Abstract
Cancer cachexia is a complex multifactorial syndrome characterized by loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. Recently, some amino acids and other amine dietary supplements have been highlighted in medical field due to positive effects upon diseases evolving skeletal muscle atrophy. Therefore, the aim of this brief review is to discuss the putative application of amines as dietary supplements to counteract skeletal muscle wasting on cancer cachexia. Specifically, we focus in two nutritional supplements: (1) branched-chain amino acids (BCAAs) and (2) creatine. Both BCAAs and creatine may attenuate proteolysis and enhance proteins synthesis in skeletal muscle. Although more experimental studies and clinical trials are still necessary to elucidate this therapeutic application, several evidences have demonstrated that amines supplementation is a promising coadjuvant treatment to cancer cachexia.
Keywords: Muscle atrophy, Wasting disease, Amino acids, BCAA, Creatine
Introduction
Cachexia is associated with approximately 80 % of severe cancer cases [1, 2] and it is responsible for more than 30 % of the deaths [3]. Cancer cachexia is a complex multifactorial syndrome characterized by loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment [4]. In fact, loss of skeletal muscle results in impaired daily activities, ultimately leading to respiratory failure and death. In this context, the lack of success of unimodal treatment led to the view that cachexia intervention should include multimodal treatment. Therefore, in order to mitigate severe changes on skeletal muscle metabolism and tropism, non-pharmacological strategies such as exercise training and nutritional supplementation have been considered as a coadjuvant treatment [3, 5, 6].
Amines are moderately polar organic substances containing a nitrogen atom with a lone pair. A large number of medically and biologically important compounds content an amine group, such as the 2-phenylethylamines (e.g., adrenaline and noradrenaline), some types of vitamins (e.g., B1 and B6) and neurotransmitters (e.g., acetycholine), and all the existing amino acids. Recently, some amino acids and other dietary supplements including amine groups (e.g., creatine) have been applied in several sports modalities due to their anabolic effects on skeletal muscle [7, 8]. Additionally, these supplements exert positive effects upon several diseases evolving skeletal muscle atrophy [9–11], including cancer [12–16].
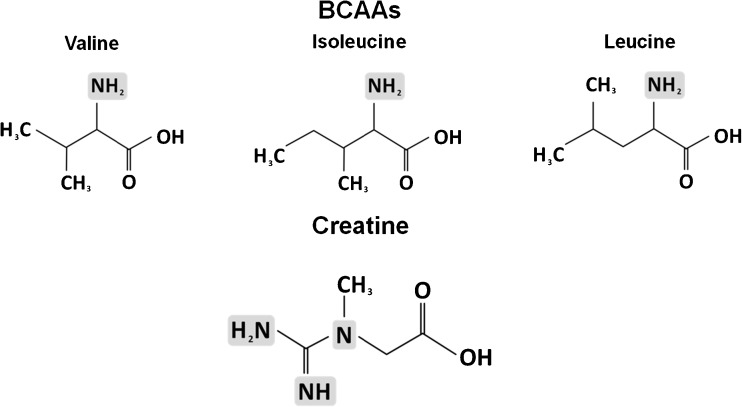
Therefore, the aim of this brief review is to discuss the putative application of amines as dietary supplements to counteract skeletal muscle wasting on cancer cachexia. Specifically, we focus in two nutritional supplements: (1) branched-chain amino acids (BCAAs) and (2) creatine. The chemical structures of these supplements are demonstrated in Fig. 1.
Fig. 1.
