Abstract
Background
In recent studies primary bowing tremor has been described; however, tremor frequency has never been quantitatively assessed. In addition to describing phenomenological aspects of tremor we thus aimed at assessing tremor frequency. Our hypothesis was that primary bowing tremor is similar to the phenomenological aspects and frequency of primary writing tremor.
Methods
We quantified primary bowing tremor in four violinists under two conditions: open strings and a G major scale. Data were analyzed using empirical mode decomposition because it takes into account non-stationarity and non-linearity of signals. For each patient we further described tremor phenomenology and assessed symptom onset, risk factors, medication, and family history with a structured anamnesis. We compare the findings to previous findings for primary bowing tremor and primary writing tremor.
Results
We mainly found a flexion–extension tremor of the wrist with a frequency range of 4.7–6.7 Hz. There was no significant difference between the conditions. Mean onset age was 43±2.4 years. Medication included trihexyphenidyl, propranolol, primidone, and botulinum toxin. We found a positive family history in two patients and an injury prior to symptom onset in another two patients. Comparison of onset age, frequency range, family history, and injuries prior to onset revealed that our findings are very similar to previous findings on primary bowing tremor and primary writing tremor.
Discussion
Our findings confirmed our hypothesis that primary bowing tremor is similar to primary writing tremor, with regard to phenomenology and epidemiology as well as tremor frequency. There was no difference in tremor frequency between conditions, suggesting that tremor is not influenced by bimanual coordination or bowing speed. Our findings thus provide new phenomenological aspects and may contribute to a better understanding of primary bowing tremor.
Keywords: Essential tremor, dystonia, dystonic tremor, empirical mode decomposition, Hilbert spectrum, musician
Introduction
Task-specific tremors occur mainly during specific activities.1 The most common form is primary writing tremor (PWT), first described by Rothwell.2 Two types exist: Type A, referring to tremor induced by writing (task specific); and type B, referring to tremor induced when holding the arm in the position for writing (position specific). It is an ongoing discussion, whether PWT is a type of focal dystonia, a subentity of essential tremor or a nosological entity of its own.1,3–6 Recent research suggests that it is a separate entity. 5–10
In bowed string instrumentalists task-specific tremors may occur unilaterally in the right arm11–13 as primary bowing tremor (PBT), a highly disabling condition that may threaten a professional career. In two recent papers it was proposed that PBT may be regarded an entity of its own, similar to PWT.12,13 However, in neither of these papers was tremor frequency quantified. Since frequency is one of the main criteria that help distinguish between tremor syndromes,1,14 our first aim was to quantify the frequency of PBT. In addition, we describe in detail PBT phenomenology with regard to tremor onset, response to medication, spreading of tremor to other activities, risk factors, and positive family history, and compare our findings with the findings from five patients with PBT described by Lederman13 and with PWT patients.4–6,8–10 Furthermore, it is known that in long bow strokes a finger change in the left hand may have an influence on the action of the bowing hand.15 Our second aim was therefore to investigate whether bimanual coordination or a change in bowing speed has an influence on tremor, to gain more insight into tremor phenomenology.
Since PBT is an action-induced tremor, difficulty arises when measuring tremor of the arm during execution of voluntary movements. One can therefore not rule out that voluntary movements interfere with the measured tremor signal. Furthermore, it is known that pathological tremor, in contrast to physiological tremor, is a nonlinear process.14,16 Finally, our signal was highly non-stationary. To address these three problems we decided to analyze the data with empirical mode decomposition (EMD),17,18 a data-driven signal-processing method that takes into account the non-linearity and non-stationarity of signals.17,19 Furthermore, it has been shown that with the EMD, voluntary movement can be distinguished from tremor.20,21
Methods
The study was approved by the local ethics committee. Written informed consent was obtained from all participants.
We assessed PBT of the wrist in four professional violinists (three men, one woman) aged 48–62 (mean 43±2.4 years) (Table 1). Since playing long, slow legato notes using the entire length of the bow (≈65 cm from the frog [i.e. the end of the bow held by the player] to the tip) evoked the most severe symptoms, we chose to measure this bowing technique. To assess whether bimanual coordination or bowing speed has an influence on tremor, we measured two conditions paced by a metronome set to 40 bpm. 1) A G major scale across three octaves using the whole bow (GM). Two beats were used per bowing direction during which eight notes were played. 2) Open strings (OS) with four beats per bowing direction. In addition, we measured postural tremor in both arms, during which the patients held the arm in position for playing the violin. Finally, rest tremor was measured for which the patient was asked to completely relax the arm.
