Abstract
Background and purpose
Radiation-esophagitis and weight loss are frequently observed toxicities in patients treated with concurrent chemo-radiotherapy (CT-RT) for non-small cell lung cancer (NSCLC) and might be related. The purpose was to investigate whether weight loss already starts early after initiation of CT-RT and precedes radiation-esophagitis.
Materials and methods
In a retrospective cohort, weight and esophagitis grade ≥2 were assessed during the first weeks of (CT-)RT in patients treated with concurrent (n = 102) or sequential (n = 92) therapy. In a prospective validation study, data on body weight, esophagitis grade ≥2, nutritional intake and muscle strength were obtained before, during and following CT-RT.
Results
In the retrospective cohort, early weight loss was observed in concurrently treated patients (p = 0.002), independent of esophagitis ≥ grade 2. Early weight loss was also observed in the prospective cohort (p = 0.003) and was not accompanied by decreases in nutritional intake. In addition lower limb muscle strength rapidly declined (p = 0.042). In the later weeks of treatment, further body weight loss occurred (p < 0.001) despite increased nutritional supplementation and body weight was only partly recovered after 4 weeks post CT-RT (p = 0.003).
Conclusions
Weight loss during concurrent CT-RT for NSCLC starts early and prior to onset of esophagitis, requiring timely and intense nutritional rehabilitation.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-013-0127-5) contains supplementary material.
Keywords: Weight loss, Chemotherapy, Concurrent, Esophagitis, Non-small cell lung cancer, Radiotherapy
Introduction
Concurrent administration of chemotherapy and radiotherapy (CT-RT) is the treatment of choice for many patients with locally advanced non-small cell lung cancer (NSCLC). It has been demonstrated that this intensive multimodal treatment regimen results in significantly longer disease free and overall survival [1, 9, 10, 18]. However, concurrent administration of CT-RT is associated with a higher incidence of severe esophagitis [11, 17]. Therefore, according to current treatment guidelines, only patients with minimal comorbidity and with a good performance status are considered eligible for concurrent CT-RT [5].
Concurrent CT-RT in NSCLC generally consists of one induction cycle of chemotherapy, which is followed by a 5-week period of concurrent CT-RT. Acute esophagitis develops during the administration of RT. Patients experience dysphagia, which continues to get worse up to 2 weeks after the end of CT-RT, with healing within 4–8 weeks. Severe (grade 3 or more) dysphagia is observed in about 25 % of patients treated with concurrent CT-RT and when taken any grade into account, over 80 % experience difficulties in swallowing [6]. Intuitively, the frequently observed body weight loss during concurrent CT-RT is a result of impaired dietary intake due to dysphagia [24]. However, alternatively, induction of systemic metabolic alterations by the intense treatment regimen could deplete body mass by altering neuroendocrine regulation of dietary intake and/or wasting of fat and muscle body compartments [12]. Because these alternative mechanisms might require different (co)interventions, the exact time course and etiology of body weight loss during CT-RT should be identified to optimize patient care [20, 21].
Based on clinical observations, we hypothesized that body weight loss starts early after initiation of therapy and precedes the presence of esophagitis. Since no published data on this clinically relevant problem is to the best of our knowledge available, we assessed weekly body weight changes during concurrent and sequential CT-RT for NSCLC and correlated this with esophagitis scores in a retrospective cohort. The findings in the retrospective cohort were validated in a prospective study design, in which body weight and esophagitis scores were assessed over a longer time period, i.e. prior, during and following concurrent CT-RT for NSCLC, and additional data on nutritional intake, muscle strength and quality of life was collected.
Patients and methods
Study population
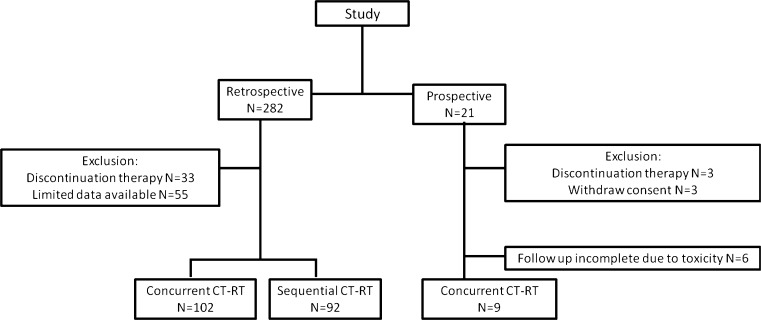
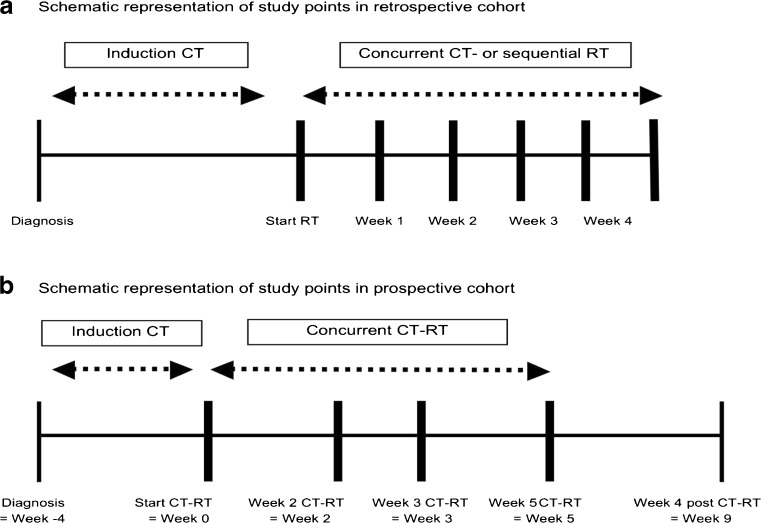
Outcome parameters were determined in a retrospective cohort and prospective study (Fig. 1). A schematic representation of the study points in the retrospective cohort is depicted in Fig. 2a and of the prospective study in Fig. 2b. Please see the supplementary data for details on inclusion criteria, study population characteristics and ethical guidelines of the study.
