Abstract
Emerging evidence suggests that microRNA (miRNA)-mediated post-transcriptional gene regulation plays an essential role in modulating embryonic stem (ES) cell pluripotency maintenance, differentiation and reprogramming of somatic cells to ES cell like state. Investigations from ES cell enriched miRNAs, such as mouse miR-290 cluster and human miR-302 cluster, and ES cell depleted miRNAs such as let-7 family miRNAs, revealed a common theme that miRNAs target diverse cellular processes including cell cycle regulators, signalling pathway effectors, transcription factors, and epigenetic modifiers and shape their protein output. The combinatorial effects downstream of miRNA action allow miRNAs to modulate cell-fate decisions effectively. Furthermore, the transcription and biogenesis of miRNAs are also tightly regulated. Thus elucidating the interplays between miRNAs and other modes of gene regulation will shed new light on the biology of pluripotent stem cells and somatic cell reprogramming.
Keywords: microRNAs, post-transcriptional gene regulation, embryonic stem cells, pluripotency regulation, somatic cell reprogramming
Introduction
Embryonic stem (ES) cells are a unique cell type due to their capacity for indefinite self-renewal and their full developmental potential to generate all somatic cell types [1]. Conversely, fully differentiated somatic cells can be reverted to this ES cell-like pluripotency state by transduction of four ES-enriched transcription factors – Oct3/4, Sox2, Klf4, and c-Myc [2]. The remarkable differentiation potential of ES cells provides an invaluable route towards regenerative medicine. The advance in somatic cell reprogramming yields great promises towards personalized cell-based therapies, as the induced pluripotent stem (iPS) cells can eliminate immune rejection and bypass the ethical dilemmas of human ES cells.
The inter-conversions of developmentally primitive and mature cells present exciting cellular systems to investigate the molecular mechanisms governing cell-fate transitions. At the molecular level, these cell-fate decisions are controlled by coordinated gene regulation at multiple levels. To date, the best understood molecular regulation underlying cell-fate conversions includes transcriptional and epigenetic regulation. The transcription-factor network can direct cell-type specific gene expression; epigenetic remodelling at the DNA or chromatin level cooperates with the transcription network to define gene expression profiles during normal development and somatic cell reprogramming.
Another important mechanism of gene regulation governing the stem cell pluripotency occurs at the post-transcriptional level. Non-coding RNAs, particularly microRNAs (miRNAs), are important molecular players in post-transcriptional gene regulation during the maintenance or establishment of pluripotency. miRNAs are small regulatory RNAs ranging from 18–25 nucleotides in length. These small RNAs recognize their target mRNAs through imperfect complementarity, and subsequently repress target mRNA expression through a combined mechanism of translational repression and mRNA degradation [3]. The small size of miRNAs, combined with their imperfect target recognition, gives them enormous capacity and versatility to regulate many genes and pathways simultaneously, thus providing an important mechanism that underlies cell-fate transitions. In this review, we focus on miRNAs as an emerging class of pluripotency regulators. Current evidence suggests that miRNA-mediated gene regulation, together with transcriptional and epigenetic gene regulation, constitute a complex molecular network for the maintenance of ES cell pluripotency and the establishment of induced pluripotency.
The molecular basis of ES cell pluripotency
Mouse ES cells were first established from the inner cell mass (ICM) of mouse pre-implantation blastocysts [4,5]. Within a transient time window during embryonic development, the ICM undergoes rapid self-renewal. Upon implantation, cells within the ICM-derived epiblast start to differentiate, giving rise to the entire embryo proper that consists of three germ layers (ectoderm, mesoderm and endoderm) and the germ-cell lineage. This capability of individual cells within the ICM to develop into any cell type of a mature organism is termed pluripotency [6]. Interestingly, this developmentally transient pluripotent state in vivo can be maintained for an extended period in vitro. ES cells cultured in vitro, with the support of irradiated mouse embryonic fibroblasts (MEFs) and leukaemia inhibitory factor (LIF), can self-renew indefinitely, and possess differentiation potential comparable to that those of ICM in vivo [7]. When re-introduced to E3.5 host blastocysts, cultured ES cells can contribute to all somatic cell types, including germ cells [1]. Moreover, when aggregated with a tetraploid embryo, ES cells can give rise to an entire mouse that is viable and fertile, demonstrating their amazing developmental potential in vivo [8].
Considerable advances have been made in identifying the genes and pathways that support ES cell self-renewal and pluripotency. The LIF and Wnt signalling pathways constitute the essential components of the core pluripotency network, in which the two pathways act synergistically [9–11]. In addition, elimination of the autocrine Fgf4 signalling can further eliminate the exit from the pluripotency state, because Fgf4 activates the mitogen-activated protein kinase (MAPK) pathway that supports growth in differentiated cells [12]. Based on these findings, germline-competent ES cells can be maintained indefinitely in feeder- and serum-free “2i/LIF” culture condition. The “2i/LIF” represents the supplementation with LIF (activator of the JAK/STAT3 pathway) and two inhibitors, namely a glycogen synthase kinase-3 (Gsk3β) inhibitor (as a Wnt agonist) and a MEK inhibitor (as a suppressor of the MAPK pathway) [13]. Under the 2i/LIF culture conditions, the LIF and Wnt pathways are both activated to converge onto several key downstream transcription factors, including Pou5f1 (or Oct3/4), Nanog, and Sox2, that form the core positive feedback circuitry to maintain pluripotency [14]. Oct3/4, Nanog, and Sox2 contribute to pluripotency regulation through three main mechanisms: (i) they bind the their own promoters cooperatively to form an auto-regulatory loop that sustains a core transcription network; (ii) they bind to genes that are actively expressed in ES cells; (iii) they bind to genes that are silent in ES cells but remain poised for expression during differentiation [15]. Altogether, downstream of the external stimuli, the coordinated actions of these transcription factors, together with chromatin modifying factors, maintain the unique transcriptional and epigenetic state that constitutes the molecular basis for pluripotency and keeps these cells poised for differentiation upon changes of the environmental stimuli.