Chemical structures of branched chain amino acids (BCAAs) and creatine
BCAAs
As showed in the Fig. 1, the BCAAs include three amino acids (i.e., valine, isoleucine, and leucine) with a similar lateral radical chain. They are transaminated in skeletal muscle and exert an important role as energy source, along with anti-inflammatory and protein synthesis stimulatory effects [10, 17, 18]. Although the dietary ingestion of BCAAs seems adequate for physically active people, the BCAAs supplementation is suggested to achieve an ergogenic dose effect [19]. In this context, studies in sports science have demonstrated an important role as donors of glucose under glycogen depletion condition during exercise. Briefly, BCAAs, especially leucine, is transaminated in skeletal muscle during exercise, generating acetyl-CoA to the Krebs Cycle. Thus, this amino group can be transaminated to alanine, which can yield glucose in liver through the glucose-alanine cycle, resulting in liver glycogen spare [20]. Moreover, it is hypothesized that BCAAs supplementation may exert a therapeutic effect in disease underlying skeletal muscle wasting. BCAAs may attenuate proteolysis and enhance proteins synthesis in skeletal muscle, mainly through activation of mammalian target of rapamycin (mTOR) pathway and modulation of inflammatory status through glutamine production in situations characterized by lack of glutamine, such as cancer [17, 21]. Considering that skeletal muscle protein synthesis is decreased and the proteolysis is extremely enhanced in cancer cachexia (mainly via inflammatory pathways), it is possible to speculate that BCAAs supplementation might counteract the negative protein turnover in this case.
In fact, for more than two decades ago, some authors [22, 23] showed that BCAAs enriched diet improved amino acid utilization for protein synthesis on skeletal muscle without stimulation of tumor development. Additionally, it is well characterized that anorexia contributes to cachexia syndrome [3, 6]. Thus, the effects of BCCAs as donors of glucose, especially leucine [19], may mitigate the anorexia condition [24, 25] and, therefore, restore the normal protein turnover. In this context, several recent studies have showed positive effects of leucine supplementation per se upon skeletal muscle. Peters et al. (2011) demonstrated that muscle mass wasting was attenuated (in a dose-dependent manner) after leucine supplementation in mice inoculated subcutaneously with tumor cells [26]. In another study, leucine supplementation in rats inoculated with Walker 256 carcinoma demonstrated a slight reduction of muscle mass versus a severe reduction in non-supplemented animals [27]. Moreover, the association of leucine supplementation with aerobic exercise training in Walker tumor-bearing rats has increased protein synthesis in gastrocnemius, leading to higher total net protein content [28]. Ventrucci et al. (2004) found a reduction in proteasome signaling pathways in muscles of pregnant tumor-bearing rats [29]. The same authors also have found that leucine supplementation prevented serum insulin decrease, increased the expression of translation inhibition factors, and caused less protein breakdown, suggesting that leucine supplementation may stimulate protein synthesis and inhibit proteolysis in pregnant tumor-bearing rats [30].
In humans, patients receiving 12 g BCAAs/day during 4 weeks before tumor-removing surgery featured reduced brain free tryptophan and improved food intake [25]. As a limitation, this study presented a small sample size and no blinded design. Moreover, Tayek et al. (1986) observed in a crossover study including ten cancer patients that a formula containing 50 % BCAA (17 % leucine) was able to increase whole body protein synthesis and leucine balance, although no differences were found in the 24-h urinary nitrogen balance between groups [31]. Treatments with different concentrations of BCAAs supplementation were studied in gastric cancer patients. An improvement of metabolism without side effects was observed [32]. Altogether, these observations lead to the hypothesis that BCAAs can ameliorate nitrogen retention on skeletal muscle in cancer patients. However, it is important to note that the most of studies have a small number of patients and/or a poor study designs. Therefore, new randomized blinded placebo-controlled clinical trials are still necessary to confirm the beneficial effects of BCAAs (or leucine per se) in cancer patients.
Finally, it is known that ~5 % of leucine is converted into β-hydroxy-β-methylbutyrate (HMβ). HMβ supplementation has also been suggested as a potential nutritional strategy to counteract muscle wasting by stimulating protein synthesis and decreasing protein breakdown [16]. In fact, HMβ supplementation caused a significant reduction in tumor growth as well as a partial restoration of weight loss and skeletal muscle mass in tumor-bearing rats. It has been suggested that HMB prevents muscle protein degradation through attenuation of proteolysis-inducing factor (PIF) [33, 34]. Unfortunately, the lack of randomized blinded placebo-controlled clinical trials limits any extrapolation to patients [35].
