Summary
Based on experience from the treatment of other autoimmune diseases and due to inherent shortcomings of the existing therapy options for Graves’ disease (GD) and thyroid associated ophthalmopathy (TAO), rituximab was recently introduced as a novel therapy option. Here we summarize the rationale for using rituximab; give an overview of the possible mechanisms of action; and give an account of its effects and side effects when used in GD and TAO. Low quality evidence, originating from few and methodologically inhomogeneous studies, suggests that rituximab may prolong remission for hyperthyroidism over that seen with antithyroid drugs, at least in mild GD. Furthermore, in TAO patients, unresponsive to conventional immunosuppressive therapy, rituximab seems efficacious. Awaiting large-scale randomized studies, rituximab, due to limited experience and high cost, should be considered experimental and reserved for patients who do not respond favourably to conventional therapy. Rituximab is the first in a series of new and emerging treatments addressing specific therapeutic targets, such as, which will hopefully lead to improved and better tailored individualized therapy for GD and especially TAO.
Keywords: Hyperthyroidism, thyroid eye disease, B cells, anti-CD20, Rituximab
Graves’ disease (GD) afflicts approximately 1–2% in the adult population.1,2 Its actiology is incompletely understood and thought to result from a complicated interaction between genetic susceptibility and environmental factors.3 This interplay might explain its epidemiologic pattern, including geographic; variation in prevalence.3 It is likely that the vast majority of clinicians will at some time be confronted with such patients, since GD can affect any organ system. During the last 50 years relatively little advance has been made in improving the therapy of this debilitating disease.4 Therapeutic options for the hyperthyroidism associated with GD - antithyroid drugs, radioiodine therapy and surgical thyroidectomy - allow us to care well for patients with the glandular aspects of the disease. Moreover, adequate data concerning efficacy, side-effect profiles, important prognostic factors (e.g. thyroid size, level of thyroid-stimulating hormone receptor antibodies [TRAbs] and smoking habits), and better patient education have improved our overall ability to individualize therapy and to obtain good clinical outcomes. However, achieving the optimal goal of a cure of the underlying autoimmune disease in a cost-effective manner devoid of therapy side-effects, remains illusive in the majority of patients. Recurrence rate following antithyroid drugs is usually more than 50%. Eventually the majority treated with radioiodine become hypothyroid as do many of those undergoing surgical and radioiodine ablation. Therefore, it is not surprising that consensus as to the best therapy for achieving euthyroidy has been difficult to reach.2,4,5
Regarding the most incapacitating manifestation of GD, thyroid associated ophthalmopathy (TAO; also called Graves’ orbitopathy), therapeutic progress has been even more difficult to identify.6,7 The established methods of treatment, including oral or intravenous glucocorticoids, orbital irradiation, surgical decompression, or a combination of these, offer only fractional improvement compared with allowing the disease to take its natural course. Cost-effectiveness is poor and the improvement of quality of life during and following therapy remains modest.8 Offering patients with GD counselling regarding the pros and cons of radioiodine ablative therapy and cessation of cigarette smoking has positively impacted the prognosis of TAO when considering cost-effectiveness of intervention.
Since the initial observations dating to 2006, we9 and others10 have reported on the beneficial effects in GD of B cell depletion with the therapeutic antibody rituximab (RTX). These and subsequent, studies have suggested that RTX may benefit with GD as well as those manifesting TAO. In the following paper, we briefly update the potential mechanisms of action of this drug and attempt to dissect the impact of therapy on thyroid function and the clinical behaviour of TAO. Despite favourable initial reports, in light of experiences from several groups of investigators, the reported side-effects, and cost of RTX therapy, we have tempered our enthusiasm and emphasize that more studies are needed before we can recommend wider scale use in GD.