Fig. 1.
Flowchart of inclusion in retrospective and prospective analysis
Fig. 2.
a Schematic representation of study points in retrospective cohort. b Schematic representation of study points in prospective cohort
Chemotherapy
Chemotherapy was administered according to national and international guidelines. Generally, patients received one or more cycles of induction therapy followed by RT alone, i.e. sequential CT-RT, or RT in combination with CT, i.e. concurrent CT-RT. Please see Table 2 for an overview of the respective CT types used. Standard dose reduction rules were applied for all CT schemes if indicated. Cycles were repeated every 21 days.
Table 2.
Treatment characteristics
| Retrospective | Prospective | ||
|---|---|---|---|
| Sequential CT-RT | Concurrent CT-RT | Concurrent CT-RT | |
| Number induction chemotherapy cycles (n (%)) | n = 88 | n = 85 | n = 9 |
| 0 | 0 (0.0 %)* | 0 (0.00 %)* | 3 (33.3 %) |
| 1 | 4 (4.5 %)* | 51 (60.0 %)* | 0 (0.00 %) |
| 2 | 4 (4.5 %)* | 21 (24.7 %)* | 6 (66.7 %) |
| 3 | 79 (89.8 %)* | 12 (14.1 %)* | 0 (0.00 %) |
| 4 | 1 (1.1 %)* | 1 (1.2 %)* | 0 (0.00 %) |
| Type induction chemotherapy (n (%)) | n = 85 | n = 84 | n = 9 |
| Carboplatin–gemcitabine | 49 (57.6 %) | 44 (52.4 %) | 0 (0.0 %) |
| Carboplatin–paclitaxel | 1 (1.2 %) | 0 (0 %) | 0 (0.0 %) |
| Carboplatin–docetaxel | 3 (3.5 %) | 0 (0 %) | 0 (0.0 %) |
| Carboplatin–etoposide | 0 (0 %) | 1 (1.2 %) | 0 (0.0 %) |
| Cisplatin–gemcitabine | 23 (27.1 %) | 25 (29.8 %) | 0 (00.0 %) |
| Cisplatin–vinorelbine | 6 (7.1 %) | 2 (2.4 %) | 4 (44.4 %) |
| Cisplatin–paclitaxel | 1 (1.2 %) | 4 (4.8 %) | 0 (00.0 %) |
| Cisplatin–etoposide | 2 (2.4 %) | 5 (6.0 %) | 5 (55.6 %) |
| Number of concurrent chemotherapy cycles (n %) | n = 87 | n = 9 | |
| 1 | 51 (60.0 %) | 1 (6.7 %) | |
| 2 | 21 (24.7 %) | 14 (93.3 %) | |
| 3 | 12 (14.1 %) | 0 (0.0 %) | |
| 4 | 1 (1.2 %) | 0 (0.0 %) | |
| Type concurrent chemotherapy (n (%)) | n = 87 | n = 9 | |
| Cisplatin–vinorelbine | 54 (62.1 %) | 4 (44.4 %) | |
| Cisplatin–etoposide | 23 (26.4 %) | 5 (55.6 %) | |
| Cisplatin–vinorelbine–cetuximab | 6 (6.9 %) | 0 (0.00 %) | |
| Carboplatin–etoposide | 3 (3.4 %) | 0 (0.0 %) | |
| Carboplatin–paclitaxel | 1 (1.1 %) | 0 (0.0 %) | |
| Treatment time RT (days) | |||
| Mean ± SD | 23 ± 6 | 31 ± 7* | 33 ± 5 |
| Range | 7–41 | 14–52 | 26–41 |
| Total dose RT (Gy) | |||
| Mean ± SD | 59.2 ± 10.8 | 61.4 ± 6.7 | 64.3 ± 6.6 |
| Range | 20–79 | 45–69 | 53 – 69 |
| Mean lung dose (Gy) | |||
| Mean ± SD | 15.3 ± 3.8 | 15.6 ± 4.6 | 19.2 ± 1.2 |
| Range | 5–21 | 4–29 | 26–41 |
| Mean esophageal dose (Gy) | |||
| Mean ± SD | 24.8 ± 10.1 | 24.7 ± 9.2 | 30.6 ± 9.5 |
| Range | 5–49 | 4–43 | 14.6–45.90 |
| Max spinal cord dose (Gy) | |||
| Mean ± SD | 45.6 ± 11.0 | 44.0 ± 12.1 | 44.4 ± 9.6 |
| Range | 16–55 | 9–56 | 22.0–53.3 |
*P < 0.05 retrospective data, significant difference between sequential and concurrent treated patients (independent sample T test or Pearson chi-square test)
Radiotherapy
RT techniques have been described previously [7, 22]. Please see the supplementary data for a short description. Patients in both the retrospective and prospective cohort treated with concurrent CT-RT received on the same target volumes first 45 Gy, delivered in twice-daily fractions of 1.5 Gy with at least 8 h of inter-fraction interval, followed by once-daily fractions of 2 Gy until a pre-defined normal tissue constraint was reached, being a mean lung dose (MLD) of 19 ± 1 Gy or a spinal cord maximal dose of 54 Gy. The maximal allowed dose was 71 Gy. Patients treated with sequential therapy received twice-daily 1.8 Gy until the normal tissue constraints reported in the supplementary data were reached.
Toxicity scoring
Toxicity of treatment was assessed by a radiation oncologist using the Common Terminology Criteria for Adverse Events version 3.0 (CTCAEv3.0). According to the CTCAEv3.0, treatment-induced dysphagia and esophagitis are almost similar. For consistency, the problems with swallowing and dietary intake associated with concurrent or sequential CT-RT are referred to as esophagitis. According to the CTCAEv3.0, esophagitis causes changes in dietary intake from grade 2 on. In order to study effects of esophagitis on dietary intake, esophagitis scores were therefore further referred to as grade <2 or ≥2. Esophagitis scores were collected at the time points depicted in Fig. 2.