Creatine
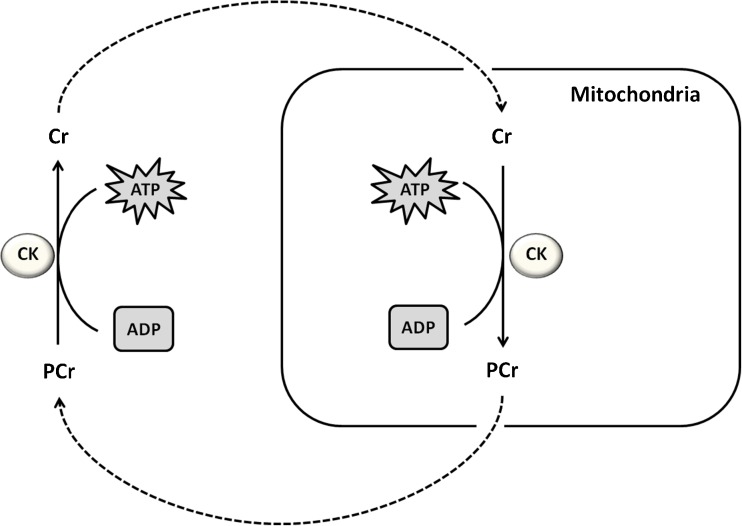
Creatine (N-aminoiminomethyl-N-methylglycine) exerts an essential role in rapid energy provision during skeletal muscle contraction. Briefly, a reversible reaction catalyzed by creatine kinase transfers an N-phosphoryl group from phosphorylcreatine to adenosine diphosphate, regenerating adenosine triphosphate [36] as illustrated in the Fig. 2.
Fig. 2.
Illustration of creatine role in rapid energy provision during skeletal muscle contraction. A reversible reaction catalyzed by creatine kinase transfers an N-phosphoryl group from phosphorylcreatine to adenosine diphosphate, regenerating adenosine triphosphate. Afterwards, phosphorylcreatine can be ressinthetized in mitochondria and transported to exert its role in adenosine triphosphate generation in citosol. Creatine kinase (CK), adenosine triphosphate (ATP), phosphocreatine (PCr), adenosine diphosphate (ADP)
Creatine is endogenously synthesized or ingested from diet. In addition, creatine can be ingested as a supplement, mainly in your monohydrate form. In fact, creatine supplementation is able to increase intramuscular creatine and phosphorylcreatine content, increasing the energy provision and the skeletal muscle mass and function [37, 38]. Thus, this supplement has been used by athletes [39], and recently, it has been emerged as a treatment to several diseases, including those characterized by skeletal muscle loss and dysfunction (for review, see [9]). For example, creatine supplementation is able to increase skeletal muscle strength in fibromyalgia patients [40] and to mitigate the decline of skeletal muscle function during aging [41].
Regarding to cancer disease, some experimental studies have been conducted to clarify the effects of creatine supplementation. These studies revealed that rodents receiving creatine in their regular diets presented a tumor regression after cancer inoculation [14, 42, 43]. Miller et al. (1993) showed that different doses of creatine supplementation attenuated tumor cell growth in mice after the inoculation of the tumor AC33TC. Although not analyzed in this study, the authors speculated that the tumor cells presented impairment in energy metabolism, resulting in low concentration of creatine as well as an exacerbated activity of the glycolytic pathway. Therefore, creatine supplementation could normalize cellular functions, acting as a therapeutic alternative for cancer control [43]. In the same year, Lillie et al., (1993) showed an increase in creatine kinase B expression in tumor cells and observed an N-phosphorylcyclocreatine accumulation. Consequently, this N-phosphorylcyclocreatine accumulation in tumor cells resulted in a detrimental effect on the tumor growth [44]. Additionally, after the creatine supplementation, Kristensen et al. (1999) also demonstrated a reduction in tumor growth of nude mice carrying a human colon adenocarcinoma LS174T [42].
Recently, Patra et al. 2008 and 2012 reported important findings to this research field. First, this group showed a progressive decrease of phosphocreatine, creatine, and creatine kinase levels on transformation of skeletal muscle into sarcoma. Second, they showed that the anticancer effect of methylglyoxal is significantly augmented in presence of creatine. In addition, the effects of methylglyoxal plus ascorbic acid were further augmented with the creatine supplementation and there were no sign of tumor. Interestingly, creatine and creatine kinase were also significantly elevated with the concomitant regression of tumor [14, 45].