In the past few decades, protein-coding genes have constituted the major components of the molecular network that regulates stem cell self-renewal and pluripotency. However, it has become increasingly clear that non-coding RNAs, particularly miRNAs, are also integral components of the same molecular network for stemness and pluripotency. The close crosstalk between miRNAs and protein-coding genes is likely to underlie the molecular basis for the complex mode of gene regulation during stem cell self-renewal and differentiation.
microRNAs: Identification, biogenesis, and post-transcriptional gene silencing
miRNAs are a family of non-coding RNAs, 18–25 nucleotides in length, which are derived from larger precursor RNA molecules with stem-loop structures [3]. Since the discovery of the first miRNA in Caenorhabditis elegans [16,17], a large number of miRNAs have been identified, mainly through high-throughput sequencing effort in a wide range of tissues and organisms [18].
Three paths to generate mature miRNAs
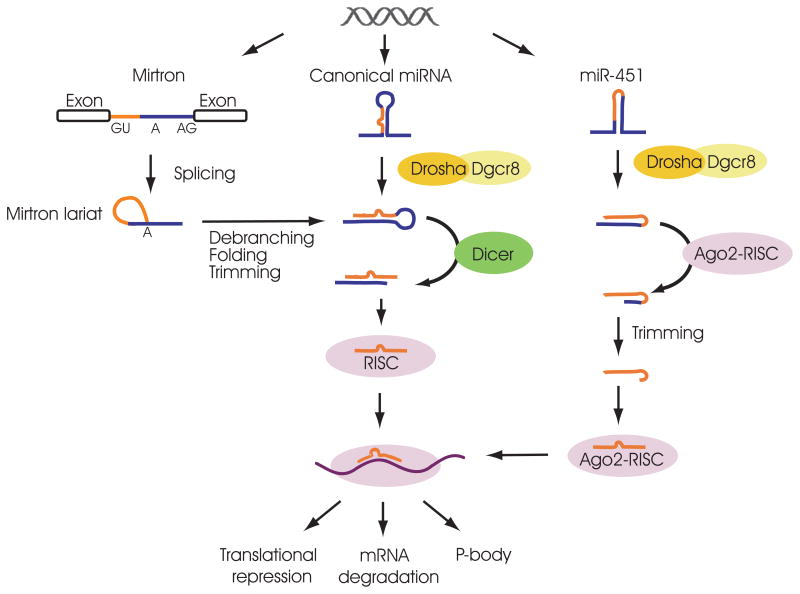
miRNAs can reside in introns and exons of protein-coding and non-coding RNA genes, or reside as independent non-coding gene loci [19]. Some miRNA genes contain a single stem-loop hairpin structure, while others are transcribed as polycistronic miRNA clusters. Most miRNA precursors, the pri-miRNAs, are transcribed by RNA polymerase II, and are subsequently processed to yield mature miRNA duplexes. Canonical miRNA biogenesis starts with the processing of pri-miRNAs into pre-miRNAs by a nuclear ribonuclease (RNase) III-like enzyme (Drosha) in a sequence-independent manner. The specificity of Drosha cleavage is guided by its partner Dgcr8, an RNA-binding protein that acts as a “molecular ruler” to direct Drosha-mediated cleavage. Dgcr8 “measures” approximately 11 bp from the base of the double-stranded RNA stem structure of the pri-miRNA, and anchors Drosha to cleave specifically at that position [20]. After initial cleavage by Drosha in the nucleus, pre-miRNAs are transported into the cytoplasm by exportin-5 (Exp5), a Ran-GTP-dependent transporter [21], and subsequently cleaved by Dicer, another RNase-III enzyme, to generate mature miRNA duplexes. Dicer exhibits little sequence specificity in its cleavage, and cuts ~22 bp away from the Drosha cleavage site in pre-miRNAs. The resulting miRNA duplex contains both the mature miRNA strand and the miRNA* strand. The RNA-binding protein TRBP recruits the Dicer-miRNA complex to the Argonaute (Ago) proteins to form the RNA-induced silencing complex (RISC), which subsequently mediate the post-transcriptional gene silencing of a variety of partially complementary mRNA targets [22] (Figure 1).
Figure 1. miRNA biogenesis pathways.
Central branch, canonical miRNA processing pathway. Left branch, the mirtron processing pathway which is Drosha-independent and Dicer-dependent. Right branch, miR-451 processing pathway which is Drosha-dependent but Dicer independent. Ago2 cleaves the pre-miR-451 to give rise to the mature miR-451.
Two alternative miRNA biogenesis pathways have also been identified, although such mechanisms only regulate the biogenesis of a small subset of miRNAs (Figure 1). Mirtrons are a class of intronic miRNAs that can be processed into pre-miRNA through a distinct splicing mechanism, independent of Drosha-mediated cleavage [23,24]. The conventional mirtron loci generate pre-miRNAs that begin and end precisely with the splice donor and splice acceptor sites. For such mirtron genes, the splicing reaction alone defines both the 5′ and 3′ end of the pre-miRNAs, which are subsequently cleaved by Dicer [24–26]. In contrast, other mirtron genes, after being spliced, yield a fragment containing either a 5′ or 3′ tail to the pre-miRNA, which needs further trimming by exonucleases before processed by Dicer [26,27]. Interestingly, there exists yet another subset of miRNAs, represented by miR-451, whose maturation is dependent on Drosha, but not on Dicer. In fish and mouse, the pre-miR-451 generated by Drosha cleavage is directly loaded into the RISC complex without Dicer processing. Subsequently, Ago2 cleaves such pre-miRNAs to generate mature miRNAs with divergent 3′ ends, possibly due to differential trimming by exonucleolytic digestion [28,29].
miRNAs functions to dampen and refine the level of protein outputs
Mature miRNAs typically recognize their mRNA targets via imperfect sequence complementarily and downregulate the target mRNA expression through a combined mechanism of translational inhibition and mRNA degradation [3,18,19]. The small size of miRNAs, combined with their imperfect target recognition mechanism, provides them enormous capacity and versatility to regulate gene expression globally. Thus on the whole, the expression profiles of miRNAs in different cell types at different times of development can shape the protein output from the transcriptome unique to the cell type and developmental stage. miRNA expression profiles can also shape their targets during evolution. For example, the 3′UTR sequences and lengths of certain genes can be evolved to adopt or avoid the miRNA recognition sites, depending the miRNA-mediated regulation is beneficial or detrimental [30].