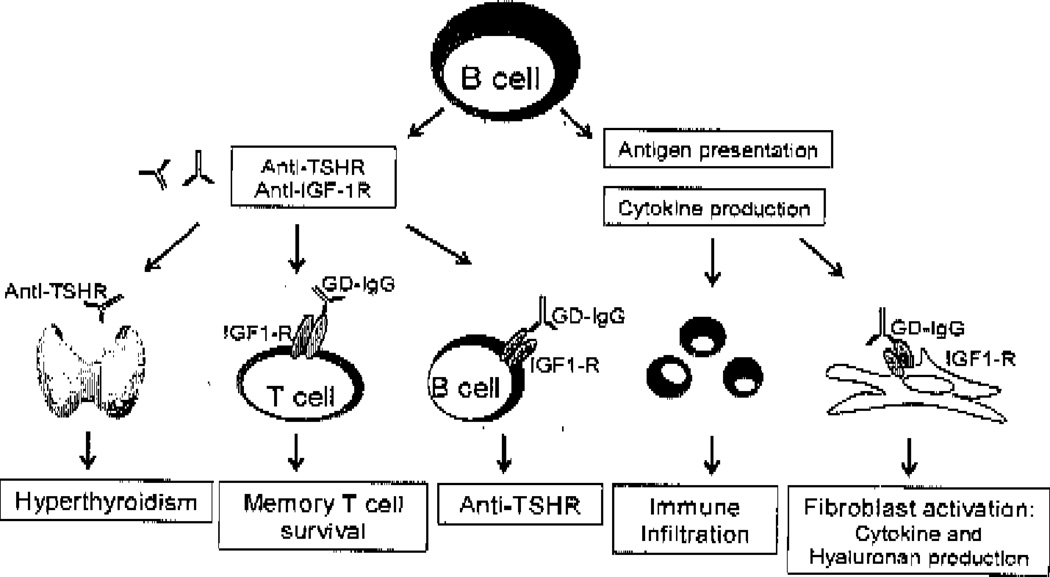
Rationale for the use of rituximab in GD (figure 1)
Figure 1.
The multifaceted role of B cells in Graves’ disease. B cells produce autoantibodies to the TSHR and IGF-IR which leads to hyperthyroidism and potentially fibroblast activation. B and T cells from Graves’ patients display increased expression of the IGF-IR and activation of this receptor via GD-IgG enhanced B cell autoantibody production and T cell survival. Furthermore, B cells are excellent antigen presenting cells and produce cytokines which may be central to immune infiltration and the exuberant inflammatory response demonstrated in patients with Graves’ disease.
RTX is a human/murine chimeric monoclonal antibody which targets CD20, a transmembrane protein present on immature and mature B cells, but absent on most pro-B cell or plasma cells,11 It was originally utilized in the treatment of B cell lymphomas, an indication first identified in 1997 (reviewed in 12). Subsequently, RTX proved useful in the treatment of a variety of autoimmune diseases13 Among the first, were IgM antibody-related polyneuropathies in 199914 and rheumatoid arthritis (RA) in 200115 The rationale behind the use of RTX in these and allied diseases remains the putative role of autoantibodies (i.e. rheumatoid factors and, as subsequently shown, anti-cyclic citrullinated peptid antibodies in RA) in the pathogenesis of these forms of autoimmunity. It was believed that disruption of pathogenic autoantibody-generation through B-cell depletion would yield long-term remission.16 Since GD is an autoantibody-mediated disease where TRAbs (or more specifically the stimulating fraction of TRAbs, known as TSAbs) are pathognomonic for GD, Hasselbalch17 and Wang and Baker18 were the first to suggest the potential benefit of RTX therapy in GD.
In addition to their role as precursors for antibody-secreting plasma cells, B cells are highly efficient antigen-presenting cells (APCs)19 Studies using animal models of RA20 and Type diabetes mellitus21,22 have pointed toward a critical role for B cells as APCs in disease pathogenesis. Accordingly, a beneficial effect of RTX has been demonstrated in classical T-cell mediated autoimmune diseases, such as multiple sclerosis23 and Type 1 diabetes.24 Because they can take up minute amounts of antigen via their high-affinity antigen receptor, antigen-specific B cells are efficient APCs, even at very low antigen concentrations.19 It should be noted that a (self) antigen associated with IgG-containing immune complexes may engage B cells expressing rheumatoid factor as antigen receptor in antigen presentation.25 Moreover, as we have previously shown regarding human thyroglobulin, B cells may take up self-antigen in the context of complement-activating immune complexes, through the complement receptor 2 (CR2, CD35), and in so doing, initiate a T cell response.26,27
In addition to their roles in antibody-production and as APCs, B cells are capable of producing a variety of cytokines. These include pro-inflammatory cytokines such as IL-6, TNF-α, and lymphotoxin28–30, and anti-inflammatory cytokines including IL-10 and TGF-β.29–31 Recently, an IL-10-producing subset of B-cells, known as regulatory B cells (Bregs) or B10 cells, has been associated with protection against experimental autoimmune encephalitis31 and arthritis.32 Moreover, B cells can induce T-cell anergy through their TGF-β production.33 Somewhat paradoxically, B cells seem to play detrimental roles under certain conditions while exerting protective effects in others. This may be explained by the protective actions of B cells during the autoimmune disease induction, prior to their pathogenic roles following disease initiation34.