In humans, Norman et al. (2006) conducted a preliminary clinical trial to evaluate the effects of 8 weeks of creatine supplementation as coadjuvant treatment in patients with colorectal cancer. The authors assessed the quality of life, muscle function, and nutritional parameters of individuals. This study did not found changes in most of the variables analyzed, however, indicated positive effect in handgrip strength test and anthropometric parameters such as capacitance and phase angle. Interestingly, these parameters were correlated with better prognosis in the patients, although these subjects were not considered cachectic during the study [46]. Conversely, it is known that creatine supplementation may counteract the side effects of glucocorticoid administration on skeletal muscle [9]. In order to assess these potential effects, Bourgeois et al. (2008) supplemented with creatine monohydrate nine children with acute lymphoblastic leukemia during two periods of 16 weeks interspaced by a wash out period of 6 weeks. During the supplementation protocol, the children also had been submitted to corticosteroids treatment. The authors found that creatine supplementation had no significant effects on the weight, body mass index, whole body bone mineral content, and fat-free mass. On the other hand, it was observed a reduced gain in body fat on supplemented patients when compared to the control group [13]. This is a pioneer study with humans; however, important limitations need to be addressed. First, the sample was highly heterogeneous (for example: age ranged from 3.5 to 17 years old). Second, different disease stages and corticosteroids treatment were used. Third, this is not a randomized and placebo-controlled trial. Finally, the children did not show weight or skeletal muscle losses. In other words, probably this is not a sample composed by cachectic patients.
Considering that creatine supplementation is able to increase strength, power, and muscle mass in healthy subjects as well as in some types of patients, we encourage the development of randomized trials to investigate the application of creatine supplementation on subjects with a well-characterized cancer cachexia state. In parallel, experiments in vitro or with animals seem absolutely necessary to understand the mechanisms underlying the effects of creatine supplementation to decrease the tumor aggressiveness as well as to mitigate the skeletal muscle atrophy. Altogether, these further studies will help us to clarify the therapeutic effects of creatine supplementation upon cancer cachexia.
Table 1 summarizes the main animal and human studies mentioned in this review.
Table 1.
Summary of main animal and human studies mentioned in this brief review
| Authors | Animals or subjects | Supplementation | Major phenotype effect | Adverse effects |
|---|---|---|---|---|
| Animal studies | ||||
| Miller et al. [43] | Rat mammary, rat sarcoma, and human neuroblastoma tumors-bearing mice | Creatine and cyclocreatine | ↓ tumor growth | None |
| Lillie et al., [44] | ME-180 tumor-bearing mice | Cyclocreatine | ↓ tumor growth | None |
| Kristensen et al., [42] | Colon adenocarcinoma LS174T tumor-bearing mice | Creatine and cyclocreatine | ↓ tumor growth | None |
| Gomes-Marcondes et al., [27] | Walker-256 tumor-bearing rats | Leucine | ↓ weight loss | None |
| Ventrucci et al., [29] | Walker-256 tumor-bearing pregnant rats | Leucine | → normal protein turnover | None |
| Salomão et al., [28] | Walker-256 tumor-bearing rats submitted or not to aerobic training | Leucine | → normal protein turnover ↓ tumor growth |
None |
| Peters et al., [26] | C26 tumor-bearing mice | Leucine | ↓ Muscle loss | None |
| Patra et al., [14] | Sarcoma 180 tumor-bearing mice | Creatine | ↑ anticancer effect of methylglyoxal | None |
| Human studies | ||||
| Tayek et al., [31] | Patients with intra-abdominal metastatic adenocarcinoma. | BCAA | ↑ whole body protein synthesis ↔ 24-h nitrogen balance |
None |
| Yamanaka et al., [32] | Gastric cancer patients | BCAA | → normal protein turnover | None |
| Norman et al., [46] | Patients with colorectal cancer undergoing chemotherapy | Creatine | ↑↔ body cell mass ↑ handgrip strength | None |
| Bourgeois et al., [13] | Children with acute lymphoblastic leukemia | Creatine | ↔ bone mineral content and fat-free mass ↓ gain in body fat |
None |
Adverse events
There were no side effects (for any supplement) reported throughout the animal or human studies included in the current review. Actually, no evidences regarding adverse events for BCAAs and creatine supplementation (in adequate doses) have been found in a large spectrum of chronic degenerative diseases. Considering that little is known regarding effects of creatine and BCAAs in tumor cells stimulation, studies showing the safety of nutritional supplements as a primary outcome on cancer cachexia patients are still necessary.