Nutritional intake
In the prospective study, dietary intake was calculated using a 24 h dietary recall assessment at the time points indicated in Fig. 2b. Energy and macronutrient intake were calculated according to Netherlands Nutrition Centre guidelines (www.voedingscentrum.nl).
Muscle strength
In the prospective study, hand and quadriceps muscle strength was assessed at the time points indicated in Fig. 2b (when physical condition of patients allowed it). Hand muscle strength was assessed using a hand grip meter. Isometric and isokinetic strength of quadriceps muscle was measured by Biodex dynamometer (Biodex system version 3.3). Please see the supplementary data for a description of the muscle strength assessment procedure.
Quality of life
For the evaluation of the quality of life in patients in the prospective cohort, the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire C30 (QLQ-C30) was used. Data on quality of life was obtained at the time points indicated in Fig. 2b.
Statistical analysis
Data was analysed using SPSS version 15.0. For descriptive statistics, results are expressed as means ± standard deviation (SD). P values lower than 0.05 were considered statistically significant.
In the retrospective cohort, continuous variables were compared using an independent samples T test. The Pearson chi-square test was used for comparing categorical variables. Associations between body mass index (BMI; body weight corrected for height) changes during concurrent or sequential CT-RT and a number of clinical and treatment parameters (age, gender, World Health Organization (WHO) performance status, Charlson comorbidity index [3], smoking incidence, duration of CT-RT (in weeks), mean lung dose (MLD), mean esophageal dose (MED), maximum spinal cord dose and grade esophagitis ≥2) were analysed with longitudinal data analysis by means of a linear mixed regression model.
In the prospective data, paired sample T test was used for statistical analysis for outcome parameter comparison between specific time points.
Results
Retrospective cohort
Study population characteristics
Baseline characteristics of the study population are shown in Table 1. No differences were observed between patients treated with sequential and concurrent CT-RT with respect to gender, height, body weight at diagnosis or at start of (CT)-RT, histology, TNM stage, Charlson Comorbidity Index and smoking incidence, whereas the mean age of patients treated with concurrent CT-RT was significantly lower than the age of sequentially treated patients (p = 0.001).
Table 1.
Study population characteristics at baseline
| Retrospective | Prospective | ||
|---|---|---|---|
| Sequential CT-RT | Concurrent CT-RT | Concurrent CT-RT | |
| Number of patients | 92 | 102 | 9 |
| Age (years) | |||
| Mean ± SDa | 65.8 ± 9.4* | 61.5 ± 8.6* | 56.9 ± 10.3 |
| Range | 42–83 | 40–80 | 38–73 |
| Gender (n (%)) | |||
| Male | 61 (66 %) | 64 (63 %) | 6 (67 %) |
| Female | 31 (34 %) | 38 (37 %) | 3 (33 %) |
| Body weight at diagnosis (kg) | |||
| Mean ± SD | 73.7 ± 15.4 | 72.8 ± 13.6 | 70.9 ± 14.1 |
| Reported body weight loss in 6 months prior to diagnosis (% of total body weight) | |||
| Mean ± SD | 6.42 ± 7.34 | 4.65 ± 5.95 | 3.4 ± 5.7 |
| Histology/cytology (n (%)) | |||
| Adenocarcinoma | 10 (10.9 %) | 22 (21.6 %) | 4 (44.4 %) |
| Squamous cell | 25 (27.2 %) | 22 (21.6 %) | 3 (33.3 %) |
| Large cell | 44 (47.8 %) | 39 (38.2 %) | 0 (00.0 %) |
| Not otherwise specified | 13 (14.1 %) | 19 (18.6 %) | 2 (22.2 %) |
| Stage TNMb (n (%)) | |||
| IIIA | 31 (33.7 %) | 31 (30.4 %) | 5 (55.6 %) |
| IIIB | 61 (66.3 %) | 71 (69.6 %) | 3 (33.3 %) |
| IV | 00 (00.0 %) | 00 (00.0 %) | 1 (11.1 %) |
| Smoking (n (%)) | |||
| Current cigarette smoker | 35 (38.0 %) | 43 (42.2 %) | 2 (22.2 %) |
| Former cigarette smoker | 49 (53.3 %) | 55 (53.9 %) | 7 (77.8 %) |
| Never smoker | 8 (8.7 %) | 4 (4.0 %) | 0 (00.0 %) |
*P < 0.05 retrospective data, significant difference between sequential and concurrent treated patients (Independent sample T test or Pearson chi-square test)
aMean ± standard deviation (SD)
bAccording to tumor-node-metastasis (TNM) International Staging System for Lung Cancer
Treatment characteristics
Treatment characteristics are depicted in Table 2. Patients treated with concurrent CT-RT received one or two cycles of induction CT in 84.7 % of the cases. Induction CT in this group consisted in 82.2 % of cases of carboplatin or cisplatin combined with gemcitabine. The majority of patients (84.7 %) of concurrently treated patients received two cycles of cisplatin-based concurrent CT, combined with either vinorelbine or etoposide. Patients treated with sequential CT-RT received three cycles of induction CT in 89.8 % of cases. This induction chemotherapy consisted in 84.7 % of patients of carboplatin or cisplatin combined with gemcitabine (Table 2).
The total radiation dose was 61.4 ± 6.7 Gy (range 45–69 Gy) in the concurrent group and 59.2 ± 10.8 Gy (range 20–79 Gy) in sequentially treated patients, which was not significantly different (Table 2). Also, the mean lung, mean esophagus and maximal spinal cord dose did not differ between the two groups (Table 2).