The proposed models for miRNA’s action include: (i) binary off-switch; (ii) tuning interaction and (iii) neutral interaction [3]. In binary off-switch model, the induction of miRNA expression dampens its target protein output to a level which does not have a functional output. In tuning interaction model, miRNA acts to dampen the target protein output to a refined range and the level of which may be important to the target functions. In the neutral interaction model, miRNA-mediated repression does not give rise to functional consequences by damping the target protein level. The details of these models of miRNA action have been detailed elsewhere [3].
miRNAs act as part of the pluripotency regulation network
There is a growing body of evidence suggesting that miRNAs play an important role in regulating the self-renewal and developmental potential of pluripotent stem cells. As previously described, ES cell self-renewal requires means of eliminating differentiation signals and acquiring signals to enforce the pluripotency state, whereas differentiation involves the means of shutting down the pluripotency programs and induction of lineage specification. Based on the modes of action of miRNAs, it is tempting to postulate that pluripotency-promoting miRNAs functions to dampen the differentiation signals during self-renewal, whereby re-enforcing the pluripotency state set by the pluripotency transcription factor and epigenetic regulation networks. Conversely, differentiation-promoting miRNAs functions to repress pluripotency regulation network, and enhancing differentiation and lineage specification. These miRNAs actions can be combinatorial or mutually exclusive, and the final outcome can be synergistic effect or a balancing act. Thus, miRNA-mediated pluripotency regulation adds an additional layer control over the transcriptional and epigenetic regulation by providing switch or tuning mechanisms to enforce the cell-fate decisions determined by transcriptional and epigenetic regulation network in response to the external stimuli. Previously, the complex molecular networks regulating stem cell pluripotency have been viewed solely from a protein-centric perspective. Thus, understanding the miRNA function and regulation in the maintenance and establishment of pluripotency is expected to provide novel insights.
microRNAs and ES cell biology
The initial recognition that miRNAs play important roles in ES cell biology comes from the functional characterization of Dicer- and Dgcr8-deficient mouse ES cells [31–33]. Loss-of-function of Dicer or Dgcr8 causes deficiency in the canonical miRNA biogenesis pathway, resulting in global depletion of the majority of miRNAs. The Dicer−/− and Dgcr8−/− ES cells share two phenotypes: (i) reduction in proliferation due to G1 cell-cycle arrest, and (ii) resistance to differentiation. During the formation of embryoid body (EB) or retinoic acid-induced differentiation, Dicer- or Dgcr8-deficient ES cells show sustained production of pluripotency markers, including Oct3/4, Nanog, and Rex1, whereas wild-type ES cells rapidly lose the expression of these markers. Consistently, defects in differentiation potential of Dicer-deficient ES cells are also observed in vivo, since these ES cells fail to generate differentiated teratomas or to contribute to chimeric mice [31]. Interestingly, Dicer−/− ES cells also show differences from Dgcr8−/− ES cells in that they exhibit unstable epigenetic silencing of centromeric repeat sequences. These Dicer-specific phenotypes could be attributed to Drosha-independent small RNAs, including the endogenous siRNAs and non-canonical miRNAs [26].
Given these observations, which miRNAs are functionally important to support ES cell-specific cell-cycle progression and differentiation potential? To address this question, one can introduce specific mature miRNA mimics into Dicer−/− or Dgcr8−/− ES cells to assay for functional rescue. Members of the miR-290 cluster and the miR-302 cluster were considered prime candidates, since they are highly enriched in ES cells, and share a specific seed sequence (AAGUGCU) (Figure 2). Indeed, re-introduction of these ES cell-specific cell cycle-regulating (ESCC) miRNAs reverses many of the defects observed in the Dicer−/− and Dgcr8−/− ES cells [34,35].
Figure 2. Several miRNAs from different clusters and families possess the same seed sequence.
These miRNAs have been termed ES cell-specific cell-cycle (ESCC) miRNAs. These miRNAs may possess similar functions. The miR-17-92, miR-106b-25, and miR-106a-363 clusters are homologues.
miR-290 cluster miRNAs as promoters of pluripotency
The miR-290 cluster is the most highly expressed miRNA cluster in mouse ES cells, contributing to over 60 %–70 % of total miRNAs expressed [36,37]. miRNAs encoded by this cluster directly target the cell-cycle inhibitors p21 and LATS2, both of which normally repress the G1-S phase transition of somatic cells [34]. The high level of the miR-290 miRNAs promotes cell-cycle transition at the G1-S phase, and possibly constitutes the molecular basis for the unusually short G1 phase cell cycle in ES cells [34]. In addition to its role in promoting cell proliferation, the miR-290 cluster also ensures the differentiation potential of ES cells by promoting DNA methylation at specific genomic loci during differentiation [35]. miR-290 miRNAs prevent the expression of Rbl2, which acts as a transcriptional repressor of de novo DNA methyltransferases [35]. Defective de novo DNA methylation during Dicer−/− ES cell differentiation, particularly at the Oct3/4 locus, is at least partially due to the loss-of-function of miR-290 [35]. This deficiency is a likely explanation for the resistance to differentiation observed in Dicer−/− or Dgcr8−/− ES cells. Finally, miR-290 miRNAs also repress the Wnt pathway inhibitor, Dkk-1, thus preventing ES cell differentiation to mesoderm and germ cell in vitro [38]. Consistent with their functional importance in ES cells, the miR-290 miRNAs also play important roles during normal embryonic development, as demonstrated by the partial lethality phenotype in miR-290−/− embryos [39].