Table 1. Phenomenology, Treatment, and Frequency of PBT for the four patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Gender | male | male | male | female |
| Age in years | 62 | 54 | 48 | 50 |
| Age when starting to play the violin | 8 | 12 | 4 | 13 |
| Age at onset of PBT | 46 | 43 | 40 | 43 |
| Tremor duration in years | 16 | 11 | 8 | 7 |
| Unilateral right tremor | y | y | y | y |
| Previous trauma | y | n | n | y |
| Spreading of tremor | y | Y | y | n |
| Postural tremor | n | n | n | y |
| Rest tremor | n | n | n | n |
| Medication | Prop, Thx, Gabap, Topi, Prim | Prim, Prop | Prim, Prop | Prop |
| Botulinum toxin | y | n | y | n |
| Ethanol responsive | y | y | little | n |
| Family history for tremor | y | y | n | n |
| Deep brain stimulation | n | n | n | n |
| Tremor frequency GM/OS | 5.9/6.7 Hz | 4.7/4.9 Hz | 6.5/5.5 Hz | 6.1/5.7 Hz |
Gabap, Gabapentin; GM, G Major Scale Condition; PBT, Primary Bowing Tremor; OS, Open-string Condition; Prim, Primidone; Prop, Propranolol; Thx, Trihexyphenidyl; Topi, Topiramat.
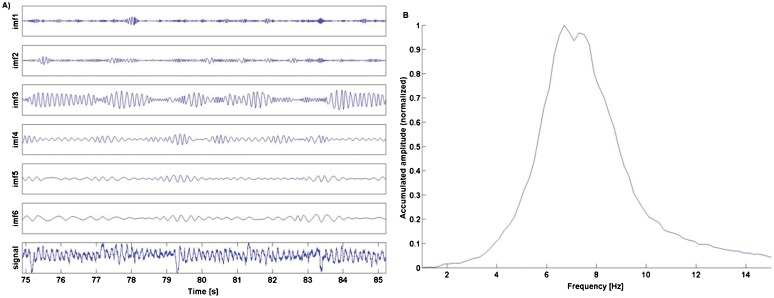
A three-dimensional (3D) accelerometer (biovision, Wehrheim, Germany, 8×8×11 mm; 4 g; DC – 500 Hz; max 50 g) was attached to the metacarpo-phalangeal (MCP) joint of the index finger of the right hand. With regard to the hand the x-axis was in a lateral-medial direction, the y-axis in a proximal-distal direction and the z-axis perpendicular to the dorsum of the hand (Figure 1). The z-axis thus represented the direction of tremor and was chosen for further analyses. Data were bandpass filtered using a fourth-order Butterworth filter (cutoff 1–50 Hz), applied back and forth to compensate for phase shift. The EMD was applied, which decomposes the signal into basic components, called intrinsic mode functions (IMFs, Figure 2A). The resulting IMFs can be considered as simple oscillatory modes with the following properties: 1) the number of extrema and the number of zero crossings is either equal or differ at most by one, and 2) at any time the mean value of the envelope defined by the local maxima and the envelope defined by the local minima is zero. Recent studies investigating tremor have shown that IMFs can be used to identify distinct frequency bands associated with physical or physiological phenomena, e.g. different types of tremor.22–24 In these studies tremor was assessed with a gyroscope; however, the EMD is viable for time-frequency data analysis without making presuppositions on how the signal is acquired.19 It has thus also been applied to accelerometer data.17,25 We therefore consider the EMD a valid approach to our data. EMD was performed in Matlab using the EMD package of Rilling et al.26 (http://perso.ens-lyon.fr/patrick.flandrin/emd.html). A Hilbert transform was applied to the IMFs to obtain the instantaneous frequency and amplitude (Hilbert spectrum). In the current study IMF3 was identified as the mode containing the tremor signal in all patients. Finally, tremor history was acquired, including symptom onset, response to medication, and spreading of tremor to other activities, as well as risk factors such as previous trauma and family history. This information was obtained with a structured anamnesis asking for the corresponding item. In all patients we assessed parkinsonism by investigating signs for rigor, rest tremor, or akinesis.