In autoimmune thyroid disease (AITD), the gland often exhibits a lymph node-like architecture, where germinal centres harbour somatic hypermutation and affinity antibody maturation.35 These germinal centers support the development of specific memory B cells and plasma cells, and thyroid autoantibody generation.35,36 We have recently shown that B cells are completely absent from thyroid and colon within one week and at 69 days, respectively, following RTX therapy.37,38 Moreover, others have shown the absence of B cells from additional examples of inflamed tissue.39,40 Taken together, these data indicate that RTX mediates complete B-cell depletion in inflamed tissues manifesting autoimmune diseases by disrupting germinal centres.
With regard to TAO, Salvi et al.10 reported an almost complete absence of lymphocytes in an orbital biopsy taken ten months after RTX therapy. In contrast, we41 found that the B-cell content in the orbital tissue following RTX in a patient with TAO was comparable to that in another patient not receiving the therapy. It is unclear whether the presence of B cells in that case reflects insufficient depletion or repopulation of lymphocytes. Clearly, additional sampling, perhaps undertaken at earlier time points, will prove necessary to establish the completeness and durability of RTX-dependent B-cell depletion in orbital tissue.
The effect of rituximab on thyroid function
Three studies have been conducted examining the effect of RTX on thyroid function in GD.42–44 We must acknowledge the limited data from the few patients thus far reported (Table 1). These preliminary observations currently do not allow conclusions to be made. Thus, enthusiasm generated by the novelty and potential benefit of RTX therapy in GD must be tempered by the shortcomings of each of these studies. Many factors varied among individual patients and between the three studies. Among them, duration of disease, interval since diagnosis, primary disease versus recurrence, familial versus sporadic autoimmune thyroid disease, duration and type of treatment for hyperthyroidism, presence of TAO, initial thyroid size, TRAb levels, smoking habits, RTX dose, follow-up intervals, and inclusion of control subjects all varied between the three studies.42–44
Table 1.
Pertinent clinical data in patients with Graves’ disease (GD), with or without thyroid associated opbthalmopathy (TAO), treated with rituximab. Influence on thyroid function and TAO.
| Study and reference number |
Number and sex |
Rituximab dose |
Thyroid State | Initial CAS |
Final CAS |
Side- effects |
Remission- rate |
|---|---|---|---|---|---|---|---|
| El Fassi et al 2007 (42) | 10 F. 2 with TAO | 375 mg/m2 weekly for 4 weeks | Euthyroid | 5 and 6 | 1 and 2 | 5 of 10 | 4 of 10 (a) |
| Salvi et al. 2007 (43) | 7 F; 2 M 7 with TAO | 1 g twice with 2 week interval | 4 hyperthyroid 5 euthyroid | Mean 4.7 | Mean 1.8 | 3 of 9 | NA |
| Heemstra et al. 2008 (44) | 9 F; 4 M 3 with mild TAO | 1 g twice with two week interval | Hyperthyroid | NA | NA | 2 of 13 | 9 of 13 (b) |
| Khanna et al. 2010(59) | 4 F; 2 M All with TAO | 1g twice with 2 week interval | Euthyroid | Mean 5.3 | Mean 1.8 | 3 of 6 | NA |
Followup 14–30 months post rituximab therapy
Followup 14–30 months post rituximab
F; females
M; males
TAO; thyroid associated ophthalmopathy
CAS; clinical activity score
NA; not applicable
El Fassi et al.42 treated prospectively a group of 20 patients with newly diagnosed but untreated hyperthyroidism with methimazole (MMI) for approximately 4 months, until they became euthyroid. Ten of these were then treated with RTX, two of whom manifested TAO. All were followed off MMI until hyperthyroidism recurred. Within approximately one year all the patients not receiving RTX had relapsed, while four of 10 patients treated with RTX were euthyroid and have subsequently remained so for up to 30 months. TRAb levels appear predictive of this sustained remission, since all four had values below 5 IU/L. Further, in support of an effect of RTX, all patients with similar low TRAb levels who did not receive RTX relapsed within a year. Salvi et al.43 studied 9 patients (Table 1), of whom 7 manifested TAO. At time of RTX therapy, four were hyperthyroid and had yet to be treated, five were euthyroid, either in remission, were currently treated with MMI, or were receiving levothyroxine following surgical thyroidectomy. Eight patients were re-assessed at 12 months while one was re-examined five months later. The authors concluded that RTX failed to affect thyroid function, since those in remission remained euthyroid while those who were hyperthyroid and remained untreated showed no improvement in their thyroid function and were thus started on MMI. Finally, Heemstra et al.44 treated 13 patients with relapsing GD of whom three manifested mild TAO. Ten patients were transiently treated with MMI There was no control group and follow-up examination occurred 26 weeks following administration of 1 g of RTX iv twice with an interval of two weeks. Four patients failed RTX treatment and received radioiodine therapy. Serum free T4 declined while serum TSH levels increased in the remaining 9 patients with normalization occurring in five. These 9 patients have remained euthyroid for a median of 18 (range 14–20) months since RXT administration. Pretreatment levels of TRAb were relatively low (median 4 IU/L, range 0.2–6.3), congruent with those reported in the study of El Fassi et al.42 Despite the small number of patients included and the lack of control subjects, the study of Heemstra et al.44 taken together with ours42 suggests that RTX may influence remission rates in GD, especially in patients with low TRAb levels.