Let-7 miRNAs act as suppressors of pluripotency, opposing the effects of miR-290 cluster
In contrast to miR-290 miRNAs, let-7 miRNAs are specifically depleted in ES cells, and the accumulation of let-7 miRNAs is a strong inducer of differentiation. Indeed, the miR-290 miRNAs functionally antagonize the let-7 family miRNAs, mostly by indirectly regulating their biogenesis. Through an as-yet unknown mechanism, miR-290 miRNAs function to maintain the expression of Lin28, an RNA-binding protein that blocks the maturation of let-7 (detailed in the next section) [40]. Upon differentiation induction, the gradual loss of the pluripotency transcriptional network results in decreased miR-290 expression and consequently, down-regulation of Lin28. As a results, let-7 maturation is restored, and increased let-7 expression further enhances differentiation by repressing pluripotency factors that would typically increase the expression of miR-290 [40] (Figure 3). In addition to their mutually exclusive expression pattern, the miR-290 and let-7 family miRNAs have opposing effects in ES cell self-renewal and pluripotency. miR-290 members promote G1-S transition by suppressing CDK inhibitors [34], while let-7 represses this transition by down-regulating Cdk4 and CyclinD [41] (Figure 3). In addition, let-7 directly targets several ES cell-enriched genes, including those encoding N-myc, C-myc, Sal4, and Lin28 [40,42]. These genes are also indirectly upregulated by the miR-290 cluster though an unknown mechanism. Thus, the mutually exclusive expression pattern and opposing functions of the miR-290 and let-7 miRNAs form a feedback regulatory circuitry, providing a switch mechanism facilitating the molecular decision between self-renewal and differentiation [40].
Figure 3. ESCC miRNAs and let-7 miRNAs have opposing functions in regulating ES cell pluripotency and differentiation.
The opposing effects of these two classes of miRNAs are mediated by regulation of the expression of cell-cycle progression and pluripotency genes. The mutually exclusive expression pattern of ESCC miRNAs and let-7 miRNAs are bridged by Lin28-mediated biogenesis regulation of let-7. In the pluripotent ES cell state, the core pluripotent factors induce the expression of ESCC miRNAs. ESCC miRNAs promote cell proliferation by enhancing G1 to S phase transition. At the same time, elevated levels of Lin28 block the maturation of let-7 miRNAs. In contrast, differentiated cells do not express ESCC miRNAs, and let-7 miRNA levels are highly elevated; consequently, the G1 to S transition is suppressed. At the same time, let-7 miRNAs also down-regulate pluripotency genes, thus pushing cells towards differentiation. let-7 also suppresses Lin28 production, a negative feedback loop that serves to reinforce the switch.
Regulation of miRNA expression in embryonic stem cells at transcriptional and post-transcriptional levels
Given the importance of the miRNA functions in pluripotent stem cells, it is increasingly clear that the regulation of miRNA expression constitutes a key molecular mechanism to sustain self-renewal and differentiation potential. Such regulation can occur at different steps during miRNA biogenesis and degradation, including miRNA transcription, miRNA maturation, and miRNA stability.
Transcriptional regulation of miRNAs determines the spatio-temporal expression of miRNAs during the self-renewal and differentiation of pluripotent stem cells. The core pluripotency transcription factors, such as Oct3/4, Nanog, and Sox2, bind to the promoter of the miR-290 cluster, inducing and maintaining cluster expression in self-renewing pluripotent stem cells [36]. On the other hand, miRNAs that promote ES cell differentiation, including miR-21 and miR-145, are transcriptionally suppressed in the self-renewal state. miR-21 is strongly induced during ES cell differentiation [36,37] and acts to suppress production of the key pluripotency factors Oct3/4, Nanog, Sox2, and c-Myc [43]. Similarly, miR-145 is induced during human ES cell differentiation and mediates repression of OCT3/4, SOX2, and KLF4. On the other hand, miR-145 promoter is bound by OCT3/4 to mediate its transcriptional repression. Thus, pluripotency factors and miR-145 form a double-negative feedback loop, reinforcing the decisions between differentiation and pluripotency [44].
Increasing evidence also suggests the regulation of miRNA biogenesis is deeply integrated in the molecular regulation of stem cell pluripotency. Such regulation can occur at the level of Drosha processing and/or Dicer processing. The best characterized example is the repression of let-7 biogenesis in ES cells. Mature let-7 miRNAs are potently induced during ES cell differentiation to promote the exit from the pluripotency state [36]. Interestingly, despite the lack of detectable mature let-7 miRNAs in ES cells, pri-let-7 miRNAs are expressed at a constant level in self-renewing and differentiating mouse ES cells, suggesting the existence of a block to let-7 maturation in self-renewing ES cells [45]. This observation is recapitulated in vivo, as mature let-7 miRNAs remain undetectable until E12.5 of mouse gestation [45]. Using pre-let-7g as a bait, RNA pull-down experiments in embryonic carcinoma (EC) cell extracts identified the RNA-binding protein Lin28 as the inhibitor of let-7 maturation [46]. Lin28 is highly expressed in ES cells, indicating that the suppression of let-7 biogenesis is essential for maintaining self-renewal and promoting pluripotency.