Figure 1. Placement of the Accelerometer on the Dorsum of the Hand.
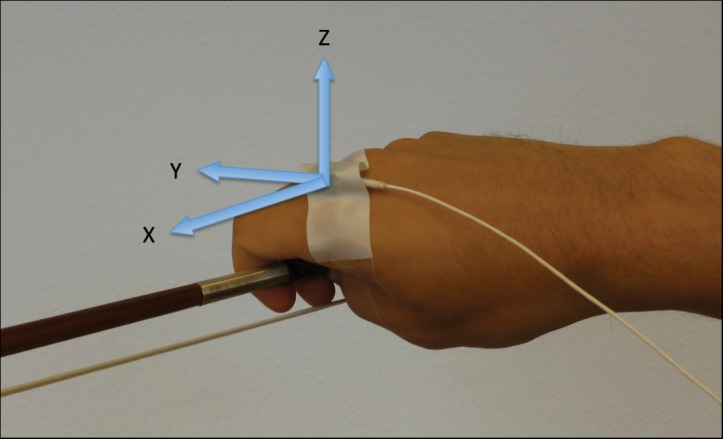
The three axes (x, y, z) are indicated by arrows. With regard to the hand the x-axis is in a lateral-medial direction, the y-axis in a proximal-distal direction and the z-axis perpendicular to the dorsum of the hand. The z-axis was thus chosen for the tremor assessment.
Figure 2. Empirical Mode Decomposition and Marginal Hilbert spectrum.
(A) Empirical mode decomposition with intrinsic mode functions (IMF) 1–6 and the original signal for patient 1 with IMF 3 containing the tremor signal. (B) Marginal Hilbert amplitude spectrum of IMF 3 for patient 1 playing the G major scale condition. The main frequency (peak value) was 6.7 Hz.
Results
Patient 1
Patient 1, male, aged 62, has played in a professional orchestra for over 30 years. First tremor symptoms occurred at the age of 46 after a car accident and a subsequent concert, which led to a pain syndrome in the right shoulder. When at the violin, a flexion–extension tremor and pronation–supination tremor became apparent that was most disturbing when long and slow notes were played. The main frequency was 5.9 Hz for the OS condition and 6.7 Hz for the GM condition (Figure 2B). There were no signs of parkinsonism or other extrapyramidal disorders. Since 2009, the tremor has spread to other activities, such as filling a glass of water; however, it is less severe and still unilateral. His father suffered from writer's cramp or PWT. Magnetic resonance imaging revealed a single, frontal white matter lesion. A dopamine transporter (DAT) scan, full blood count, and electrophysiological measures for motor evoked potentials were normal. Somatosensory evoked potentials were marginally prolonged. Tremor responded to beta-blockers (propranolol 20 mg); however, the effect wore off after 1 year. Alcohol reduced tremor. Trihexiphenidyl (6 mg) had no effect. Botulinum toxin injections had a slight effect. Primidone (62.5 mg) helped but had to be discontinued due to side effects. Neither cabapentin (2400 mg) nor topiramat (200 mg) were useful. Deep brain stimulation (DBS) was declined.
Patient 2
Patient 2, male, aged 54, has played in a professional orchestra for over 20 years. First tremor symptoms appeared at the age of 43, only while playing the violin. When at the violin, a flexion–extension tremor became apparent that was most severe for long legato notes. The main frequencies for tremor were 4.7 Hz in the OS condition and 4.9 Hz in the GM condition. No parkinsonism or signs of other extrapyramidal symptoms were present. Two years ago, the tremor spread to shaving, but less severely. His mother suffered from a right-sided postural tremor. Whether the tremor was action induced or task specific was not remembered. Primidone (60 mg) and beta-blockers (propranolol, dosage not remembered) led to an improvement but had to be discontinued due to side effects. The tremor responded to alcohol. Botulinum toxin was declined.
