Abstract
Background
We sought to determine the association between maternal vitamin D status at ≤26 weeks gestation and the risk of preeclampsia separately by clinical subtype.
Methods
We conducted a case-cohort study among women enrolled at 12 U.S. sites from 1959 to 1966 in the Collaborative Perinatal Project. In 717 women who later developed preeclampsia (560 mild and 157 severe cases) and in 2986 mothers without preeclampsia, we measured serum 25-hydroxyvitamin D at ≤26 weeks gestation (median 20.9 weeks) over 40 years later using liquid-chromatography-tandem mass spectrometry.
Results
Half of women in the subcohort had 25(OH)D <50 nmol/L. Maternal 25(OH)D 50–<75 nmol/L was associated with a reduction in the absolute and relative risk of preeclampsia and mild preeclampsia compared with 25(OH)D <30 nmol/L, but the effects were no longer present after adjustment for confounders including race, prepregnancy body mass index, and parity. For severe preeclampsia, 25(OH)D ≥50 nmol/L was associated with a reduction of 3 cases per 1,000 pregnancies (adjusted RD −.003, 95% CI: −.005, .0002) and a 40% reduction in risk (adjusted RR .65, 95% CI .43, .98) compared with 25(OH)D <50 nmol/L. The conclusions were the same after restricting to women with 25(OH)D measured at <22 weeks gestation and after formal sensitivity analyses for unmeasured confounding.
Conclusions
Maternal vitamin D deficiency may be a risk factor for severe preeclampsia, but it is not associated with preeclampsia overall or its mild subtypes. Contemporary cohorts with large numbers of severe preeclampsia cases are needed to confirm or refute these findings.
Preeclampsia is a multisystem disorder diagnosed by new-onset hypertension and proteinuria. In developed countries, the perinatal mortality rate among preeclamptic pregnancies is five times as great as non-preeclamptic pregnancies, 1 and indicated preterm deliveries for preeclampsia account for 15% of preterm births.2 Mothers who develop preeclampsia are at elevated risk of abruptio placentae, acute renal failure, and neurologic and cardiovascular complications. 1 Moreover, preeclampsia contributes to 18% of maternal deaths in the U.S. and 20–80% of maternal deaths in developing countries. 1,3 Delivery is the only known cure for preeclampsia, and few interventions have been effective in preventing the disorder.
There is a growing interest in the role of maternal vitamin D status in the development of preeclampsia. Vitamin D is a prohormone that is either made in the skin through ultraviolet B radiation exposure or ingested orally. 4 Vitamin D deficiency is widespread in U.S. pregnant women 5–8 due to inadequate sunlight exposure, limited vitamin D-rich food sources, and use of prenatal vitamins with low doses of vitamin D. 4 Vitamin D has diverse and protean functions that may be relevant in the pathophysiology of preeclampsia, including abnormal placental implantation and angiogenesis, excessive inflammation, hypertension, and immune dysfunction. 4,9–12 Unfortunately, most vitamin D-preeclampsia research has been conducted in predominantly Caucasian populations with small numbers of preeclampsia cases, and results have been inconsistent. 13–21
We sought to determine the association between maternal vitamin D status at ≤26 weeks and the risk of preeclampsia in a large, geographically-diverse U.S. multicenter cohort of pregnant women.
Methods
We conducted a case-cohort study 22 in the Collaborative Perinatal Project (CPP, 1959–65). 23 A total of 55,908 pregnant women were enrolled at their first prenatal visit at 12 U.S. medical centers after providing verbal informed consent for participation (as was common at the time the CPP was conducted). Detailed data were collected via in-person interviews on maternal sociodemographic factors, medical history, and obstetric history. Mothers provided non-fasting blood samples every 8 weeks. At each visit, medical and obstetric events were recorded and random urine samples were tested for albumin. Blood pressures were measured at enrollment and each prenatal visit, during labor and delivery, and postpartum. Korotkoff phase 4 (muffling) or phase 5 (disappearance) was used for diastolic blood pressure. 24 A validation study in which information on blood pressure and urinary albumin was checked against that in the original medical records showed a high degree of accuracy. 24 A labor and delivery summary was recorded by the obstetrician responsible for each patient’s care.