Lin28 has two paralogs in the mammalian genome, Lin28a and Lin28b, both of which bind let-7 miRNAs through conserved motifs within their terminal loops. However, Lin28a and Lin28b inhibit let-7 biogenesis through different molecular mechanisms at different subcellular locations [47]. Specifically, Lin28a blocks let-7 biogenesis in the cytoplasm by recruiting the TUT4 terminal uridylyl transferase (TUTase) to the pre-let-7 precursors [48–50]. TUT4 then catalyses the polyuridylation of pre-let-7 and subjects the modified pre-let-7 to degradation [49]. Additionally, Lin28a also binds to pre-let-7 miRNAs, and inhibits Dicer processing by inducing a conformational change within pre-let-7 that unwinds a double-stranded region at the Dicer processing site [51]. In contrast, Lin28b inhibits let-7 processing at the Drosha processing step in the nucleus. Lin28b sequesters primary let-7 transcripts in a TUT4-independent manner to inhibit pri-let7 processing by the Drosha/Dgcr8 microprocessor [47]. In addition to Lin28, another RNA binding protein, Musashi1 (Msi1), is also highly expressed in ES cells and regulates the biogenesis of the let-7 family miRNAs. On one hand, Msi1 enhances the nuclear localization of Lin28 to suppress let-7 processing by Drosha; on the other hand, Msi1 directly inhibits the nuclear cropping of miR-98, a let-7 homologue [52].
In the cytoplasm, miRNA maturation can also be modulated at the Dicer-processing step. For example, KH-type splicing regulatory protein (KSRP) is a Dicer-processing activator, promoting the maturation of a subset of miRNAs [53]. KSRP is speculated to optimize positioning and recruitment of proteins that enhance Dicer processing [53]. KSRP binds to the loop of a cohort of miRNAs containing G-rich stretches in the terminal loop, including pre-let-7a-1. It remains to be determined whether dicer-processing modulators, such as KSRP, play an important role in ES cell self-renewal and pluripotency. Taken together, miRNA biogenesis regulation offers a critical control for cell-fate decisions.
miRNA functions in induced pluripotency
Somatic cells can be reprogrammed to ES-cell like pluripotent states via somatic nuclear transfer to enucleated oocytes, or cell fusion between somatic cells and pluripotent ES cells [14]. The success of such reprogramming approaches indicates the existence of cytoplasmic factors in oocytes or ES cells which can revert the differentiated somatic nuclei to pluripotency. In fact, reprogramming of somatic cells into pluripotent stem cells can be achieved by ectopically expressing only four ES cell-enriched transcription factors, namely Oct3/4, Sox2, Klf4, and C-myc [2]. In addition to these four “classic” reprogramming factors, recent studies have identified other ES cell-enriched protein-coding genes that either enhanced reprogramming efficiency or improved the iPSC pluripotency [54–57]. Despite these exciting breakthroughs, reprogramming still occurs with low efficiency and compromised pluripotency using current technologies, suggesting the existence of road blocks during somatic reprogramming.
The routes to pluripotency
Elucidating the mechanism of induced pluripotency has been one of the major challenges for the stem cell community. The reprogramming process is postulated to occur in two phases [58]. The early phase involves the reprogramming of somatic cells to a pre-pluripotent state with enhanced proliferation rate and a conversion towards an epithelial-like cellular state. The p53-induced cell-cycle block [59–62] and Tgf-β promoted epithelial-to-mechysemal transition (EMT) [58,63,64] constitute the two major road blocks during this phase. The subsequent phase is the establishment of pluripotency from the pre-pluripotent state by inducing pluripotency genes, such as Nanog, and stabilizing the core pluripotency network [58,65]. The fully reprogrammed iPS cells no longer depend on the exogenous reprogramming factors to maintain their self-renewal and pluripotency. Reducing the barriers for the first phase often results in a rapid kinetics and an increased efficiency of iPSC production, whereas overcoming the barriers in the second phase lead to the generation of authentic iPSCs that functionally resemble more to the embryo-derived ES cells (Figure 4).
Figure 4. Roles of miRNAs in enhancing or suppressing somatic reprogramming.
ESCC miRNAs can enhance the reprogramming process by promoting cell-cycle progression as well as by suppressing the Tgfβ signalling, thus promoting MET. These events occur at the early stage of reprogramming. miR-200b and miR-200c, acting downstream of BMP signalling, can also promote MET synergistically with the reprogramming factors. Let-7 inhibition promotes reprogramming, possibly through the de-repression of cell-cycle progression and pluripotency gene expression. miR-34 family miRNAs act as a barrier to reprogramming by suppressing expression of pluripotency gene, including those encoding Nanog, Sox2, and N-myc. miR-34 family members are direct targets of p53; thus these miRNAs may also pose a barrier in promoting somatic cell survival and proliferation. miRNAs marked in red are enhancers of reprogramming, whereas miRNAs in blue are suppressors of reprogramming.
Pluripotency-promoting miRNAs enhances reprogramming
Given the functional importance of miRNAs in ES cell biology, it is not surprising that miRNAs also act to modulate reprogramming barriers to promote or suppress this process (Table 1, Figure 4). During major cell-fate conversions, re-structuring the existing mRNA expression profile at post-transcriptional level is as important as re-establishing the new transcriptome. Thus, miRNA-mediated post-transcriptional gene regulation acts coordinately with transcriptional regulation and epigenetic regulation to shape gene expression profiles during somatic reprogramming.
Table 1.
miRNAs that promote or suppress somatic reprogramming.
| miRNAs | Cluster or family | ES-cell expression | Roles in reprogramming | Mechanism of action | Species | Ref |
|---|---|---|---|---|---|---|
| mmu-miR-291-3p, 294 and 295 | mmu-miR-290~295 | Yes | Enhancer | Indirectly activate pluripotent factors. Promote G1-S transition | M,H | [66] |
| hsa-miR-302b, hsa-miR-372 | hsa-miR-302~367; hsa-miR-371~373 | Yes | Enhancer | Suppress inhibitors of G1-S transition and Tgf-β signalling induced EMT | M,H | [67] |
| miR-200b,c, miR-205 | miR-200, miR-205 | Yes | Enhancer | Suppress Tgf-β signalling induced EMT | M | [58] |
| miR-93, miR-106 | miR-106b~25 and miR-106a~363 | Yes | Enhancer | Suppress inhibitors of G1-S transition and Tgf-β signalling induced EMT | M | [68] |
| miR-34a,b,c | miR-34 | miR-34a: Yes | Suppressor | Suppress Nanog, Sox2 and N-myc | M | [75] |
| Let-7c | Let-7 | No | Suppressor | Suppress G1-S transition and pluripotent factors | M | [40] |
| miR200c+mir302+mir369 | miR-302~367, miR-200 and miR-154 | Yes | Direct reprogramming | Yet to be determined | M,H | [73] |
| mmu-miR-302a, b,c,d, 367 | mmu-miR-302~367 | Yes | Direct reprogramming | Yet to be determined | M,H | [72] |
M, mouse; H, human.