Patient 3
Patient 3, male, aged 48, has played in a professional orchestra for over 20 years. He had the first symptoms at the age of 20. However, at that time the tremor appeared exclusively under stress and did not interfere with his violin playing until the age of 40, when the tremor occurred independently of the stress level or the situation. Given the commonness of stress-induced bowing tremor in string instrument players, we thus consider the age of 40 as PBT onset. At the violin, a flexion–extension tremor became apparent, which was most severe for long legato notes. Interestingly, postural tremor could be provoked when bringing a fast up-bow movement to a sudden stop at the frog. The main tremor frequency was 6.5 Hz for the OS condition and 5.5 Hz for the GM condition. Further clinical examination did not reveal any signs of parkinsonism or other extrapyramidal disorders. A DAT scan, magnetic resonance imaging, and a full blood count were normal. Over about 4 years, tremor spread to when filling a glass of water. Family history with regard to movement disorders was negative. The combination of propranolol (60 mg) and primidone (62.5 mg) reduced tremor. Alcohol had little effect. Botulinum toxin had a slight effect. DBS was declined.
Patient 4
Patient 4, female, aged 50, had the first tremor symptoms at the age of 43, following a playing-induced overstrain injury of the right shoulder. At the violin, a flexion–extension tremor became apparent, which was most prominent and disturbing when playing long and slow legato notes. There was a slight position-dependent tremor. The tremor main frequency was 6.1 Hz for the OS condition and 5.7 Hz for the GM condition. There were no signs of parkinsonism or other extrapyramidal disorders. The tremor had not spread to other activities. Family history with regard to movement disorders was negative. The tremor responded to beta-blockers (propranolol 20 mg). Trihexiphenidyl (6 mg) did not have an effect. Alcohol responsiveness could not be confirmed. Botulinum toxin and DBS were declined.
Statistical analysis
The frequency range was 4.7–6.7 Hz. Mean frequency (± SD) for the GM condition was 5.8 Hz (±0.8 Hz) and for the OS condition 5.7 Hz (±0.8 Hz). The difference between the conditions was not significant (Wilcoxon signed-rank-test, p = 0.875).
Discussion
We report on four violinists with PBT, assessing tremor frequency, describing its phenomenology, and comparing our findings to previous findings in PBT and PWT. We found a mean frequency (±SD) of 5.8 (±0.8) Hz (GM) and 5.7 (±0.8) Hz (OS). Tremor frequency ranged from 4.7 to 6.7 Hz. Two recent studies, one case series including four violinists and one violist13 and one case study of a violinist,12 addressed the phenomenology of PBT in detail. The case series described features that were atypical for essential tremor and dystonia, and it was thus concluded that, analogous to PWT, PBT may be an entity of its own.13 The mean onset age of PBT in this study was 45±23.2 years, which is almost identical to the mean onset age of our patients (43±2.4 years) and only slightly younger than the mean onset age in PWT of 50.1 years.4 Two of our patients (3 and 4) had type A and two (3 and 4) had type B tremor, which is similar to findings by Lederman,13 where two patients had type A and three patients type B tremor. Thus, as in PWT,2 both types of task-specific tremors exist in PBT and seem to occur almost equally often. In three of our patients, tremor had spread to other tasks. However, it remained most prominent and caused most impairment when the patient was at the violin. The same finding was described by Lederman, where in two patients tremor had spread. This phenomenon has been described in PWT4,27 and a strict task specificity has been questioned in recent discussions.10,27 An “increasing tendency to appear the more the act resembles writing”10 (or in our case playing the violin) and as an evolution of the disorder in the course of time27 have been discussed. Two of our patients (1 and 2) and two of the patients described by Lederman13 had a positive family history of movement disorders, which alludes to a genetic for PBT risk factor in PWT.4,27
In two patients (1 and 4) trauma preceded the onset of PBT. Similarly, a coincidence between previous trauma and PWT was described in 19% of cases,4 alluding to environmental risk factors playing a role in the development of PBT as well. We cannot rule out, however, that the trauma merely unmasked the tremor rather than being causative. The study by Lederman did not mention a traumatic event prior to PBT onset.