The effect of rituximab on autoantibodies
Our study42 demonstrated that TRAb levels decrease at similar rates in patients regardless of whether they are receiving RTX as a single agent (median 29%), RTX combined with MMI (36%), or only MMI (64%) at 13 weeks. Subsequent levels were found to decline in patients treated with RTX while the levels remained relatively constant in those not receiving the drug. Salvi et al.43 found a mean decrease of 40% in TRAb levels in RTX-treated patients after 30 weeks, similar to that observed in patients treated with i.v. glucocorticoids. In the study by Heemstra et al,44 the median TRAb levels of the 9 responders decreased by 52% within 26 weeks following RTX administration. Thus, a moderate decrease in TRAb levels occurred following RTX therapy in all three studies, but the decline was similar to that in patients treated with MMI or prednisolone.
The report by El Fassi et al.45 of a decrease (median 84%) in cAMP-production in TSHR-transfected CHO cells elicited by sera collected from patients treated with RTX, Strongly suggests that TSAb activity had declined within 20 weeks of therapy. Those patients not treated with RTX failed to exhibit a decrease despite similar decreases in total TRAbs in the two groups.42 Due to the small number of sera with TRAb levels sufficiently high to allow this kind of analysis, the findings of a differential effect of RTX on TSAb level compared with that of TRAbs need to be confirmed in a larger study.45
The data presented by Salvi et al.43 indicate an approximately 65% decrease in TPOAb levels by 50 weeks following RTX administration, findings congruent with those of us.45 While the effect of RTX on anti-thyroglobutin antibodies was rot examined in the study by El Fassi et al42. Salvi et al.43 indicated a non-significant 50% decline in those antibodies within 8 weeks in the patients treated with RTX. To date no reports have appeared concerning potential effects of RTX on other autoantibodies in TAO, including those against collagen XII46 or insulin-like growth factor receptor.47
Treatment with RTX, usually in combination with cyclophosphamide and prednisolone generally has modest effects on levels of circulating IgG and IgA. This includes anti-microbial and self-directed antibodies.45,48–54 In contrast, RTX often lowers levels of IgM, including rheumatoid factor46,54,55 We know now that plasma cells can be especially long-lived56, which explains why detectable levels of IgGs persist, even during B-cell depletion. On the other hand, levels of some autoantibodies, including anti-dsDNA, anti-Clq, and ANCA can fall markedly after initiation of RTX treatment.53,54,57
The effect of rituximab on TAO
When considering evidence for a positive impact of RTX on the clinical course of TAO, concerns similar to those articulated with regard to the glandular component of CD appear to apply. In addition, the well-recognized limitations in evaluating and classifying patients with TAO confound any assessment of the studies performed. These barriers merit attention.58 In the three studies, only 17 patients with TAO, were included9,43,59 (Table 1). While these studies report remarkable improvement in the activity and severity of TAO following RTX therapy, the substantial limitations of all three preclude drawing any firm conclusions.
The study of El Fassi et al. included two female ex-smokers who had previously received both glucocorticoids and retrobulbar irradiation.9 Both were euthyroid and had completed their course of steroids at time of RTX therapy. Eight months after RTX administration, the CAS had decreased from 6 to 2 in one patient and from 5 to 1 in the other. Disease severity (soft tissue changes and eye motility) was significantly improved in both patients, as was proptosis. Effects were evident as early as one month post-therapy and have lasted for more than one year without additional therapy.
Seven of 9 patients followed prospectively by Salvi et al.43 had active TAO while the others manifested lid signs. Evaluated 7 months after RTX therapy, the CAS decreased from a mean of 4.7 to 1.8. Disease severity, including soft tissue inflammation, was reduced significantly and ocular motility improved. The effect was evident at one month after therapy and has lasted for at least one year.