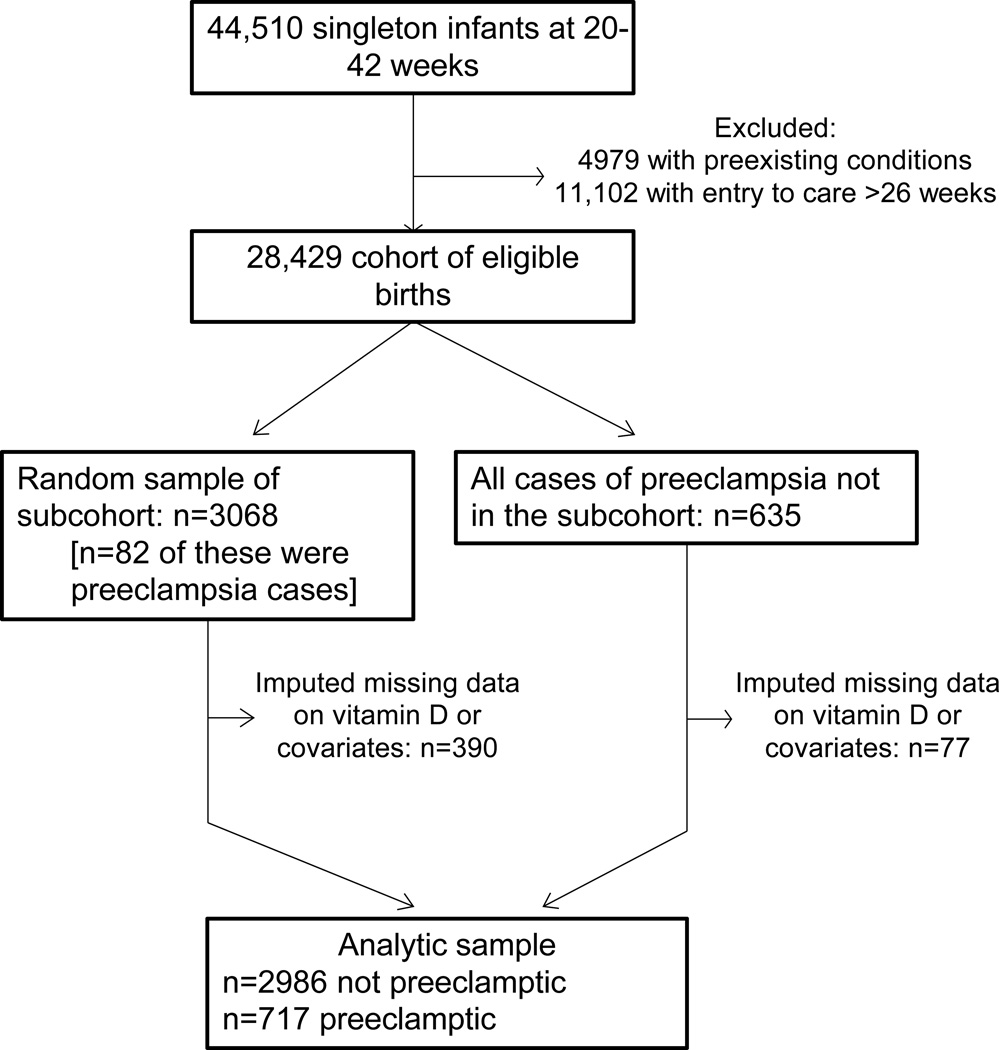
Figure 1 describes the selection of the sample. There were 44,510 singleton deliveries to white, black, or Puerto Rican mothers at 20 to 42 weeks gestation in CPP. We excluded women with pregestational diabetes, hypertension, or cardiovascular disease and women who entered the study after 26 weeks, which led to a cohort of 28,429 eligible women. We randomly selected 11% of the eligible cohort and augmented this subcohort with all remaining cases of preeclampsia. We used multiple imputation (described below) to address missing data on 25(OH)D concentrations, prepregnancy body mass index, socioeconomic status, or other covariates in 12% of pregnancies. After imputation, the analytical sample included 717 preeclamptics (560 mild and 157 severe cases) and 2986 non-preeclamptics. This study used de-identified data and was exempt from ethics review.
Figure 1.
Case-cohort subject selection
We applied a contemporary definition 25 of preeclampsia to measurements of blood pressure and urinary protein taken at the time of the study. Preeclampsia was defined as gestational hypertension and proteinuria, and return of abnormalities to normal in the postpartum period. 25 Gestational hypertension was defined as two or more measurements of systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg for the first time after 24 weeks of gestation. In the intrapartum period, the first five pressures obtained after hospital admission for delivery were averaged. Proteinuria was defined as two random urine dipsticks of 1+ protein or one dipstick of 2+ protein. Cases of preeclampsia were considered severe if they had at least one of the following symptoms: a systolic blood pressure ≥160 mmHg, a diastolic blood pressure ≥110 mmHg, proteinuria of 5g/24 hours, proteinuria of 3+ or more, oliguria, pulmonary edema, or convulsions/eclampsia. All other cases were considered mild. The HELLP syndrome had not yet been described at the time of the CPP, and liver function tests and platelet counts were not included in the database.