With regard to treatment, Botulinum toxin led to an improvement in two of our patients (1 and 3), which is in accordance with studies reporting improvement in PWT with Botulinum toxin.28,29 Oral medication included propranolol, primidone, gabapentin and trihexiphenidyl, of which primidone and/or propranolol were most effective for all patients. Trihexiphenidyl had an effect in patient 1. Likewise, in the case series by Lederman, a beneficial effect of propranolol and trihexyphenidyl (patient 1 and 4, respectively) was reported.13 This is in accordance with reports that about 60% of patients with PWT benefit from oral medication6 and 33% respond specifically to primidone or propranolol.4 Alcohol relieved symptoms in patient 1 and 2, as well as in patient 5 described by Lederman,13 which is similar to PWT, where an improvement has been reported in about 33% of the cases.4
What is new in our study is that by quantifying tremor we could include a comparison of tremor frequency ranges between PBT and PWT. We found a frequency range of 4.7–6.7 Hz, which is comparable to the range reported in PWT (4.1–7.3 Hz).4 We are aware, however, that this frequency range is not specific for PWT and that a conclusion with regard to whether or not PBT may be an entity of its own cannot be drawn on the basis of our data. Further electrophysiological studies are necessary to investigate this hypothesis.
Finally, we found no difference of tremor frequency between the conditions. This suggests a robustness of tremor against bimanual coordination or bowing speed, at least for the conditions we measured, and thus adds another phenomenological feature to PBT.
Video 1. Unmarked festgelegt von lee.
First a short up-bow movement on the g string and then a short up- and down-bow movement on the d string are visible and audible. Tremor is clearly visible and audible as well and most severe at the frog of the bow.
Footnotes
Funding: None.
Financial Disclosures: EA: Research-grants from the German Research Foundation (Al 269/5-3, Al 269/7-3), from the European Marie Curie Actions, from the Lichtenberg Scholarship Program of Lower Saxony and from the Dystonia Medical Research Foundation. He receives royalties from the publication of the book “Music, Motor Control and the Brain”, Oxford University Press 2006. He received honoraria for teaching courses on the application of botulinum toxin A from the following: Allergan (Botox), Ipsen Pharma (Dysport), Desitin/Merz (Xeomin).
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell JC, Traub MM, Marsden CD. Primary writing tremor. J Neurol Neurosurg Psychiatry. 1979;42:1106–1114. doi: 10.1136/jnnp.42.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum F, Jankovic J. Focal task-specific tremor and dystonia: Categorization of occupational movement disorders. Neurology 198. 38:522–527. doi: 10.1212/WNL.38.4.522. [DOI] [PubMed] [Google Scholar]
- 4.Bain PG, Findley LJ, Britton TC, et al. Primary writing tremor. Brain J Neurol. 1995;118(Pt 6):1461–1472. doi: 10.1093/brain/118.6.1461. [DOI] [PubMed] [Google Scholar]
- 5.Meunier S, Bleton JP, Mazevet D, et al. TENS is harmful in primary writing tremor. Clin Neurophysiol. 2011;122:171–175. doi: 10.1016/j.clinph.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Hai C, Yu-ping W, Hua W, Ying S. Advances in primary writing tremor. Parkinsonism Relat Disord. 2010;16:561–565. doi: 10.1016/j.parkreldis.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Berg D, Preibisch C, Hofmann E, Naumann M. Cerebral activation pattern in primary writing tremor. J Neurol Neurosurg Psychiatry. 2000;69:780–786. doi: 10.1136/jnnp.69.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modugno N, Nakamura Y, Bestmann S, Curra A, Berardelli A, Rothwell J. Neurophysiological investigations in patients with primary writing tremor. Mov Disord. 2002;17:1336–1340. doi: 10.1002/mds.10292. [DOI] [PubMed] [Google Scholar]