Final considerations
BCAAs and creatine may attenuate proteolysis and enhance proteins synthesis in skeletal muscle. Although more experimental studies and clinical trials are still necessary to elucidate this therapeutic application, several evidences have demonstrated that amines supplementation is a promising coadjuvant treatment to cancer cachexia. Furthermore, the nutritional supplementation is a non-pharmacological strategy; hence, we also encourage the researchers to assess the safety of supplements in further studies underlying cancer cachexia.
Acknowledgment
The authors are supported by FAPESP (São Paulo, Brazil). Grant numbers: PLCF 12/02682-9, WN 13/04744-4, CRRA 12/25240-1, AHLJ 12/07319-0. All authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010; 1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Disclosure of potential conflict of interest
Patrícia Lopes de Campos-Ferraz, Isabel Andrade, Willian das Neves, Isabela Hangai, Christiano Robles Rodrigues Alves, and Antonio Herbert Lancha Junior declare that they have no conflict of interests.
References
- 1.Tan BHL, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008:11:400–07. [DOI] [PubMed]
- 2.Warren S. The immediate cause of death in cancer. Am J Med Sci. 1932;184:610–13.
- 3.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 4.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 5.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Argilés JM, López-Soriano FJ, Busquets S. Mechanisms and treatment of cancer cachexia. Nutr Metab Cardiovasc Dis. 2013;23(S1):S19–S24. [DOI] [PubMed]
- 7.de Campos-Ferraz PL, Ribeiro SM, Luz SS, Lancha Jr AH, Tirapegui J. Exercise x BCAA supplementation in young trained rats: What are their effects on body growth? J Sports Sci Med. 2011;10:483–90. [PMC free article] [PubMed]
- 8.Wilson GJ, Wilson JM, Manninen AH. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: a review. Nutr Metab (Lond). 2008;5:1. [DOI] [PMC free article] [PubMed]
- 9.Tarnopolsky MA. Creatine as a therapeutic strategy for myopathies. Amino Acids. 2011;40:1397–1407. doi: 10.1007/s00726-011-0876-4. [DOI] [PubMed] [Google Scholar]
- 10.Nicastro H, Artioli GG, Costa Ados S, Solis MY, da Luz CR, Blachier F, Lancha AH., Jr An overview of the therapeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids. 2011;40:287–300. doi: 10.1007/s00726-010-0636-x. [DOI] [PubMed] [Google Scholar]
- 11.Wagenmakers AJ. Amino acid metabolism, muscular fatigue and muscle wasting, Speculation on adaptations at high altitude. Int J Sports Med. 1992;13:S110–S113. doi: 10.1055/s-2007-1024611. [DOI] [PubMed] [Google Scholar]
- 12.Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr. 2006;136:314S–318S. doi: 10.1093/jn/136.1.314S. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeois JM, Nagel K, Pearce E, Wright M, Barr RD, Tarnopolsky MA. Creatine monohydrate attenuates body fat accumulation in children with acute lymphoblastic leukemia during maintenance chemotherapy. Pediatr Blood Cancer. 2008;51:183–187. doi: 10.1002/pbc.21571. [DOI] [PubMed] [Google Scholar]
- 14.Patra S, Ghosh A, Roy SS, Bera S, Das M, Talukdar D, Ray S, Wallimann T, Ray M. A short review on creatinecreatine kinase system in relation to cancer andsome experimental results on creatineas adjuvant in cancer therapy. Amino Acids. 2012;42:2319–2330. doi: 10.1007/s00726-011-0974-3. [DOI] [PubMed] [Google Scholar]
- 15.Baracos VE, Mackenzie ML. Investigations of branched-chain amino acids and their metabolites in animal models of cancer. J Nutr. 2006;136:237S–242S. doi: 10.1093/jn/136.1.237S. [DOI] [PubMed] [Google Scholar]