Most recently, we reported that in six patients unresponsive to glucocorticoid therapy and presenting with severe and active TAO, RTX had a rapid and sustained therapeutic effect on both activity and severity of TAO.59 CAS decreased from a mean of 5.3 to 1.8 after 8 weeks had elapsed following therapy and continuing for an average of 6 months. Four of the six patients had presented with optic neuropathy. Visual acuity improved in all within 4 weeks of RTX therapy and returned to pre-morbid levels within two months. Tapering of glucocorticoids, given concomitantly in all patients, was uniformly well-tolerated, Not surprisingly none of the patients experienced improvement in extra-ocular motility or proptosis. In contrast to the other reports, failure of RTX in improving TAO in a single patient was recently reported.60
Taken together, these limited studies on a heterogeneous cohort of patients, suggest that RTX may prove efficacious in those patients with TAO who are most needy. But controlled studies must now be conducted. In view of the high cost of RTX and the reportedly serious side-effects, such studies must focus on both efficacy and on carefully defining the subgroup of patients most appropriate for inclusion. Awaiting such studies, it is our opinion that RTX be used when evidence-based therapy has failed or is contraindicatcd.
Possible mechanisms for RTX action
A series of animal studies20,22,60,61 and clinical trials of RTX in T-cell mediated autoimmune23–24 have taught us that RTX targets the APC functions of B cells. It remains likely that abolished antigen-presentation by B cells also plays a significant role in the clinical improvement observed in GD and TAO following RTX therapy, leading to abrogated CD4+ T-helper cell recognition of thyroid self-antigens. In TAO, attenuation of antigen-presentation by B cells seems a plausible explanation for the clinical effect of RTX. This is entirely consistent with the view that TAO is T-cell mediated62, and because a role for autoantibodies in the pathogenesis of this component of GD has yet to be established convincingly.
RTX therapy alters the behaviour of other immune cells besides B cells. For instance, administration of the drug to patients with RA upregulated expression of B cell activating factor, IL-10, and CD86 in monocyte-derived macrophages63. Another important potential aspect of RTX actions on immune function concerns its impact on B cell cytokine production. In patients with RA, B cell depletion results in a dramatic elevation of serum IL-8 levels64. The markedly elevated levels of IL-5, IL-6, and IL-10 found in untreated individuals with HIV infections were reduced following RTX therapy (65). Serum TNF-α levels were also reduced within two days of RTX administration to patients with systemic lupus erythematosus (66).
Of particular relevance for GD, it is conceivable that B cells support inflammatory plasma cell niches where the production of autoantibodies occurs, and that RTX therapy destroys such inflammatory niches.66 This mechanism might enhance the turnover of autoantibody-producing plasma cells, and in so doing reduce autoantibody production. The available data from three studies of RTX in GD or TAO suggest that TRAb levels decrease following B-cell depletion, but not to a greater extent than following treatment with MMI or prednisolone.42–44 However, the prolonged remission observed in patients with low baseline TRAb levels indicate that pathogenic antibody-specificities may have been temporarily removed from the repertoire of the repopulating B-cells, as shown in SLE where RTX treatment causes a temporary decrease in the proportion of specific CD27 memory B cells producing disease-associated autoantibodies encoded from the VH4-34-gene67
Side effects of rituximab
In deciding on disease management, the potential side effects of RTX therapy need to be balanced with disease severity and suitability of therapetic alternatives. In uncomplicated GD, inexpensive and time-proven therapies make the use of RTX relatively unattractive (4,69,70) The threshold for its use differs substantially in severe TAO, where our options are considerably more limited. All studies examining RTX therapy in GD and TAO have reported the occurrence of side effects, but with varying prevalence and severity.37,42,43,44,58 Pre-treating with 1 g of acetaminophen and 2 mg of iv clemastine, El Fassi et al. encountered side effects in 5 of 10 patients following the initial RTX infusion (42). Four patients developed hypotension, two became nauseated, one became febrile, another complained of chills and one developed sinus tachycardia. Two of these patients received antihistamine and one was given glucocorticoids. Four days after the second infusion, two patients developed serum sickness (joint pain and fever), one of whom subsequently developed diarrhoca and iridocyclitis a year later. Another had recurrent fever, symmetric polyarthritis, and ulcerative colitis diagnosed 1–2 months after the second infusion.38 The latter process is remarkable since administration of RTX to patients with ulcerative colitis has lead to its exacerbation (71). This finding suggests that B cells might play a protective role mediated by IL-10, which may then override any detrimental aspects of B cell function. Therefore, RTX should be administered with caution in patients with concomitant inflammatory bowel disease.