Maternal serum was stored in glass at −20°C with no recorded thaws. We randomly selected one banked serum sample drawn at ≤26 weeks for each mother. A 26-week gestational age cut-off was chosen for two reasons. First, many women in CPP registered late for prenatal care, and this allowed us to capture a large number of preeclamptics while also assessing vitamin D status before the clinical onset of disease in most cases. Second, when the study was designed, there was no information available to determine the window of gestation that was critical for vitamin D exposure. With limited resources, we chose to randomly select one sample and perform analyses stratified by gestational age at blood sampling.
Sera were shipped to the laboratory of Dr. Michael Holick at Boston University, which is DEQAS (Vitamin D External Quality Assessment Scheme)-proficient laboratory. Samples were assayed for total 25-hydroxyvitamin D (25(OH)D) [25(OH)D2 + 25(OH)D3] using liquid-chromatography-tandem mass spectrometry based on National Institute of Standards and Technology (NIST) standards. 26 The assay had a coefficient of variation of 6.0%. 25(OH)D has been proven to be highly stable. No loss of 25(OH)D has been noted after leaving uncentrifuged blood as long as 72 hours at 24°C; after storage of serum for years at 20°C; after exposure to ultraviolet light; or after up to 11 freeze-thaw cycles. 27 A pilot study comparing 25(OH)D in CPP serum with serum frozen for ≤2 years found that 25(OH)D in CPP is unlikely to show significant degradation. 28 There is no universally-accepted definition of vitamin D deficiency, so we used multiple cut-points. 29,30
The CPP defined race as white, black, or Puerto Rican. Prepregnancy body mass index [BMI, weight (kg)/height (m)2] was based on maternal self-reported pregravid weight and measured height at enrollment. Season of blood sample collection was defined as winter (December–February), spring (March–May), summer (June–August), and fall (September-November). Education, occupation, and family income data were combined into a composite socioeconomic status score. 23 Data were also available on parity (primiparous, multiparous), maternal age (<20, 20–29, ≥30 years), smoking status at entry (smoker, nonsmoker), and marital status (unmarried, married). Gestational age was based on the mother’s report of the first day of her last menstrual period.
Statistical analysis
To address the missing data, we used multiple imputation. We created 5 imputed datasets that assumed a multivariable normal distribution with a Markov chain Monte Carlo approach to jointly address the missing data in 25(OH)D and key covariates of interest. 31,32 We imputed prepregnancy weight, height, parity, smoking, socioeconomic status, month of blood sampling, and 25(OH)D (all of which were log-transformed) by including preeclampsia, race, age, marital status, gestational age at prenatal care entry, gestational age at blood sampling, study site, and sample weight in the imputation model. We compared the results based on multiple imputation with those generated using the complete dataset (n=632 preeclamptics and 2609 non-preeclamptics).
Absolute risks, adjusted risk differences (RD), adjusted risk ratios (RR) and 95% confidence intervals (CI) were calculated from multivariable log-binomial regression. All subjects were weighted using the inverse of the sampling fraction, 22 and a clustered robust variance was used to account for the cases in the subcohort. 22 Nonlinearity in 25(OH)D was tested using splines. Effect modification on the risk-difference scale by sample gestational age, parity, race, and pregravid overweight was tested using the synergy index.33 We assessed effect modification rather than interaction because we believe that these are not variables on which one could intervene (as is required for tests of biologic interaction34), and studied that additive scale because it is the scale that is argued to be of greatest public health importance.35 Potential confounders (race, prepregnancy BMI, trimester of entry to prenatal care, smoking, parity, age, socioeconomic status, marital status, season of blood draw, gestational age at blood draw, and latitude of study site) were identified using theory-based causal models. 36 Only season and gestational age of blood sampling and trimester of entry to prenatal care met our definition of confounding (≥10% change in the magnitude of the association after removal of the variable from the full model 37). However, we also included race, BMI, smoking, latitude of study site, and parity in models to ensure that our results were comparable to previous literature. For some models, there were too few cases to include indicator variables for study site. Therefore, we tested for confounding by study site using multivariable conditional logistic regression conditioned on site.
Finally, we conducted a probabilistic bias analysis for unmeasured confounding by leisure-time physical activity and fish intake using published methods 38 (eAppendix). These factors are are associated with higher 25(OH)D concentrations 39–41 and preeclampsia, 42,43 but were unmeasured in our dataset. We compared the estimates from the conventional regression model of maternal 25(OH)D ≥50 vs. <50 nmol/L and preeclamsia risk with estimates obtained from the sensitivity analysis iterations, which reflected systematic error and random error associated with missing data on each unmeasured covariate. 38
Results
The subcohort was 49% white, 44% black, and 7% Puerto Rican. A majority of women in the subcohort were 20 to 29 years old, normal-weight before pregnancy, married, less than high-school educated, smokers, and had at least one previous live-born child (Table 1). In unadjusted analyses, mothers who developed preeclampsia tended to be black, nulliparous, <20 years or ≥30 years, overweight, unmarried, non-smokers, less educated, of lower socioeconomic position, and from study centers at ≤40°N latitude. They were more likely to enter care after the first trimester and have blood drawn in the winter months.