Body weight loss during concurrent and sequential CT-RT
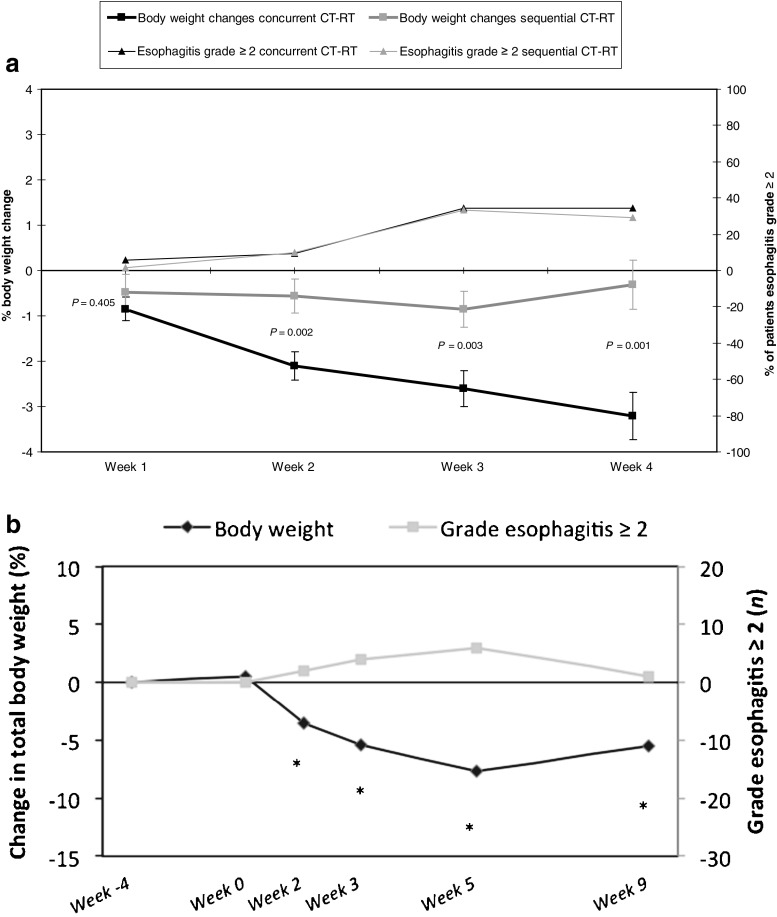
A significantly higher number of sequentially than concurrently treated patients showed a decrease in body weight during the period between diagnosis and start of (CT-)RT, which represents the induction chemotherapy period (p = 0.012; Table 3). However, the mean loss of body weight during induction chemotherapy was not significantly different between both study groups (p = 0.736). From week 2 of (CT-)RT on, body weight loss was significantly more frequent in concurrently treated patients than in sequentially treated patients (p = 0.005; Table 3). Also, the mean body weight loss, both in absolute values (Table 3) as well as calculated as percentage of total body weight (Fig. 3a), was significantly higher in concurrently treated patients in week 2 (p = 0.002), week 3 (p = 0.003) and week 4 (p = 0.001) of (CT-)RT when compared to start of (CT-)RT.
Table 3.
Body weight changes and grade esophagitis prior and during (CT-)RT
| Retrospective | Prospective | ||
|---|---|---|---|
| Sequential CT-RT | Concurrent CT-RT | Concurrent CT-RT | |
| Weight loss diagnosis—start (CT-)RT (kg) | n = 83 | n = 94 | n = 6 |
| Mean ± SDa | −1.31 ± 10.78 | −0.50 ± 3.03 | −0.13 ± 1.97 |
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 51 (61.4 %)* | 75 (81.5 %)* | 4 (66.7 %) |
| 0–5 % loss of total body weight | 17 (20.5 %)* | 8 (8.7 %)* | 2 (33.3 %) |
| ≥ 5 % loss of total body weight | 15 (18.1 %)* | 9 (9.8 %)* | 0 (0.00 %) |
| Weight loss week 1 (CT-)RT (kg) | n = 68 | n = 91 | |
| Mean ± SDb | 0.38 ± 2.48 | 0.61 ± 1.69 | |
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 39 (57.4 %) | 42 (46.2 %) | |
| 0–5 % loss of total body weight | 26 (38.2 %) | 45 (49.5 %) | |
| ≥ 5 % loss of total body weight | 3 (4.4 %) | 4 (4.4 %) | |
| Weight loss week 2 (CT-)RTb (kg) | n = 71 | n = 94 | n = 9 |
| Mean ± SD | 0.36 ± 2.27* | 1.58 ± 2.2* | 2.53 ± 1.75† |
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 37 (52.1 %)* | 27 (28.7 %) | 1 (11.1 %) |
| 0–5 % loss of total body weight | 29 (40.8 %)* | 50 (53.2 %) | 6 (66.7 %) |
| ≥ 5 % loss of total body weight | 5 (7.0 %)* | 17 (18.1 %) | 2 (22.2 %) |
| Weight loss week 3 (CT-)RT (kg) | n = 65 | n = 87 | n = 9 |
| Mean ± SD | 0.54 ± 2.21* | 1.95 ± 2.67* | 4.07 ± 3.07† |
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 31(47.7 %)* | 23 (26.4 %)* | 1 (11.1 %) |
| 0–5 % loss of total body weight | 29 (44.6 %)* | 47 (54.0 %)* | 3 (33.3 %) |
| ≥ 5 % loss of total body weight | 5 (7.7 %)* | 17 (19.5 %)* | 5 (55.6 %) |
| Weight loss week 4 (CT-)RT (kg) | n = 29 | n = 71 | |
| Mean ± SD | 0.22 ± 2.02* | 2.56 ± 3.36* | |
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 17 (60.7 %)* | 17 (23.9 %)* | |
| 0–5 % loss of total body weight | 9 (32.1 %)* | 33 (46.5 %)* | |
| ≥ 5 % loss of total body weight | 2 (7.1 %)* | 21(29.6 %)* | |
| Weight loss week 5 (CT-)RT (kg) | n = 9 | ||
| Mean ± SD | 5.62 ± 2.43† | ||
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 2 (22.2 %) | ||
| 0–5 % loss of total body weight | 0 (0.00 %) | ||
| ≥ 5 % loss of total body weight | 7 (77.8 %) | ||
| Weight loss week 4 post (CT-)RT (kg) | n = 9 | ||
| Mean ± SD | 4.41 ± 3.11† | ||
| Number of patients losing weight (n (%)): | |||
| ≤ 0 % loss of total body weight | 1 (11.1 %) | ||
| 0–5 % loss of total body weight | 2 (22.2 %) | ||
| ≥ 5 % loss of total body weight | 6 (66.7 %) | ||
| Grade esophagitis week 1 RT (n (%)) | n = 74 | n = 86 | |
| < 2 | 73 (98.7 %) | 81 (94.2 %) | |
| ≥ 2 | 1 (1.4 %) | 5 (5.8 %) | |
| Grade esophagitis week 2 RT (n (%)) | n = 79 | n = 85 | n = 9 |
| < 2 | 71(89.9 %) | 77 (90.6 %) | 7 (77.8 %) |
| ≥ 2 | 8 (10.1 %) | 8 (9.4 %) | 2 (22.2 %) |
| Grade esophagitis week 3 RT (n (%)) | n = 72 | n = 81 | n = 9 |
| < 2 | 48 (66.6 %) | 53 (65.4 %) | 5 (55.6 %) |
| ≥ 2 | 24(33.3 %) | 28(34.5 %) | 4 (44.4 %) |