The ESCC miRNAs, which share a common seed sequence, comprise a number of polycistronic miRNA clusters including miR-290-295 (and its human homologue miR-371-373) [66,67], miR-302a-367 [67], and miR-17-92 (and its homologue miR-106b-25 and miR-106a-363) [68] (Figure 2). The introduction of the four classic reprogramming factors into mouse embryonic fibroblasts (MEFs) directly induces the expression of the ESCC miRNAs [68]. These ESCC miRNAs suppress cell-cycle inhibitors to accelerate the G1-S transition during cell-cycle progression [34,68]. In addition, these ESCC miRNAs repress Tgfβ signalling to promote mesenchymal to epithelial transition (MET) by directly targeting the Tgfβ type II receptor, TgfβR2 [67,68]. Not only do the ESCC miRNAs act to remove the major road blocks during the early phase of reprogramming, these miRNAs also enhance reprogramming at the later stage. For example, miR-302 miRNAs suppress epigenetic regulators AOF2, AOF1, MECP1-p66, and MECP2 to trigger global demethylation [69]; such demethylation serves as a prerequisite for establishing pluripotency. Additionally, ESCC miRNAs may promote the re-activation of endogenous pluripotency factors, such as OCT3/4, by repressing NR2F2, a transcriptional repressor of OCT3/4 [70]. Due to their ability to regulate multiple targets, which are effectors of different pathways, these ESCC miRNAs can modulate the reprogramming process in a co-ordinated fashion.
Besides ESCC miRNAs, several other miRNAs, including miR-200b, miR-200c, miR-141, and miR-205, also have been shown to promote the early phase of reprogramming by suppressing Tgfβ signalling to induce MET [58]. Upon the introduction of the reprogramming factors, cell-intrinsic BMP signalling is activated, which induces the expression of miR-205 and miR-200 family miRNAs to enhance MET [58]. BMP signalling also functions to promote ES cell self-renewal by acting as a potent blocker of differentiation [71]; it remains to be determined if BMP signalling is also mediated through miRNAs.
Given the importance of these pluripotency-promoting miRNAs in somatic reprogramming, several groups have taken a step further to explore the possibility of using miRNAs alone to achieve somatic reprogramming. Stable lentiviral delivery of miR-302/367 miRNAs can generate iPSCs without any protein-encoding reprogramming factors. Surprisingly, the efficiency of reprogramming was over 100-fold higher than the “classic” reprogramming method that used retroviral infection to introduce genes encoding the four standard transcription factors [72]. Another study demonstrated successful somatic reprogramming by serial transfection with miR-200c, miR-302, and miR-369 miRNA mimics [73]. Debates still exist as to whether miRNAs alone can replace protein-coding reprogramming factors entirely. Nonetheless, it is clear that selected miRNAs can enhance reprogramming efficiency in combination with the four classic reprogramming factors through as-yet partly defined mechanism(s).
Suppression of differentiation-promoting miRNAs enhances reprogramming
Consistent with the opposing effects of the ESCC miRNAs and let-7 miRNAs in regulating ES cell pluripotency and differentiation, let-7 miRNAs inhibit somatic reprogramming by repressing important cell proliferation genes and pluripotency genes [40]. Interestingly, the negative regulator of let-7 biogenesis, Lin28, is a potent reprogramming factor. It is among the very few reprogramming factors that is not a transcription factor itself [74]. Thus, let-7 miRNAs constitute a major barrier for somatic reprogramming to maintain a differentiated cell fate (Figure 4)
Another important miRNA barrier for somatic reprogramming is a component in the p53 tumor suppressor pathway (Figure 4). Members of the miR-34 family, including miR-34a, b, and c, are direct transcriptional targets of p53. These p53-regulated miRNAs, together with the canonical p53 target p21, are essential mediators to repress reprogramming downstream of p53 [75]. MEFs deficient in miR-34a, in particular, exhibit an increased efficiency and kinetics during reprogramming. Unlike p53 deficiency, which enhances reprogramming at the expense of iPSC pluripotency, genetic ablation of miR-34a promoted iPSC generation without compromising self-renewal or differentiation [75]. The suppression of reprogramming by miR-34a is, at least in part, attributed to its repression of the pluripotency factors Nanog, Sox2, and N-myc. Thus, miR-34 miRNAs act downstream of p53 to suppress the establishment of pluripotency at the later stage of reprogramming. Interestingly, miR-34a is highly expressed in ES cells [36]. It is plausible that miR-34a acts in ES cells to fine-tune the dosage of pluripotency factors, enabling ES cells to maintain pluripotency and to be primed to differentiate upon appropriate induction. The well-known p53 target, p21, also represses the efficiency of iPSC generation, yet p21 exerts this effect mostly through negative regulation of cell proliferation. Taken together, the two branches of the p53 effector pathway, p53/p21 and p53/miR-34, can act synergistically, providing separate barriers at different steps of somatic reprogramming (Figure 4).
Future studies and challenges
Despite the demonstrated importance of miRNAs in regulating pluripotency, there are several challenges to elucidate their underlying mechanisms. First, regulation of pluripotency has been shown to be dose-sensitive. For example, the correct level of Oct3/4 expression is important in maintenance of ES cell pluripotency. A 50 % increase in Oct4 levels causes cells to commit to trophectoderm fate; a 50 % reduction causes cells to differentiate into mesendoderm lineages [76]. miRNA-mediated regulatory effects could be a powerful mechanism to fine-tune the final gene products to a specific dosage [3]. However, when using mimics or antagomirs to overexpress or down-regulate miRNAs, the level of target protein may vary depending on the level of mimics or antagomirs in individual cells. The output of these genetic manipulations could vary significantly within individual experiments, thus complicating the interpretation of observed phenotypes.