Table 1.
Characteristics of mothers in the subcohort and mothers who developed preeclampsia, Collaborative Perinatal Project (1959–65).
| Subcohort n=3068 % |
Preeclampsia cases n=717 % |
|
|---|---|---|
| Maternal race | ||
| White | 49 | 38 |
| Black | 44 | 53 |
| Puerto Rican | 7 | 9 |
| Parity | ||
| 0 | 33 | 52 |
| 1 or more | 67 | 48 |
| Maternal age, years | ||
| <20 | 23 | 37 |
| 20–29 | 61 | 43 |
| ≥30 | 16 | 20 |
| Prepregnancy body mass index, kg/m2 | ||
| <18.5 | 10 | 9 |
| 18.5–24.9 | 72 | 64 |
| 25–29.9 | 14 | 18 |
| ≥30 | 4 | 9 |
| Marital status | ||
| Married | 81 | 67 |
| Unmarried | 19 | 33 |
| Maternal education, years | ||
| ≤8 | 16 | 23 |
| 9–11 | 37 | 39 |
| 12 | 33 | 30 |
| >12 | 14 | 8 |
| Socioeconomic status score | ||
| 0–20 | 7 | 11 |
| 20–39 | 27 | 34 |
| 40–59 | 33 | 33 |
| 60–79 | 20 | 15 |
| 80–100 | 13 | 7 |
| Smoking during pregnancy | ||
| Yes | 53 | 35 |
| No | 47 | 65 |
| Entry to prenatal care | ||
| <13 weeks | 28 | 20 |
| 13–26 weeks | 72 | 80 |
| Gestational age of blood sampling | ||
| <13 weeks | 13 | 8 |
| 13–<20 weeks | 33 | 28 |
| 20–26 weeks | 54 | 64 |
| Season of blood sampling | ||
| Winter | 23 | 26 |
| Spring | 26 | 25 |
| Summer | 26 | 25 |
| Fall | 25 | 24 |
| Latitude of study site | ||
| ≥41°N | 61 | 50 |
| 38–40°N | 31 | 39 |
| ≤35°N | 8 | 11 |
In the subcohort, the mean (95% CI) maternal 25(OH)D at ≤26 weeks was 50.7 (49.7, 51.7) nmol/L, and 24%, 57%, and 84% of women had serum 25(OH)D <30, <50, and <75 nmol/L, respectively. As expected, 25(OH)D <30 nmol/L was most common among black women (32%) compared with Puerto Rican (23%) and white (13%) mothers, and among those blood samples drawn in December through May (33%) compared with June through November (15%).
The cumulative incidence of preeclampsia was 2.6% in the cohort, and most cases were mild (incidence 2.0%). Incidence of overall preeclampsia was lowest among gravidae with 25(OH)D 50–<75 nmol/L at ≤26 weeks and highest with 25(OH)D <30 nmol/L, but there was no absolute or relative difference in risk after adjustment for race, prepregnancy BMI, socioeconomic position, parity, smoking, latitude, and season of blood sampling (Table 2). Results were similar for mild preeclampsia.
Table 2.
Association between maternal 25-hydroxyvitamin D (25(OH)D) at ≤26 weeks and preeclampsia, mild preeclampsia, and severe preeclampsia, Collaborative Perinatal Project (1959–1965)
| Maternal serum 25(OH)D, nmol/L |
Cases (n) |
Unadjusted Incidencea |
Unadjusted risk difference (95% CI) |
Adjustedb risk difference (95% CI) |
Unadjusted risk ratio (95% CI) |
Adjustedb risk ratio (95% CI) |
|---|---|---|---|---|---|---|
| Preeclampsia (mild and severe) | ||||||
| <30 | 185 | .028 | ref | ref | ref | ref |
| 30-<50 | 247 | .027 | −.0009 (−.006, .005) | .001 (−.003, .006) | .97 (.78, 1.2) | 1.1 (.84, 1.3) |
| 50-<75 | 171 | .022 | −.005 (−.011, −.0001) | −.002 (−.006, .003) | .79 (.63, .99) | .90 (.69, 1.2) |
| ≥75 | 114 | .026 | −.002 (−.008, .005) | .002 (−.004, .008) | .93 (.71, 1.2) | 1.1 (.89, 1.5) |
| 1-standard deviation c increase | −.002 (−.004, .0003) | .002 (−.015, .010) | .93 (.85, 1.0) | .99 (.89, 1.1) | ||
| Mild preeclampsia | ||||||
| <30 | 144 | .022 | ref | ref | ref | ref |
| 30-<50 | 179 | .020 | −.002 (−.006, .003) | 0 (−.003, .003) | .91 (.72, 1.2) | 1.0 (.77, 1.3) |
| 50-<75 | 137 | .018 | −.004 (−.009, .001) | −.001 (−.004, .002) | .80 (.62, 1.0) | .92 (.69, 1.2) |
| ≥75 | 100 | .023 | .001 (−.005, .007) | .003 (−.001, .007) | 1.0 (.79, 1.4) | 1.3 (.90, 1.7) |
| 1-standard deviation c increase | −.0004 (−.002, .001) | .003 (−.007, .012) | .98 (.89, 1.1) | 1.0 (.93, 1.2) | ||
| Severe preeclampsia | ||||||
| <30 | 41 | .0063 | ref | ref | ref | ref |
| 30-<50 | 68 | .0076 | .001 (−.002, .004) | .003 (−.002, .008) | 1.2 (.76, 1.7) | 1.3 (.86, 2.0) |