| Grade esophagitis week 4 RT (n (%)) | n = 31 | n = 66 | |
| < 2 | 22 (71.0 %) | 43 (65.2 %) | |
| ≥ 2 | 9 (29.0 %) | 23 (34.8 %) | |
| Grade esophagitis week 5 RT (n (%)) | n = 8 | ||
| < 2 | 2 (25 %) | ||
| ≥ 2 | 6 (75 %) | ||
| Grade esophagitis week 4 post RT (n (%)) | n = 12 | ||
| < 2 | 8 (88.9 %) | ||
| ≥ 2 | 1 (11.1 %) | ||
*P < 0.05 in retrospective study, comparison between sequential and concurrent treated patients (independent sample T test or Pearson chi-square test)
† P < 0.05 in prospective study, comparison between body weight at a specific time point compared to body weight at diagnosis (paired sample T test)
aMean ± standard deviation (SD)
bIn the retrospective cohort, body weight loss during (CT-)RT is depicted relative to body weight at start of (CT-)RT. In the prospective cohort, the body weight is depicted relative to diagnosis
Fig. 3.
a Body weight changes and grade dysphagia during concurrent and sequential CT-RT in the retrospective cohort. Left Y axis: body weight loss as percentage of total body weight during concurrent and sequential CT-RT. Right Y axis: percentage of patients with esophagitis grade ≥ 2 during concurrent and sequential CT-RT. b Body weight changes and grade dysphagia during concurrent CT-RT in the prospective cohort. Left Y axis: body weight loss as percentage of total body weight. Right Y axis: number of patients with esophagitis grade ≥ 2 during concurrent and sequential CT-RT. Week 4: diagnosis, Week 0: start of concurrent CT-RT, Week 2: week 2 of concurrent CT-RT, Week 3: week 3 of concurrent CT-RT, Week 5: week 5 of concurrent CT-RT, Week 9: week 4 post CT-RT. *Significant difference between indicated time point and diagnosis (P < 0.05)
Grade esophagitis during concurrent and sequential CT-RT
No significant differences were observed in the frequency of esophagitis symptoms during the first weeks of (CT-)RT between patients treated with sequential CT-RT vs. patients treated with concurrent CT-RT (Table 3 and Fig. 1). Because esophagitis grade ≥2 is associated with altered nutritional intake according to the CTCAE 3.0, longitudinal data analysis by means of a linear mixed model was performed to assess whether or not the observed body weight loss during concurrent CT-RT was associated with esophagitis grade ≥2. Associations between body weight loss and grade ≥2 esophagitis were tested in two separate models, one for patients treated with sequential and one for patients treated with concurrent CT-RT. BMI was used in these models to calculate associations between grade ≥2 esophagitis and body weight loss independent of height. No significant associations were observed between body weight loss and esophagitis grade ≥2 in the first 3 weeks of concurrent CT-RT (estimated effect = 0.47, 95 % C.I. = −0.03–0.49, p = 0.096), while significant associations were observed between body weight loss and esophagitis grade ≥2 in patients treated with sequential therapy (estimated effect = 0.55, 95 % C.I. = 0.28–0.82, p < 0.001). In concurrently treated patients, significant associations were observed between decreases in BMI and duration of treatment (estimated effect week 2 = 0.28, 95 % C.I. = 0.14–0.43, p < 0.001; estimated effect week 3 = 0.39, 95 % C.I. = 0.16–0.61, p = 0.001).
Prospective study
Study population characteristics
Baseline characteristics of the prospective validation group are shown in Table 1.
Treatment characteristics
Treatment characteristics are depicted in Table 2. Most patients (n = 6) received one cycle of induction CT. The other patients did not receive any induction therapy (n = 3). The type induction chemotherapy as well as the concurrent CT consisted of cisplatin combined with either vinorelbine (44 %) or etoposide (56 %). Patients received two cycles of cisplatin-based concurrent CT. Total concurrent radiation dose was 64.3 ± 6.6 Gy (range 53–69).
