neurophysiol. 2007;97(1):572–578. doi: 10.1152/jn.00957.2006. [DOI] [PubMed] [Google Scholar]
- 21.Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53(1):65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liu PP, Li LY, Zhang HM, Li T. Hypothermia reduces brain edema, spontaneous recurrent seizure attack, and learning memory deficits in the kainic acid treated rats. CNS Neurosci Ther. 2011;17(5):271–280. doi: 10.1111/j.1755-5949.2010.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;4(4):CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Shah PS. Hypothermia: A systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15(5):238–246. doi: 10.1016/j.siny.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Harbert MJ, Tam EW, Glass HC, et al. Hypothermia is correlated with seizure absence in perinatal stroke. J Child Neurol. 2011;26(9):1126–1130. doi: 10.1177/0883073811408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low E, Boylan GB, Mathieson SR, et al. Cooling and seizure burden in term neonates: An observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F267–F272. doi: 10.1136/archdischild-2011-300716. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: Electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013 Feb 26; doi: 10.1016/j.jpeds.2013.01.041. pii: S0022-3476(13)00116-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341(7):485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 30.Bonifacio SL, Glass HC, Vanderpluym J, et al. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011;158(3):360–365. doi: 10.1016/j.jpeds.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37(12):1837–1844. doi: 10.1212/wnl.37.12.1837. [DOI] [PubMed] [Google Scholar]
- 32.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76(6):556–562. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpe J. Neurology of the newborn. 5th ed. Philadelphia: Elsevier; 2008. [Google Scholar]
- 35.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: An updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–566. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]