| 50-<75 | 34 | .0045 | −.002 (−.004, .001) | −.001 (−.006, .003) | .74 (.46, 1.2) | .87 (.87, 1.4) |
| ≥75 | 14 | .0032 | −.003 (−.006, −.0004) | −.003 (−.009, .003) | .51 (.27, .96) | .69 (.32, 1.4) |
| 1-standard deviation c increase | −.002 (−.003, −.0006) | −.002 (−.003, .0002) | .73 (.61, .90) | .79 (.62, .99) | ||
Incidence is based on the weighted sample.
Adjusted for maternal race, prepregnancy body mass index, parity, socioeconomic status, smoking status, latitude of study site, season of blood sampling, gestational age at blood sampling, and trimester of entry to prenatal care.
1 SD is 28 nmol/L.
There were no differences in the relation between 25(OH)D and preeclampsia across strata of gestational age at blood draw or parity (Table 3). Alternate cut-points for gestational age had no effect on conclusions. There was effect modification on the risk-difference scale for prepregnancy overweight (synergy index .46 95% CI: .01, .92). Serum 25(OH)D ≥75 nmol/L was associated with an absolute and relative increase in the risk of preeclampsia compared with concentrations <30 nmol/L among overweight (BMI ≥25), but not leaner women, but the estimates were highly imprecise.
Table 3.
Association between maternal 25-hydroxyvitamin D (25(OH)D) and preeclampsia by maternal characteristics, Collaborative Perinatal Project (1959–66)
| Maternal serum 25(OH)D, nmol/L |
Cases (n) |
Unadjusted incidence |
Adjusted risk difference (95% CI) |
Adjustedb risk ratio (95% CI) |
|---|---|---|---|---|
| <18 weeks at blood sampling | ||||
| <30 | 53 | .023 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 61 | .019 | −.001 (−.009, .007) | .96 (.62, 1.5) |
| 50–<75 | 41 | .014 | −.004 (−.013, .006) | .81 (.49, 1.3) |
| ≥75 | 24 | .018 | .002 (−.010, .014) | 1.1 (.60, 1.9) |
| 18–22 weeks at blood sampling | ||||
| <30 | 53 | .029 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 70 | .031 | .0002 (−.008, .009) | 1.0 (.64, 1.6) |
| 50–<75 | 44 | .026 | −.002 (−.011, .008) | .91 (.55, 1.5) |
| ≥75 | 33 | .031 | .004 (−.009, .017) | 1.2 (.64, 2.2) |
| 22–26 weeks at blood sampling | ||||
| <30 | 79 | .031 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 116 | .032 | .002 (−.004, .009) | 1.1 (.80, 1.6) |
| 50–<75 | 86 | .028 | −.001 (−.007, .005) | .93 (.62, 1.4) |
| ≥75 | 57 | .029 | .002 (−.006, .010) | 1.1 (.69, 1.8) |
| Pregravid BMI<25 kg/m2 | ||||
| <30 | 130 | .025 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 180 | .024 | .001 (−.004, .005) | 1.1 (.79, 1.4) |
| 50–<75 | 134 | .021 | −.002 (−.006, .003) | .91 (.67, 1.2) |
| ≥75 | 81 | .021 | 0 (−.006, .006) | .99 (.69, 1.4) |
| Pregravid BMI≥25 kg/m2 | ||||
| <30 | 55 | .037 | .0 (ref) | 1.0 (ref) |
| 30–<50 | 67 | .040 | .003 (−.010, .016) | 1.1 (.69, 1.7) |
| 50–<75 | 37 | .030 | −.005 (−.019, .009) | .80 (.46, 1.4) |
| ≥75 | 33 | .056 | .015 (−.006, .037) | 1.6 (.87, 2.8) |
| White race | ||||
| <30 | 36 | .020 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 83 | .021 | −.002 (−.010, .007) | .92 (.58, 1.5) |
| 50–<75 | 86 | .018 | −.004 (−.013, .004) | .79 (.50, 1.3) |
| ≥75 | 65 | .020 | −.003 (−.012, .006) | .87 (.54, 1.4) |
| Nonwhite race | ||||
| <30 | 149 | .031 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 164 | .032 | .002 (−.003, .007) | 1.1 (.83, 1.4) |
| 50–<75 | 85 | .028 | −.002 (−.008, .004) | .91 (.66, 1.3) |
| ≥75 | 49 | .040 | .007 (−.001, .014) | 1.4 (.91, 2.1) |
| Nulliparous | ||||
| <30 | 94 | .046 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 130 | .046 | .007 (−.009, .024) | 1.2 (.82, 1.6) |
| 50–<75 | 92 | .033 | −.006 (−.022, .011) | .87 (.60, 1.3) |
| ≥75 | 56 | .037 | .002 (−.018, .023) | 1.0 (.67, 1.6) |
| Parous | ||||
| <30 | 91 | .020 | 0 (ref) | 1.0 (ref) |
| 30–<50 | 117 | .019 | 0 (−.006, .006) | .98 (.72, 1.3) |