- 16.Aversa Z, Bonetto A, Costelli P, Minero VG, Penna F, Baccino FM, Lucia S, Rossi Fanelli F, Muscaritoli M. ß-hydroxy-ß-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol. 2011;38:713–720. [DOI] [PubMed]
- 17.Nicastro H, da Luz CR, Chaves DF, Bechara LR, Voltarelli VA, Rogero MM, Lancha AH Jr. Does branched chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J Nutr Metab. 2012;136937 [DOI] [PMC free article] [PubMed]
- 18.Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, Kobayashi H, Mawatari K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr. 2006;136:529S–532S. doi: 10.1093/jn/136.2.529S. [DOI] [PubMed] [Google Scholar]
- 19.Burke LM, Castell LM, Stear SJ. BJSM reviews: A-Z of supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance Part 1. Br J Sports Med. 2009;43:728–729. doi: 10.1136/bjsm.2009.063941. [DOI] [PubMed] [Google Scholar]
- 20.Campos-Ferraz PL, Bozza T, Nicastro H, Lancha AH., Jr Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition. 2013;29:1388–1394. doi: 10.1016/j.nut.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Eley HL, Russell ST, Tisdale MJ. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem J. 2007;407:113–120. doi: 10.1042/BJ20070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura I, Sato H, Ogoshi S, Blackburn GL. Use of an Intravenous branched chain amino acid enriched diet in the tumor-bearing rat. Jpn J Surg. 1985;15:471–476. doi: 10.1007/BF02470093. [DOI] [PubMed] [Google Scholar]
- 23.Crosby LE, Bistrian BR, Ling PR, Istfan NW, Blackburn GL, Hoffman SB. Effects of branched chain amino acid-enriched total parenteral nutrition on amino acid utilization in rats bearing Yoshida sarcoma. Cancer Res. 1988;48:2698–2702. [PubMed] [Google Scholar]
- 24.Le Bricon T. Effects of administration of oral branched-chain amino acids on anorexia and caloric intake in cancer patients. Clin Nutr. 1996;15:337. doi: 10.1016/S0261-5614(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 25.Cangiano C, Laviano A, Meguid MM, Mulieri M, Conversano L, Preziosa I, Rossi-Fanelli F. Effects of administration of oral branched-chain amino acids on anorexia and caloric intake in cancer patients. J Natl Cancer Inst. 1996;88:550–552. doi: 10.1093/jnci/88.8.550. [DOI] [PubMed] [Google Scholar]
- 26.Peters SJ, van Helvoort A, Kegler D, Argilès JM, Luiking YC, Laviano A, van Bergenhenegouwen J, Deutz NE, Haagsman HP, Gorselink M, van Norren K. Dose-dependent effects of leucine supplementation on preservation of muscle mass in cancer cachectic mice. Oncol Rep. 2011;26:247–254. doi: 10.3892/or.2011.1269. [DOI] [PubMed] [Google Scholar]
- 27.Gomes-Marcondes MC, Ventrucci G, Toledo MT, Cury L, Cooper JC. A leucine-supplemented diet improved protein content of skeletal muscle in young tumor-bearing rats. Braz J Med Biol Res. 2003;36:1589–1594. doi: 10.1590/S0100-879X2003001100017. [DOI] [PubMed] [Google Scholar]
- 28.Salomão EM, Toneto AT, Silva GO, Gomes-Marcondes MC. Physical exercise and a leucine rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr Cancer. 2010;62:1095–1104. doi: 10.1080/01635581.2010.492082. [DOI] [PubMed] [Google Scholar]
- 29.Ventrucci G, Mello MA, Gomes-Marcondes MC. Proteasome activity is altered in skeletal muscle tissue of tumour-bearing rats a leucine-rich diet. Endocr Relat Cancer. 2004;11:887–895. doi: 10.1677/erc.1.00828. [DOI] [PubMed] [Google Scholar]