In the study of Salvi et al. 1 g of paracetamol and 10 mg chlorphenamine were given as pre-treatment.43 Only three of 9 patients had mild side effects during the first RTX infusion, such as a mild fever, which was treated by 100 mg hydrocortisone i.v. Heemstra et al. gave 10 mg dexamethasone and 2 mg clemastine i.v. and reported no other side effects than temporary joint pain in two patients who had no clinical signs (44). Khanna et al administered 100 mg i.v. methylprednisolone, 1 g acetaminophen, and 50 mg diphenhydramine as pre-medication (59). One patient developed a urinary tract infection, one had worsening of hypertension, and one died from sudden cardiac arrest three months after the Second infusion.
The relatively small numbers of cases thus far reported make any valid conclusions impossible to draw. Reconciling the types and severities of the side effects thus far encountered in patients with GD with the experience in other diseases where RTX is administered may provide valuable insights into what we can expect prospectively with wider use of the drug. Until results from a randomized study with standardized recruitment becomes available, any reassurances or concerns about the scope of side effects remains speculative.
Acknowledgments
This work was supported by an unrestricted grant from the Novo Nordisk Foundation and Roche A/S, Denmark, National Institutes of Health grants EY08976, EY011708, EY016339, DK063121, an unrestricted grant and a career development award from Research to Prevent Blindness and the Bell Charitable Foundation.
LH and CHN have received consultancy fees from Roche A/S Denmark.
Footnotes
Financial disclosure:
The authors have no proprietary or commercial interest in any material discussed in this article.
The present manuscript constitutes an invited review for LH as the 2009 Pitt-Rivers Lecturer at the Society for Endocrinology BES, Harrogate 18 March 2009.
References
- 1.Weetman AP. Graves’ disease. The New England Journal of Medicine. 2000;343:1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 2.Brent GA. Clinical practice. Graves’ disease. The New England Journal of Medicine. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 3.Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. The Journal of Clinical Endocrinology and Metabolism. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 4.Hegedus L. Treatment of Graves’ hyperthyroidism: evidence-based and emerging modalities. Endocrinology and Metabolism Clinics of North America. 2009;38:355–371. doi: 10.1016/j.ecl.2009.01.009. ix. [DOI] [PubMed] [Google Scholar]
- 5.Wartofsky L, Glinoer D, Solomon B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129–135. doi: 10.1089/thy.1991.1.129. [DOI] [PubMed] [Google Scholar]
- 6.Bartalcna L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. The New England Journal of Medicine. 2009;360:994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 7.Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, et al. Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. The Journal of Clinical Endocrinology and Metabolism. 2009;94:2708–2716. doi: 10.1210/jc.2009-0376. [DOI] [PubMed] [Google Scholar]
- 8.Watt T, Grocnvold M, Rasmussen AK, et al. Quality of life in patients with benign thyroid disorders. A review. European Journal of Endocrinology. 2006;154:501–510. doi: 10.1530/eje.1.02124. [DOI] [PubMed] [Google Scholar]
- 9.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedus L. Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16:709–710. doi: 10.1089/thy.2006.16.709. [DOI] [PubMed] [Google Scholar]
- 10.Salvi M, Vannucchi G, Campi I, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. European Journal of Endocrinology. 2006;154:511–517. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 11.Reff ME, Carner K, Chambers KS. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 12.Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Annals of Oncology. 2003;14:520–535. doi: 10.1093/annonc/mdg175. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen CH, El Fassi D, Hasselbalch HC, et al. B-cell depletion with rituximab in the treatment of autoimmune diseases. Graves’ ophthalmopathy the latest addition to an expanding family. Expert Opinion in Biological Therapy. 2007;7:1061–1078. doi: 10.1517/14712598.7.7.1061. [DOI] [PubMed] [Google Scholar]
- 14.Levine TD, Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using Rituximab. Neurology. 1999;52:1701–1704. doi: 10.1212/wnl.52.8.1701. [DOI] [PubMed] [Google Scholar]
- 15.Edwards JC, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 2001;40:205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- 16.Edwards JC, Cambridge G, Abrahams VM. Do self-perpetuating B lymphocytes drive human autoimmune disease? Immunology. 1999;97:188–196. doi: 10.1046/j.1365-2567.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasselbalch HC. B-cell depletion with rituximab - a targeted therapy for Graves’ disease and autoimmune thyroiditis. Immunology Letters. 2003;88:85–86. doi: 10.1016/s0165-2478(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang SH, Baker JR., Jr Targeting B cells in Graves’ disease. Endocrinology. 2006;147:4559–4560. doi: 10.1210/en.2006-0852. [DOI] [PubMed] [Google Scholar]