| 50–<75 | 79 | .016 | −.001 (−.008, .006) | .94 (.66, 1.3) |
| ≥75 | 58 | .020 | .004 (−.004, .013) | 1.2 (.82, 1.8) |
Adjusted for maternal race, prepregnancy body mass index, parity, socioeconomic status, smoking status, and season of blood sampling. A statistical interaction term was included in models to generate linear combinations of interest.
For severe preeclampsia, each 1-standard deviation increase in 25(OH)D was associated with absolute and relative reductions in risk before and after confounder adjustment (Table 2). Compared with 25(OH)D <50 nmol/L, mothers who had concentrations ≥50 nmol/L had a reduction of 3 cases per 1,000 pregnancies (adjusted RD −.003, 95% CI: −.005, .0002) and a 40% reduction in risk (adjusted RR .65, 95% CI .43, .98). In formal sensitivity analysis, this conventional RR was attenuated slightly when accounting for unmeasured confounding by exercise (.71, 95% simulation interval .46, 1.1) or fish intake (.74, 95% simulation interval: .48, 1.1).
None of our results were meaningfully different when 25(OH)D was specified using flexible spline terms or quantiles; when preeclampsia was defined based only on antepartum blood pressures and protein measurements; when other covariates including study site were considered (data not shown). Restriction to women who had complete vitamin D and covariate data or who delivered at 26 to 42 weeks of pregnancy also did not alter conclusions (data not shown).
Discussion
Preeclampsia causes maternal morbidity and infant morbidity and mortality, but it is the severe cases and those with early onset that are associated with the highest risks of adverse perinatal outcomes, including preterm birth and fetal growth restriction 44,45. We found that the risk of severe preeclampsia was lower for women with vitamin D sufficiency at ≤26 weeks gestation compared with those who were deficient. This finding was robust to adjustments for prepregnancy body mass index, race, parity and other measured confounders, as well as exercise and fish intake, which were unmeasured. We found no association with preeclampsia overall or mild preeclampsia after controlling for confounders.
The literature on vitamin D status in relation to preeclampsia is mixed 14,15,18 13,16,17,19,46,47. Two recent meta-analyses on vitamin D deficiency and preeclampsia arrived at different conclusions about the this body of research, 21,48 but neither separated studies based on severity of the disease. There is increasing conviction that separating cases of preeclampsia into more homogenous groups based on severity, gestational age, recurrence, or pathophysiologic markers may lead to a greater understanding of how exposures play a role in the pathogenesis of this complex syndrome. 49 We are aware of three studies of maternal vitamin D in relation to severe or early-onset preeclampsia that adjusted for confounders. Although none had more than 50 cases of severe disease, most of the findings are consistent with ours. In a study of 43 severe preeclampsia cases and 198 controls, investigators reported that 25(OH)D <50 nmol/L was associated with an 80% reduction in risk compared with ≥75 nmol/L after adjustment for key confounders. 15 Canadian researchers studied 32 women who subsequently developed preeclampsia (23 of whom had severe disease) and 665 controls and found no association between 25(OH)D and preeclampsia at 16–18 weeks, but a 30% reduction in risk with each 1-standard deviation increase in 25(OH)D at 24–26 weeks.43 A 70% reduction in severe preeclampsia risk per 25-nmol/L increase in 25(OH)D was reported in a study of 50 severe preeclamptics and 100 controls,50 but this study measured 25(OH)D after clinical onset of the disease and may not reflect exposures relevant in the pathophysiology of severe preeclampsia.