- 9.Espay AJ, Chen R. Primary writing tremor and writer's cramp: Distinct phenomenology, diverging pathophysiology. Clin Neurophysiol. 2011;122:5–6. doi: 10.1016/j.clinph.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Bain PG. Task-specific tremor. In: Vinken PJ, Bruyn GW, . Handbook of clinical neurology. Elsevier, New York, Edinburgh. Vol. 100. 2011. pp. 711–718. editors. [DOI] [PubMed] [Google Scholar]
- 11.Lederman, R J. Tremor in instrumentalists: Influence of tremor type on performance. Med Probl Perform Art. 2007;22:70–3. [Google Scholar]
- 12.Lee A, Altenmüller E. Primary task-specific tremor: An Entity of its own? Med Probl Perform Art. 2012;27:224–226. [PubMed] [Google Scholar]
- 13.Lederman RJ. Primary bowing tremor: A task-specific movement disorder of string instrumentalists. Med Probl Perform Art. 2012;27:219–223. [PubMed] [Google Scholar]
- 14.Deuschl G. Tremor. Das Neurophysiol-Labor. 2008;30:49–57. [Google Scholar]
- 15.Winold H, Thelen E. Coordination and control in the bow arm movements of highly skilled cellists. Ecol Psychol. 1994;6:1–31. doi: 10.1207/s15326969eco0601_1. [DOI] [Google Scholar]
- 16.Gantert C, Honerkamp J, Timmer J. Analyzing the dynamics of hand tremor time series. Biol Cybern. 1992;66:479–484. doi: 10.1007/BF00204112. [DOI] [PubMed] [Google Scholar]
- 17.Huang NE, Shen Z, Long SR, et al. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Math Phys Eng Sci. 1998;454:903–995. doi: 10.1098/rspa.1998.0193. [DOI] [Google Scholar]
- 18.Hogan N. Adaptive control of mechanical impedance by coactivation of antagonist muscles. IEEE Trans Autom Control. 1984;29:681–690. doi: 10.1109/TAC.1984.1103644. [DOI] [Google Scholar]
- 19.Huang NE. Singapore, Hackensack, NJ, London: World Scientific; Introduction to the Hilbert–Huang transform and its related mathematical problems; pp. 1–26. 2005p. [Google Scholar]
- 20.Lima ER, Andrade AO, Pons JL, Kyberd P, Nasuto SJ. Empirical mode decomposition: A novel technique for the study of tremor time series. Med Biol Eng Comput. 2006;44:569–582. doi: 10.1007/s11517-006-0065-x. [DOI] [PubMed] [Google Scholar]
- 21.Gallego JA, Rocon E, Koutsou AD, Pons JL. Analysis of kinematic data in pathological tremor with the Hilbert-Huang transform. IEEE. 2011:80–3. [cited 2011]. p. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber = 5910493. [Google Scholar]
- 22.Rocon E, Pons JL, Andrade AO, Nasuto SJ. Application of EMD as a novel technique for the study of tremor time series. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc. 2006;(Suppl):6533–6536. doi: 10.1109/IEMBS.2006.260871. [DOI] [PubMed] [Google Scholar]
- 23.Silchenko AN, Adamchic I, Pawelczyk N, et al. Data-driven approach to the estimation of connectivity and time delays in the coupling of interacting neuronal subsystems. J Neurosci Methods. 2010;191:32–44. doi: 10.1016/j.jneumeth.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Li K, Hogrel J-Y, Duchêne J, Hewson DJ. Analysis of fatigue and tremor during sustained maximal grip contractions using Hilbert-Huang Transformation. Med Eng Phys. 2012;34:832–8340. doi: 10.1016/j.medengphy.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Zhang Y, Zheng H. Hilbert-Huang transform and marginal spectrum for detection and diagnosis of localized defects in roller bearings. J Mech Sci Technol. 2010;23:291–301. [Google Scholar]
- 26.Rilling G, Flandrin P, Gonçalves P. On empirical mode decomposition and its algorithms. Grado, Italy; 2003 Jun. [Google Scholar]
- 27.Ondo WG, Satija P. Task-specific writing tremor: Clinical phenotypes, progression, treatment outcomes, and proposed nomenclature. Int J Neurosci. 2012;122:89–91. doi: 10.3109/00207454.2011.630544. [DOI] [PubMed] [Google Scholar]
- 28.Singer C, Papapetropoulos S, Spielholz NI. Primary writing tremor: Report of a case successfully treated with botulinum toxin A injections and discussion of underlying mechanism. Mov Disord. 2005;20:1387–1388. doi: 10.1002/mds.20590. [DOI] [PubMed] [Google Scholar]
- 29.Papapetropoulos S, Singer C. Treatment of primary writing tremor with botulinum toxin type a injections: Report of a case series. Clin Neuropharmacol. 2006;29:364–367. doi: 10.1097/01.WNF.0000236765.00785.9C. [DOI] [PubMed] [Google Scholar]