Secondly, the identification of the miRNA-regulated direct targets and pathways is challenging. miRNA-mediated gene repression is inherently complex. The seed sequences that miRNAs use for target recognition are only 6–8 bases long, and miRNA target recognition is imperfect. Thus, one miRNA can regulate the expression of many targets in a given cellular context, and the target sets can differ in different cell types and cellular contexts. Although the extent of miRNA-mediated gene repression tends to be moderate at the level of each individual target, several components of a single pathway could be targeted by one miRNA simultaneously, thus creating a potent cumulative effect on the functional readout of the pathway itself. Based on these observations, elucidating the molecular mechanisms downstream of important miRNA enhancers or repressors of somatic reprogramming will remain a challenge in the near future.
Finally, despite similarities between the murine and human pathways, mouse ES cells and human ES cells exhibit notable differences in the molecular regulation of pluripotency. Human ES cells resemble mouse epiblast stem cells (EpiSCs) more than mouse ES cells. EpiSCs are derived from E5.5 mouse post-implantation epiblasts [77,78], which differ from mouse ES cells in terms of growth dependency and genetic and epigenetic profiles [13,77,78]. Thus, the miRNA network in the mouse and human ES cells may overlap only partially. For example, miR-290 and miR-302 orthologues are expressed differently in mouse and human ES cells: members of the miR-290 cluster are the most highly expressed miRNAs in mouse ES cells [36], while members of the miR-302 cluster are the most enriched miRNAs in human ES cells [79]. It is thus important to determine if miR-290 and miR-302, despite their identical seed, contribute to the functional difference between mouse and human ES cells by targeting overlapping yet distinct sets of mRNA targets.
Beyond miRNAs, emerging evidence suggests that other classes of non-coding RNAs may also play a role in regulating ES-cell pluripotency and differentiation. Genome-wide approaches have identified hundreds of large intervening non-coding RNAs (lincRNAs) which are expressed in mouse ES cells [80]. Systematic loss-of-function studies of these lincRNAs seem to produce global gene expression alterations comparable to those resulting from well-known regulators of ES cell pluripotency and differentiation [81]. Thus, these lincRNAs could offer novel mechanisms to regulate stem cell pluripotency.
Non-coding RNAs could also affect the process of somatic reprogramming. In one study, the expression level of multiple maternally imprinted non-coding RNAs residing in the mouse imprinted locus, Dlk1–Dio3, correlates with the authenticity of iPS cells [82]. iPS cells in which this locus has been aberrantly silenced display impeded in vivo developmental potential. Interestingly, another recent study suggested that the predictive value of the Dlk1–Dio3 for iPSC pluripotency is dependent on the reprogramming methodology [83]. Although it is yet to be determined whether these non-coding RNAs in the Dlk1–Dio3 locus are causal to the full-developmental capability, further characterization of these non-coding RNAs will be an exciting field of exploration in stem cell biology in the near future.
Conclusion and outlook
Over the past three decades, we have gained a tremendous amount of insight into the molecular mechanisms regulating the self-renewal, pluripotency, and differentiation of vertebrate cells. These insights also have guided us in achieving reprogramming from developmentally more mature cellular states to pluripotency. Non-coding RNA regulation in stem-cell pluripotency is a relatively young field. Yet the function of a family of non-coding RNA, miRNA, has emerged as an integral part of the intertwined network of gene regulation during stem cell self-renewal and differentiation. As described in this review, miR-290 clusters and other ESCC miRNAs function to promote self-renewal and somatic cell reprogramming; let-7 miRNAs and p53-regulated miRNAs, such as miR-34 family, suppress pluripotency and self-renewal and act as road-blocks during pluripotency induction. Moreover, these miRNAs act as a functional network, such as the opposing effect between miR-290 cluster and let-7 family as described, and the result of which further shape and refine the transcriptional and epigenetic regulation during differentiation. Future investigations into the molecular basis of non-coding RNA function in pluripotent stem cells will offer novel insights into the complex mode of gene regulation required for the maintenance and establishment of pluripotency.
Acknowledgments
We would like to thank the members of the He lab for helpful discussion. M.A.L. is supported by Siebel Stem Cell Postdoctoral Fellowship. L.H. is supported in part by a RO1 grant from National Cancer Institute, and by a new faculty award from the California Institute of Retentive Medicine.