Our finding was observed using samples at a median of 21 weeks, and it is possible that like two aforementioned studies,18,50 the low 25(OH)D was a consequence of the disease process in some individuals. Preeclampsia is thought to originate from reduced placental perfusion caused by abnormal placentation. 51 The resultant oxidative stress, antiangiogenic factors and/or inflammation are hypothesized to lead to maternal systemic disease. 51 The placenta expresses 1-α-hydroxylase for the metabolism of 25(OH)D, 11 and abnormal placental functioning could alter the influx and efflux of 25(OH)D into maternal circulation. More research is needed to understand the effect of placental vitamin D metabolism on maternal circulating 25(OH)D.
Our results suggesting an increased risk of overall preeclampsia at 25(OH)D ≥75 nmol/L among mothers with a pregravid BMI ≥25 and among nonwhite mothers is puzzling, particularly because there was no dose-response relationship. We have no obvious explanation for this finding, but our ongoing research studying polymorphisms in key vitamin D metabolizing genes may shed light on this contradictory result.
Our study could not determine the association between vitamin D and subtypes other than those based on mild and severe symptoms because we lacked adequate numbers of preeclampsia cases delivered at <34 weeks (n=17) and data on preeclampsia recurrence (though our results did not differ by parity). We also did not measure biological intermediates of preeclampsia or other biomarkers in the vitamin D pathway, including vitamin D binding protein. Observational studies of vitamin D are susceptible to confounding bias because sufficient 25(OH)D may be a marker of more time spent outdoors or other behaviors that investigators did not measure. We attempted to account for unmeasured confounding by outdoor leisure-time physical activity and fish intake. We did not consider calcium intake, which was also unmeasured, because we have previously shown that it is unlikely to meaningfully confound the vitamin D-preeclampsia relationship. 14 However it is possible that unmeasured confounding by genetic factors or other lifestyle variables may exist, and those that were available may have been measured with error. Selection bias is unlikely to be a major problem in our study because results generated after multiple imputation of vitamin D and covariate data on 13% of our sample agreed with those from the complete-case analysis.
Results from CPP may not generalize to today’s general obstetric population because of differences in population characteristics (e.g., more smoking, less obesity in the 1960s and potentially their interrelationships with vitamin D) and preeclampsia management. However, there is no evidence the pathophysiology of preeclampsia has changed over time. The large sample size and the proven validity of the hypertension and proteinuria data in CPP 24 afforded us the unique opportunity to prospectively study preeclampsia using rigorous, contemporary definitions separately by severity. As expectant management of preeclampsia did not typically involve iatrogenic preterm delivery in the 1960s, these data offered the chance to study the natural progression of the disease.
While the maternal sera was stored for decades, our pilot work suggests that the long-term storage is unlikely to have caused deterioration of 25(OH)D, 28 and the high prevalence of maternal vitamin D deficiency that we observed is similar to racially-diverse modern cohorts. 6–8 If 25(OH)D had deteriorated over time, it would have misclassified both cases and controls and led to bias towards the null. Our ability to replicate factors generally regarded as associated with vitamin D (e.g., race, season) and preeclampsia (e.g., parity, obesity, smoking) supports the validity of our measurements.
Future research in large, contemporary pregnancy cohorts with rigorous definitions of preeclampsia separated by clinical subtype are needed to advance the field. Quantifying vitamin D metabolites and preeclampsia biomarkers along with 25(OH)D longitudinally during gestation will not only help to elucidate pathways linking vitamin D to pregnancy outcome, but also identify subsets of preeclampsia that may be responsive to vitamin D treatment.
Acknowledgments
Sources of financial support:
This research was supported by NIH grant HD 056999 (PI: Bodnar).