Body weight loss during concurrent CT-RT
Data on body weight in the prospective cohort are shown in Fig. 3b and Table 3. Body weight remained relatively stable between diagnosis and start of concurrent CT-RT. As Fig. 3b indicates, body weight loss started early after initiation of therapy (p = 0.002) and the body weight reached its lowest level at the end of concurrent CT-RT (p < 0.001). In the 4 weeks following CT-RT, body weight partly recovered but was still significantly lower than the body weight at diagnosis (p = 0.003).
Grade esophagitis and nutritional intake during concurrent CT-RT
In Table 3, it can be observed that the number of patients in the prospective analysis suffering from grade esophagitis that interferes with nutritional intake (grade ≥2) is low at 2 weeks after initiation of concurrent CT-RT (∼20 %) and increases in the later weeks of treatment (44 % in week 3 and 75 % in week 5). As in the retrospective analysis, the number of patients losing weight was consistently higher than the percentage of patients having grade esophagitis ≥2 (Table 3).
Total dietary intake remained relatively stable until week 3 of CT-RT, albeit that the proportion of calories consumed from nutritional support was already significantly increased and the proportion of calorie consumption from regular diet was already decreased in week 3 of CT-RT (Fig. 4a). Despite continuation of nutritional support, total calorie consumption further decreased to about -15 % at week 5 of CT-RT (statistically not significant from start of treatment; Fig. 4a). After 4 weeks of follow up, total calorie intake was comparable to the dietary intake at diagnosis, though some patients still partly relied on supplemental nutrition. The findings in Fig. 4b, c indicated that macronutrient intake did not significantly alter during concurrent CT-RT, i.e. total carbohydrate, fat and protein intake (per kg body weight) was not significantly decreased during concurrent CT-RT (Fig. 4c).
Fig. 4.
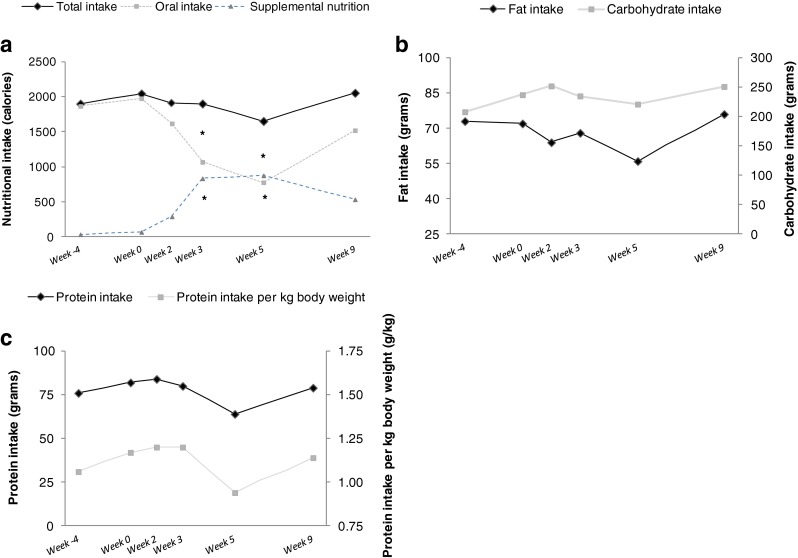
a Caloric intake. The solid line represent total calorie intake which consist of oral intake (grey line) and supportive nutrition (drink supplementation or tube feeding). b Changes in dietary carbohydrate (grams) and fat (grams) intake (oral intake and supportive nutrition). c Changes in total dietary protein (grams) and protein intake per kg body weight (grams/kilograms). Week 4: diagnosis, Week 0: start of concurrent CT-RT, Week 2: week 2 of concurrent CT-RT, Week 3: week 3 of concurrent CT-RT, Week 5: week 5 of concurrent CT-RT, Week 9: week 4 post CT-RT. *Significant difference between indicated time point and diagnosis (P < 0.05)
Physical performance during concurrent CT-RT
Patients demonstrated a significant decline in muscle strength in the first weeks of concurrent CT-RT (Fig. 5a). For quadriceps muscle, the strength declined immediately after initiation of concurrent CT-RT and reached its minimum at week 2 of concurrent CT-RT (p = 0.042; Fig. 5a). For handgrip strength, the decline also started at initiation of concurrent CT-RT but the lowest muscle strength was observed at week 3 of CT-RT (p = 0.002; Fig. 5a). Both hand and quadriceps muscle strength improved in the 4 weeks following concurrent CT-RT and nearly reached the level of muscle strength at diagnosis at that time point.
Fig. 5.
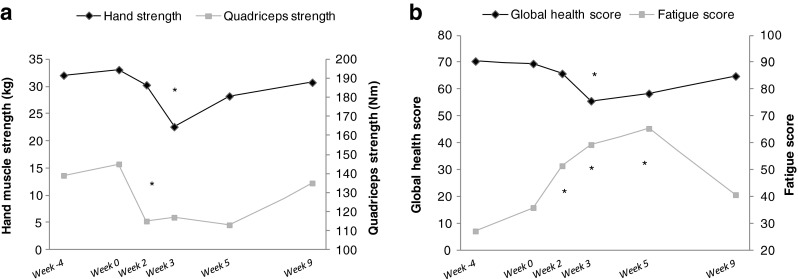
Quadriceps not all patients due to weakness, indicating even lower numbers. a Muscle strength dominant hand (kilograms) and quadriceps (Nm). b Quality of life scores (global health score and fatigue score) assessed using QLQ-C30 questionnaire. Week 4: diagnosis, Week 0: start of concurrent CT-RT, Week 2: week 2 of concurrent CT-RT, Week 3: week 3 of concurrent CT-RT, Week 5: week 5 of concurrent CT-RT, Week 9: week 4 post CT-RT. *Significant difference between indicated time point and diagnosis (P < 0.05)
In a similar pattern, global health score was decreased (p = 0.024) and fatigue score was increased (p = 0.012) in the first weeks of concurrent CT-RT. Both global health and fatigue score improved in the 4 weeks following concurrent CT-RT (global health: p = 0.438, fatigue score: p = 0.200; Fig. 5b).