References
- 1.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–6. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 5.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith A. A glossary for stem-cell biology. Nature. 2006;441:1060. [Google Scholar]
- 7.Smith AG, Heath JK, Donaldson DD, Wong GG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–90. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 8.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, et al. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwa H, Burdon T, Chambers I, Smith a. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes & Development. 1998;12:2048–60. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–22. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, et al. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochemical and biophysical research communications. 2006;343:159–66. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 12.Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development (Cambridge, England) 2007;134:2895–902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 13.Ying Q-L, Wray J, Nichols J, Batlle-Morera L, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–23. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–82. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer La, Lee TI, Cole MF, Johnstone SE, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–16. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- 17.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 18.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews. Genetics. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 19.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nature reviews. Molecular cell biology. 2009;10:126–39. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Lee Y, Yeom K-H, Nam J-W, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Lund E, Güttinger S, Calado A, Dahlberg JE, et al. Nuclear export of microRNA precursors. Science (New York, NY ) 2004;303:95–8. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 22.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–4. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamura K, Hagen JW, Duan H, Tyler DM, et al. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448:83–6. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamura K, Hagen JW, Duan H, Tyler DM, et al. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babiarz JE, Ruby JG, Wang Y, Bartel DP, et al. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes & development. 2008;22:2773–85. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flynt AS, Greimann JC, Chung WJ, Lima CD, et al. MicroRNA biogenesis via splicing and exosome-mediated trimming in Drosophila. Mol Cell. 2010;38:900–7. doi: 10.1016/j.molcel.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheloufi S, Dos Santos CO, Chong MMW, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–9. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cifuentes D, Xue H, Taylor DW, Patnode H, et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science (New York, NY ) 2010;328:1694–8. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayr C, Bartel DP. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–84. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanellopoulou C, Muljo Sa, Kung AL, Ganesan S, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes & development. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murchison EP, Partridge JF, Tam OH, Cheloufi S, et al. Characterization of Dicer-deficient murine embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12135–40. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Medvid R, Melton C, Jaenisch R, et al. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nature genetics. 2007;39:380–5. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Baskerville S, Shenoy A, Babiarz JE, et al. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nature genetics. 2008;40:1478–83. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, et al. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nature structural & molecular biology. 2008;15:259–67. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 36.Marson A, Levine SS, Cole MF, Frampton GM, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–33. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Developmental cell. 2003;5:351–8. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 38.Zovoilis A, Smorag L, Pantazi A, Engel W. Members of the miR-290 cluster modulate in vitro differentiation of mouse embryonic stem cells. Differentiation; research in biological diversity. 2009;78:69–78. doi: 10.1016/j.diff.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros La, Dennis LM, Gill ME, Houbaviy H, et al. Mir-290-295 deficiency in mice results in partially penetrant embryonic lethality and germ cell defects. Proceedings of the National Academy of Sciences. 2011;108:1–6. doi: 10.1073/pnas.1111241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–6. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz J, Lorenz P, Gross G, Ibrahim S, et al. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell research. 2008;18:549–57. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 42.Zhong X, Li N, Liang S, Huang Q, et al. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. The Journal of biological chemistry. 2010;285:41961–71. doi: 10.1074/jbc.M110.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh SK, Kagalwala MN, Parker-Thornburg J, Adams H, et al. REST maintains self-renewal and pluripotency of embryonic stem cells. Nature. 2008;453:223–7. doi: 10.1038/nature06863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, et al. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 45.Thomson JM, Newman M, Parker JS, Morin-kensicki EM, et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes & Development. 2006:2202–7. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science (New York, NY ) 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piskounova E, Polytarchou C, Thornton James E, LaPierre Robert J, et al. Lin28A and Lin28B Inhibit let-7 MicroRNA Biogenesis by Distinct Mechanisms. Cell. 2011;147:1066–79. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heo I, Joo C, Cho J, Ha M, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Molecular cell. 2008;32:276–84. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Heo I, Joo C, Kim Y-K, Ha M, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138:696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–49. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rybak A, Fuchs H, Smirnova L, Brandt C, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–93. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 52.Kawahara H, Okada Y, Imai T, Iwanami A, et al. Musashi1 cooperates in abnormal cell lineage protein 28 (Lin28)-mediated let-7 family microRNA biogenesis in early neural differentiation. The Journal of biological chemistry. 2011;286:16121–30. doi: 10.1074/jbc.M110.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010–4. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng B, Jiang J, Kraus P, Ng J-H, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nature cell biology. 2009;11:197–203. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 55.Han J, Yuan P, Yang H, Zhang J, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heng J-CD, Feng B, Han J, Jiang J, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell stem cell. 2010;6:167–74. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, et al. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–9. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 58.Samavarchi-Tehrani P, Golipour A, David L, Sung H-K, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell stem cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Hong H, Takahashi K, Ichisaka T, Aoi T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawamura T, Suzuki J, Wang YV, Menendez S, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–4. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Collado M, Villasante A, Strati K, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–9. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Utikal J, Polo JM, Stadtfeld M, Maherali N, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–8. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li R, Liang J, Ni S, Zhou T, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell stem cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Maherali N, Hochedlinger K. Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Current biology : CB. 2009;19:1718–23. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Theunissen TW, van Oosten AL, Castelo-Branco G, Hall J, et al. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol. 2011;21:65–71. doi: 10.1016/j.cub.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nature biotechnology. 2009;27:459–61. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subramanyam D, Lamouille S, Judson RL, Liu JY, et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nature Biotechnology. 2011:29. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z, Yang C-S, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. The EMBO journal. 2011;30:823–34. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin S-L, Chang DC, Lin C-H, Ying S-Y, et al. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Research. 2010 doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosa A, Brivanlou AH. A regulatory circuitry comprised of miR-302 and the transcription factors OCT4 and NR2F2 regulates human embryonic stem cell differentiation. EMBO J. 2011;30:237–48. doi: 10.1038/emboj.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–92. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 72.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, et al. Highly Efficient miRNA-Mediated Reprogramming of Mouse and Human Somatic Cells to Pluripotency. Cell stem cell. 2011;8:376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyoshi N, Ishii H, Nagano H, Haraguchi N, et al. Reprogramming of Mouse and Human Cells to Pluripotency Using Mature MicroRNAs. Cell stem cell. 2011;8:633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Yu J, Vodyanik Ma, Smuga-Otto K, Antosiewicz-Bourget J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY ) 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 75.Choi YJ, Lin C-P, Ho JJ, He X, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nature Cell Biology. 2011;13:1353–60. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niwa H, Miyazaki J, Smith aG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature genetics. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 77.Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 78.Tesar PJ, Chenoweth JG, Brook Fa, Davies TJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 79.Lipchina I, Elkabetz Y, Hafner M, Sheridan R, et al. Genome-wide identification of microRNA targets in human ES cells reveals a role for miR-302 in modulating BMP response. Genes & Development. 2011;25:2173–86. doi: 10.1101/gad.17221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guttman M, Amit I, Garber M, French C, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guttman M, Donaghey J, Carey BW, Garber M, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011 doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–81. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carey BW, Markoulaki S, Hanna JH, Faddah DA, et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell stem cell. 2011;9:588–98. doi: 10.1016/j.stem.2011.11.003. [DOI] [PubMed] [Google Scholar]