References
- 1.Roberts JM. Pregnancy related hypertension. In: Creasy RK, Resnik R, editors. Maternal Fetal Medicine. Philadelphia: W.B. Saunders; 1998. pp. 883–872. [Google Scholar]
- 2.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339(5):313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 3.Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin No. 33. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. [DOI] [PubMed] [Google Scholar]
- 9.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010;74(1):71–75. doi: 10.1016/j.mehy.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11(5):263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Liu NQ, Ouyang Y, Bulut Y, Lagishetty V, Chan SY, Hollis BW, Wagner C, Equils O, Hewison M. Dietary vitamin D restriction in pregnant female mice is associated with maternal hypertension and altered placental and fetal development. Endocrinology. 2013;154(7):2270–2280. doi: 10.1210/en.2012-2270. [DOI] [PubMed] [Google Scholar]
- 13.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, Thadhani R. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56(4):758–763. doi: 10.1161/HYPERTENSIONAHA.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95(11):5105–5109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010;117(13):1593–1598. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu CK, Ertl R, Skyfta E, Akolekar R, Nicolaides KH. Maternal serum vitamin D levels at 11–13 weeks of gestation in preeclampsia. Journal of Human Hypertension. 2012 doi: 10.1038/jhh.2012.1. [DOI] [PubMed] [Google Scholar]
- 18.Wei SQ, Audibert F, Hidiroglou N, Sarafin K, Julien P, Wu Y, Luo ZC, Fraser WD. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG : an international journal of obstetrics and gynaecology. 2012;119(7):832–839. doi: 10.1111/j.1471-0528.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 19.Hypponen E, Hartikainen AL, Sovio U, Jarvelin MR, Pouta A. Does vitamin D supplementation in infancy reduce the risk of pre-eclampsia? Eur J Clin Nutr. 2007 doi: 10.1038/sj.ejcn.1602625. [DOI] [PubMed] [Google Scholar]
- 20.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, Meltzer HM. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20(5):720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 21.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 22.Cologne J, Preston DL, Imai K, Misumi M, Yoshida K, Hayashi T, Nakachi K. Conventional case-cohort design and analysis for studies of interaction. Int J Epidemiol. 2012;41(4):1174–1186. doi: 10.1093/ije/dys102. [DOI] [PubMed] [Google Scholar]
- 23.Niswander K. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The women and their pregnancies. Philadelphia, PA: WB Saunders; 1972. [Google Scholar]
- 24.Friedman EA, Neff RK. Pregnancy hypertension: A systematic evaluation of clinical diagnostic criteria. Littleton, MA: PSG Publishing Co; 1977. [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 26.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 27.Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41(Pt 4):272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101(2):278–284. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IOM. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C: National Academy Press; 2010. [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 31.Royston P. Multiple imputation of missing values: Further update of ice, with an emphasis on categorical variables. Stata Journal. 2009;9(3):466–4477. [Google Scholar]
- 32.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman KJ. Synergy and antagonism in cause-effect relationships. Am J Epidemiol. 1974;99(6):385–388. doi: 10.1093/oxfordjournals.aje.a121626. [DOI] [PubMed] [Google Scholar]
- 34.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology. 2009;20(6):863–871. doi: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 36.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82:669–688. [Google Scholar]
- 37.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169(10):1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 38.Lash TL, Fink AK. Semi-automated sensitivity analysis to assess systematic errors in observational data. Epidemiology. 2003;14(4):451–458. doi: 10.1097/01.EDE.0000071419.41011.cf. [DOI] [PubMed] [Google Scholar]
- 39.Freedman DM, Cahoon EK, Rajaraman P, Major JM, Doody MM, Alexander BH, Hoffbeck RW, Kimlin MG, Graubard BI, Linet MS. Sunlight and Other Determinants of Circulating 25-Hydroxyvitamin D Levels in Black and White Participants in a Nationwide US Study. American Journal of Epidemiology. 2013;177(2):180–192. doi: 10.1093/aje/kws223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dam RM, Snijder MB, Dekker JM, Stehouwer CD, Bouter LM, Heine RJ, Lips P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr. 2007;85(3):755–761. doi: 10.1093/ajcn/85.3.755. [DOI] [PubMed] [Google Scholar]
- 41.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 42.Kasawara KT, do Nascimento SL, Costa ML, Surita FG, e Silva JL. Exercise and physical activity in the prevention of pre-eclampsia: systematic review. Acta obstetricia et gynecologica Scandinavica. 2012;91(10):1147–1157. doi: 10.1111/j.1600-0412.2012.01483.x. [DOI] [PubMed] [Google Scholar]
- 43.Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane database of systematic reviews. 2006;(3):CD003402. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, Klebanoff M, Vandorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66–71. doi: 10.1067/mob.2002.120080. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet Gynecol. 2001;97(2):261–267. doi: 10.1016/s0029-7844(00)01125-x. [DOI] [PubMed] [Google Scholar]
- 46.Haugen G, Helland I. Influence of preeclampsia or maternal intake of omega-3 fatty acids on the vasoactive effect of prostaglandin F-two-alpha in human umbilical arteries. Gynecol Obstet Invest. 2001;52(2):75–81. doi: 10.1159/000052947. [DOI] [PubMed] [Google Scholar]
- 47.Wei SQ, Audibert F, Luo ZC, Nuyt AM, Masse B, Julien P, Fraser WD. Maternal plasma 25-hydroxyvitamin D levels, angiogenic factors, and preeclampsia. American journal of obstetrics and gynecology. 2013;208(5):390, e1–e6. doi: 10.1016/j.ajog.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 48.Tabesh M, Salehi-Abargouei A, Esmaillzadeh A. Maternal vitamin d status and risk of pre-eclampsia: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2013;98(8):3165–3173. doi: 10.1210/jc.2013-1257. [DOI] [PubMed] [Google Scholar]
- 49.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 50.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366, e1–6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]