Discussion
In order to provide optimal care to patients being treated with concurrent CT-RT for NSCLC, the course of treatment-induced weight loss and its dependence on the most important acute dose-limiting side effect, i.e. esophagitis, needs to be determined. It is believed that weight loss and radiation-esophagitis are causally linked because esophagitis can lead to decreased dietary intake and subsequently, to loss of body weight [24]. However, no data on the incidence and pattern of weight loss or its association with treatment-induced esophagitis is available in patients treated with concurrent CT-RT for locally advanced NSCLC.
In a large retrospective cohort in which patients treated with concurrent CT-RT for locally advanced NSCLC were compared to patients treated with sequential CT-RT, we show that loss of body weight is a common systemic complication of concurrent CT-RT, starts early after initiation of treatment and surprisingly and is independent of treatment-induced esophagitis in the first weeks of CT-RT. These data on early body weight loss were confirmed in a prospective validation study, i.e. body weight loss was also observed during the first 3 weeks of concurrent CT-RT in the prospective study population, while the number of patients suffering from grade ≥2 esophagitis was still low at that time and importantly, total caloric intake was not decreased. In addition, no correlations were found between weight loss and esophagitis until week 3 of concurrent CT-RT in the prospective cohort (data not shown). Combined, these findings suggest that processes different from esophagitis-induced nutritional intake problems may contribute to “early” body weight loss during concurrent CT-RT. The loss of body weight despite stable energy intake suggests that energy needs are increased, i.e. dysbalance between energy intake and energy expenditure leads to an energy deficit and subsequently, weight loss.
The observations in the prospective dataset further indicate that body weight progressively deteriorates from week 3 to 5 of concurrent CT-RT, together with an increase in patients exhibiting esophagitis grade ≥2. This ‘late’ body weight loss is accompanied by a significant decrease of spontaneous oral intake and increased reliance on supplemental nutrition. Although administration of supplemental nutrition prevents the total calorie intake to decline significantly, administration of supplemental nutrition is not sufficient to prevent further progression of body weight loss.
Another important finding in the current study is that after 4 weeks of completion of concurrent CT-RT, the body weight has not completely returned to the level at diagnosis. This, despite the fact that some of the patients are still using supplemental nutrition, indicates that body weight loss is a problem that extends over at least 2 months. Since significant body weight loss in cancer patients has been associated with negative effects on therapy response, survival as well physical as emotional wellbeing [8], the weight loss during and following concurrent CT-RT requires intense management to prevent these negative consequences.
Our observations that body weight loss occurs frequently during concurrent administration of CT-RT are consistent with literature on body weight changes in concurrent CT-RT treatment regimens in other malignancies. Several studies in head and neck cancer show a decrease in body weight during concurrent CT-RT starting from the first week on and continuing in the weeks after concurrent CT-RT [13, 19]. These changes in body weight are also addressed in the Clinical Practice Guideline of the American Society of Clinical Oncology (ASCO) for Laryngeal Cancer [4]. In addition, comparison between radiation treatment alone and concurrent CT-RT in cervical cancer patients showed increased body weight loss during concurrent CT-RT [14]. However, none of these studies have addressed the etiology or systemic effects of this treatment-related body weight loss.
An observation in the current study that could be of importance for optimization of patient care is that the ‘early’ weight loss during concurrent CT-RT is accompanied by a significant decline in muscle strength. As muscle strength strongly correlates with muscle mass, it is possible that the early weight loss originates from a disturbed muscle protein turnover and subsequent loss of muscle mass, i.e. the balance between muscle protein synthesis and degradation is disturbed in favor of protein degradation. Undergoing the aggressive concurrent CT-RT treatment regimen may increase energy needs, which could induce catabolism and deplete muscle mass. Therefore, increased energy needs require adequate balancing by nutritional intake to maintain muscle mass. Yet, not only energy balance should be maintained but also specific attention is warranted to the role of dietary protein intake, as this can maintain muscle mass by stimulation of protein synthesis. However, the recommended protein intake between 1.2 and 1.5 g/kg body weight was not reached during the concurrent treatment regimen [15, 25], which indicates that more aggressive supplementation of dietary protein is needed. More precisely, provision of branched chain amino acids (BCAA) might by indicated, since BCAA’s can stimulate muscle protein synthesis downstream of muscle anabolic integrator Akt [23] and we recently reported an impairment in protein synthesis signaling at the level of Akt (as part of the anabolic PI3K/Akt/mTOR pathway) in muscle of cachectic cancer patients with NSCLC [16].
Continuation of aggressive nutritional support during the ‘late’, esophagitis-associated weight loss (weeks 3–5 of concurrent CT-RT) seems plausible, as patients are at an even higher risk of dietary uncompensated muscle catabolism due to decreased esophagitis-related energy intake. Therefore, it seems conceivable to initiate aggressive nutritional support, possibly with specific amino acid formulation from the start of concurrent CT-RT to prevent or attenuate ‘early’ and ‘late’ weight loss associated with concurrent CT-RT. As simultaneous administration of exercise training or neuromuscular electrical stimulation significantly enhances the positive effects of nutritional support on muscle synthesis, these physical interventions should be considered as co-intervention [2].
In conclusion, the current study shows that ‘early’ body weight loss is a common complication of concurrent CT-RT in patients with locally advanced NSCLC and is independent of decreased intake due to esophagitis. This suggests that other treatment-dependent metabolic alterations contribute to this ‘early’ weight loss. A further decline in body weight is observed during the later weeks of concurrent CT-RT, when the incidence of esophagitis increases, and body weight is still not totally recovered after 4 weeks post treatment. Since the ‘early’ body weight loss is accompanied by a significant decline in muscle strength, which may implicate active catabolism, more supportive and early initiated nutritional intervention, preferably combined with tailored exercise could be suggested to optimize concurrent CT-RT management. The efficacy of such interventions needs to be explored in adequately designed clinical trials.