- 30.Ventrucci G, Mello MA, Gomes-Marcondes MC. Leucine-rich diet alters the eukaryotic translation initiation factors expression in skeletal muscle of tumour-bearing rats. BMC Cancer. 2007;7:42. doi: 10.1186/1471-2407-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tayek JA, Bistrian BR, Hehir DJ, Martin R, Moldawer LL, Blackburn GL. Improved protein kinetics and albumin synthesis by branched chain amino acid-enriched total parenteral nutrition in cancer cachexia: a prospective randomized crossover trial. Cancer. 1986;58:147–157. doi: 10.1002/1097-0142(19860701)58:1<147::AID-CNCR2820580126>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka H, Kanemaki T, Tsuji M, Kise Y, Hatano T, Hioki K, Yamamoto M. Branched-chain amino acid-supplemented nutritional support after gastrectomy for gasric cancer with special reference to plasma amino acid profiles. Nutrition. 1990;6:241–245. [PubMed] [Google Scholar]
- 33.Nunes EA, Kuczera D, Brito GA, Bonatto SJ, Yamazaki RK, Tanhoffer RA, Mund RC, Kryczyk M, Fernandes LC. Beta-hydroxy-beta-methylbutyrate supplementation reduces tumor growth and tumor cell proliferation ex vivo and prevents cachexia in Walker 256 tumor-bearing rats by modifying nuclear factor-kappaB expression. Nutr Res. 2008;28:487–493. doi: 10.1016/j.nutres.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Smith HJ, Wyke SM, Tisdale MJ. Mechanism of the attenuation of proteolysis-inducing factor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Cancer Res. 2004;64:8731–8735. doi: 10.1158/0008-5472.CAN-04-1760. [DOI] [PubMed] [Google Scholar]
- 35.Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids. 2013;45:1273–1292. doi: 10.1007/s00726-013-1592-z. [DOI] [PubMed] [Google Scholar]
- 36.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 37.Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH. Exploring the therapeutic role of creatine supplementation. Amino Acids. 2010;38:31–44. doi: 10.1007/s00726-009-0263-6. [DOI] [PubMed] [Google Scholar]
- 38.Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 39.Tarnopolsky MA. Caffeine and creatine use in sport. Ann Nutr Metab. 2010;57:1–8. doi: 10.1159/000322696. [DOI] [PubMed] [Google Scholar]
- 40.Alves CR, Santiago BM, Lima FR, Otaduy MC, Calich AL, Tritto AC, de Sá Pinto AL, Roschel H, Leite CC, Benatti FB, Bonfá E, Gualano B. Creatine supplementation in fibromyalgia: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res. 2013;65:1449–1459. doi: 10.1002/acr.22020. [DOI] [PubMed] [Google Scholar]
- 41.Rawson ES, Venezia AC. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids. 2011;40:1349–1362. doi: 10.1007/s00726-011-0855-9. [DOI] [PubMed] [Google Scholar]
- 42.Kristensen CA, Askenasy N, Jain RK, Koretsky AP. Creatine and cyclocreatine treatment of human colon adenocarcinoma xenografts: 31P and 1H magnetic resonance spectroscopic studies. Br J Cancer. 1999;79:278–285. doi: 10.1038/sj.bjc.6690045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller EE, Evans AE, Cohn M. Inhibition of rate of tumor growth by creatine and cyclocreatine. Proc Natl Acad Sci U S A. 1993;90:3304–3308. doi: 10.1073/pnas.90.8.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lillie JW, O'Keefe M, Valinski H, Hamlin HA, Jr, Varban ML, Kaddurah-Daouk R. Cyclocreatine (1-carboxymethyl-2-iminoimidazolidine) inhibits growth of a broad spectrum of cancer cells derived from solid tumors. Cancer Res. 1993;53:3172–3178. [PubMed] [Google Scholar]
- 45.Patra S, Bera S, SinhaRoy S, Ghoshal S, Ray S, Basu A, Schlattner U, Wallimann T. Ray. Progressive decrease of phosphocreatine, creatine and creatine kinase in skeletal muscleupon transformation to sarcoma. FEBS J. 2008;275:3236–3247. doi: 10.1111/j.1742-4658.2008.06475.x. [DOI] [PubMed] [Google Scholar]
- 46.Norman K, Stübler D, Baier P, Schütz T, Ocran K, Holm E, Lochs H, Pirlich M. Effects of creatine supplementation on nutritional status, muscle function and quality of life in patients with colorectal cancer–a double blind randomised controlled trial. Clin Nutr. 2006;25:596–605. doi: 10.1016/j.clnu.2006.01.014. [DOI] [PubMed] [Google Scholar]