- 19.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 20.Takemura S, Klimiuk PA, Braun A, et al. T cell activation in rheumatoid synovium is B cell dependent. Journal of Immunology. 2001;167:4710–4718. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 21.Serreze DV, Fleming SA, Chapman HD, et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. Journal of Immunology. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 22.Falcone M, Lee J, Patstone G, et al. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. Journal of Immunology. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 23.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. The New England Journal of Medicine. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 24.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. The New England, Journal of Medicine. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rifkin IR, Leadbetter EA, Beaudette BC, et al. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. Journal of Immunology. 2000;165:1626–1633. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen C-H, Leslie RG, Jepsen BS, et al. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. European Journal of Immunology. 2001;31:2660–2668. doi: 10.1002/1521-4141(200109)31:9<2660::aid-immu2660>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen CH, Hegedus L, Leslie RG. Autoantibodies in autoimmune thyroid disease promote immune complex formation with self antigens and increase B cell and CD4+ T cell proliferation in response to self antigens. European Journal of Immunology. 2004;34:263–272. doi: 10.1002/eji.200324413. [DOI] [PubMed] [Google Scholar]
- 28.Smeland EB, Blomhoff HK, Funderud S, et al. Interleukin 4 induces selective production of interleukin 6 from normal human B lymphocytes. Journal of Exerimental Medicine. 1989;170:1463–1468. doi: 10.1084/jem.170.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skok J, Poudrier J, Gray D. Dendritic cell-derived IL-12 promotes B cell induction of Th2 differentiation: a feedback regulation of Th1 development. Journal of Immunology. 1999;163:4284–4291. [PubMed] [Google Scholar]
- 30.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Annals of Neurology. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 31.Fillatrea S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10. Nature Immunology. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 32.Mauri C, Gray D, Mushtaq N, et al. Prevention of arthritis by interleukin 10-producing B cells. Journal of Experimental Medicine. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekh VV, Prasad DV, Banerjee PP, et al. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CDS+ T cells: role of TGF-beta 1. Journal of Immunology. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 34.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunology Reviews. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 35.Armengol MP, Juan M, Lucas-Martin A, et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. American Journal of Pathology. 2001;159:861–873. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segundo C, Rodriguez C, Aguilar M, et al. Differences in thyroid-infiltrating B lymphocytes in patients with Graves’ disease: relationship to autoantibody detection. Thyroid. 2004;14:337–344. doi: 10.1089/105072504774193159. [DOI] [PubMed] [Google Scholar]
- 37.El Fassi D, Clemmensen O, Nielsen CH, Silkiss RZ, Hegedus L. Evidence of intrathyroidal B-lymphocyte depletion after rituximab therapy in a patient with Graves’ disease. The Journal of Clinical Endocrinology and Metabolism. 2007;92:3762–3763. doi: 10.1210/jc.2007-1238. [DOI] [PubMed] [Google Scholar]
- 38.El Fassi D, Nielsen CH, Kjeldsen J, et al. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut. 2008;57:714–715. doi: 10.1136/gut.2007.138305. [DOI] [PubMed] [Google Scholar]
- 39.Paran D, Trej'o L, Caspi D. Clinical images: B cell depletion in the appendix following rituximab treatment. Arthritis and Rheumatism. 2006;54:2151. doi: 10.1002/art.21871. [DOI] [PubMed] [Google Scholar]
- 40.Teng YK, Levarht EW, Hashemi M, et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis and Rheumatism. 2007;56:3909–3918. doi: 10.1002/art.22967. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen JF, El Fassi D, Nielsen CH, et al. Evidence of orbital B and T cell depletion after rituximab therapy in Graves’ ophthalmopathy. Acta Ophthalmologica. 2009;87:927–929. doi: 10.1111/j.1755-3768.2009.01647.x. [DOI] [PubMed] [Google Scholar]
- 42.El Fassi D, Nielsen CH, Bonnema SJ, et al. B lymphocyte depletion with the monoclonal antibody Rituximab in Graves’ disease. A controlled pilot study. The Journal of Clinical Endocrinology and Metabolism. 2007;92:1769–1772. doi: 10.1210/jc.2006-2388. [DOI] [PubMed] [Google Scholar]
- 43.Salvi M, Vannucchi G, Campi J, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. European Journal of Endocrinology. 2007;156:33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 44.Heemstra KA, Toes RE, Sepers J, et al. Rituximab in relapsing Graves’ disease, a phase II study. European Journal of Endocrinology. 2008;159:609–615. doi: 10.1530/EJE-08-0084. [DOI] [PubMed] [Google Scholar]