Electronic supplementary material
(DOCX 137 kb)
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7-8).
Conflict of interest
Céline M.H. Op den Kamp, Dirk K.M. De Ruysscher, Marieke van den Heuvel, Meike Elferink, Ruud M.A. Houben, Cary J.G. Oberije, Gerben P. Bootsma, Wiel H. Geraedts, Cordula C.M. Pitz, Ramon C. Langen, Emiel F.M. Wouters, Annemie M.W.J. Schols, and Anne-Marie C. Dingemans declare that they have no conflict of interest.
Sources of support
No financial support was received by the authors.
The author contributions were as follows:
Conception and design of the study: Céline Op den Kamp, Dirk De Ruysscher, Annemie Schols, Anne-Marie Dingemans.
Acquisition of data, or analysis and interpretation of data: Céline Op den Kamp, Dirk De Ruysscher, Marieke van den Heuvel, Meike Elferink, Ruud Houben, Cary Oberije, Gerben Bootsma, Wiel Geraedts, Cordula Pitz, Ramon Langen, Emiel Wouters, Annemie Schols, Anne-Marie Dingemans.
Drafting the article or revising it critically for important intellectual content and final approval of the version to be submitted: Céline Op den Kamp, Dirk De Ruysscher, Marieke van den Heuvel, Meike Elferink, Ruud Houben, Cary Oberije, Gerben Bootsma, Wiel Geraedts, Cordula Pitz, Ramon Langen, Emiel Wouters, Annemie Schols, Anne-Marie Dingemans.
References
- 1.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol;28:2181-2190. [DOI] [PubMed]
- 2.Cermak NM, Res PT, de Groot LC, van Saris WH, Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–64. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 3.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG SAL, Weinstein GS, Mendenhall WM, Adelstein DJ, Ang KK, Clayman GL, Fisher SG, Forastiere AA, Harrison LB, Lefebvre J-L, Leupold N, List MA, O’Malley BO, Patel S, Marshall RP, Schwartz MA, and Wolf GT. American Society of Clinical Oncology Clinical Practice Guideline for the Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer. J Clin Oncol. 2006;24:3693–704. [DOI] [PubMed]
- 5.De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20:98–102. doi: 10.1093/annonc/mdn559. [DOI] [PubMed] [Google Scholar]
- 6.De Ruysscher D, Dehing C, Bremer RH, et al. Maximal neutropenia during chemotherapy and radiotherapy is significantly associated with the development of acute radiation-induced dysphagia in lung cancer patients. Ann Oncol. 2007;18:909–16. doi: 10.1093/annonc/mdm005. [DOI] [PubMed] [Google Scholar]
- 7.De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol. 2010;28:5301–10. doi: 10.1200/JCO.2010.30.3271. [DOI] [PubMed] [Google Scholar]
- 8.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 9.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–7. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 10.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–9. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 11.Jain AK, Hughes RS, Sandler AB, et al. A phase II study of concurrent chemoradiation with weekly docetaxel, carboplatin, and radiation therapy followed by consolidation chemotherapy with docetaxel and carboplatin for locally advanced inoperable non-small cell lung cancer (NSCLC) J Thorac Oncol. 2009;4:722–7. doi: 10.1097/JTO.0b013e3181a5275c. [DOI] [PubMed] [Google Scholar]
- 12.Johnke RM, Edwards JM, Evans MJ, et al. Circulating cytokine levels in prostate cancer patients undergoing radiation therapy: influence of neoadjuvant total androgen suppression. In Vivo. 2009;23:827–33. [PubMed] [Google Scholar]
- 13.McRackan TR, Watkins JM, Herrin AE, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope. 2008;118:1180–5. doi: 10.1097/MLG.0b013e31816fca5c. [DOI] [PubMed] [Google Scholar]
- 14.Ohno T, Kato S, Wakatsuki M, et al. Incidence and temporal pattern of anorexia, diarrhea, weight loss, and leukopenia in patients with cervical cancer treated with concurrent radiation therapy and weekly cisplatin: comparison with radiation therapy alone. Gynecol Oncol. 2006;103:94–9. doi: 10.1016/j.ygyno.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Op den Kamp CM, Langen RC, Schols AM HA. Muscle atrophy in cachexia: can dietary protein tip the balance? Curr Opin Clin Nutr Metab Care. 2009;12:611–6. doi: 10.1097/MCO.0b013e3283319399. [DOI] [PubMed] [Google Scholar]
- 16.Op den Kamp CM, Langen RC, Snepvangers FJ, et al. Nuclear transcription factor kappaB activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr 2013. [DOI] [PubMed]
- 17.Price KA, Azzoli CG, Gaspar LE. Chemoradiation for unresectable stage III non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2008;20:204–9. doi: 10.1053/j.semtcvs.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Rowell NP, O’Rourke N P. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004:CD002140. [DOI] [PubMed]
- 19.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29:893–900. doi: 10.1002/hed.20607. [DOI] [PubMed] [Google Scholar]
- 20.Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11:400–7. doi: 10.1097/MCO.0b013e328300ecc1. [DOI] [PubMed] [Google Scholar]
- 21.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 22.van Baardwijk A, Wanders S, Boersma L, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol;28:1380-1386. [DOI] [PubMed]
- 23.Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137:1835–43. doi: 10.1093/jn/137.8.1835. [DOI] [PubMed] [Google Scholar]
- 24.Werner-Wasik M. Treatment-related esophagitis. Semin Oncol. 2005;32:S60–6. doi: 10.1053/j.seminoncol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27:675–84. doi: 10.1016/j.clnu.2008.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 137 kb)