- 45.El Fassi D, Banga JP, Gilbert JA, et al. Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clinical Immunology. 2009;130:252–258. doi: 10.1016/j.clim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.De BA, Sansone D, Coronella C, et al. Serum antibodies to collagen XIII: a further good marker of active Graves’ ophthalmopathy. Clinical Endocrinology (Oxf) 2005;62:24–29. doi: 10.1111/j.1365-2265.2004.02167.x. [DOI] [PubMed] [Google Scholar]
- 47.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. The Journal of Clinical Endocrinology and Metabolism. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 48.Leandro MJ, Edwards JC, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Annals of Rheumatic Diseases. 2002;61:883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis and Rheumatism. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 50.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. The New England Journal of Medicine. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 51.Gottenberg JE, Guillevin L, Lambotte O, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Annals of Rheumatic Diseases. 2005;64:913–920. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis and Rheumatism. 2006;54:1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 53.Cambridge G, Leandro MJ, Teodorescu M, et al. B cell depletion therapy in systemic lupus erythematosus: effect on autoantibody and antimicrobial antibody profiles. Arthritis and Rheumatism. 2006;54:3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 54.Vallerskog T, Gunnarsson I, Widhe M, et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clinical Immunology. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis and Rheumatism. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 56.Moser K, Muehlinghaus G, Manz R, et al. Long-lived plasma cells in immunity and immunopathology. Immunology Letters. 2006;103:83–85. doi: 10.1016/j.imlet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Stasi R, Stipa E, Del Poeta G, et al. Long-term observation of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis treated with rituximab. Rheumatology (Oxford) 2006;45:1432–1436. doi: 10.1093/rheumatology/kel098. [DOI] [PubMed] [Google Scholar]
- 58.Douglas RS, Tsirbas A, Gordon M, et al. Development of criteria for evaluating clinical response in thyroid eye disease using a modified Delphi technique. Archives of Ophthalmology. 2009;127:1155–1160. doi: 10.1001/archophthalmol.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khanna D, Chong KK, Afifyan NF, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010;117:133–139. doi: 10.1016/j.ophtha.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krassas GE, Stafilidou A, Boboridis KG. Failure of rituximab treatment in a case of severe thyroid ophthalmopathy unresponsive to steroids. Clinical Endocrinology. 2010 doi: 10.1111/j.1365-2265.2009.03762.x. in press. [DOI] [PubMed] [Google Scholar]
- 61.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD Ig mu null mice. Journal of Experimental Medicine. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo J, Wang Y, Rapoport B, et al. Evidence for antigen presentation to sensitized T cells by thyroid peroxidase (TPO)-specific B cells in mice injected with fibroblasts co-expressing TPO and MHC class II. Clinical and Experimental Immunology. 2000;119:38–46. doi: 10.1046/j.1365-2249.2000.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toubi E, Kessel A, Slobodin G, et al. Changes in macrophage function after rituximab treatment in patients with rheumatoid arthritis. Annals of Rheumatic Diseases. 2007;66:818–820. doi: 10.1136/ard.2006.062505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keren Z, Braun-Moscovici Y, Markovits D, et al. Depletion of B lymphocytes in rheumatoid arthritis patients modifies IL-8-anti-IL-8 autoantibody network. Clinical Immunology. 2009;113:108–116. doi: 10.1016/j.clim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Bower M, Veralitch O, Szydlo R, et al. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood. 2009;113:4521–4524. doi: 10.1182/blood-2008-12-197053. [DOI] [PubMed] [Google Scholar]
- 66.Tamimoto Y, Horiuchi T, Tsukamoto H, et al. A dose-escalation study of rituximab for treatment of systemic lupus crythematosus and Evans’ syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology. 2008;47:821–827. doi: 10.1093/rheumatology/ken071. [DOI] [PubMed] [Google Scholar]
- 67.Ferraro AJ, Drayson MT, Savage CO, MacLennan IC. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. European Journal of Immunology. 2008;38:292–298. doi: 10.1002/eji.200737557. [DOI] [PubMed] [Google Scholar]
- 68.Anolik JH, Barnard J, Cappione A, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus crythematosus. Arthritis and Rheumatism. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 69.Smith TJ. B cell depletion in Graves’ disease: The right answer for the wrong question? The Journal of Clinical Endocrinology and Metabolism. 2007;92:1620–1622. doi: 10.1210/jc.2007-0463. [DOI] [PubMed] [Google Scholar]
- 70.Hegedüs L. Optimizing remission rates using antithyroid drugs for Graves’ disease. Clinical Endocrinology. 2010 doi: 10.1111/j.1365-2265.2009.03778.x. in press PMID:20050856. [DOI] [PubMed] [Google Scholar]
- 71.Goetz M, Atreya R, Ghalibafian M, et al. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflammatory Bowel Disease. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]