Abstract
Background
Sarcopenia, the age-related, progressive loss of skeletal muscle mass, strength, and function, is a considerable socioeconomic burden by increasing risks of falls, fractures, and frailty. Moreover, sarcopenic patients are often obese and therapeutic options are very limited.
Methods
Here, we assessed the efficacy of espindolol on muscle mass in 19-month-old male Wistar Han rats (weight, 555 ± 18 g), including safety issues. Rats were randomized to treatment with 3 mg/kg/day espindolol (n = 8) or placebo (n = 14) for 31 days.
Results
Placebo-treated rats progressively lost body weight (−15.5 ± 7.2 g), lean mass (−1.5 ± 4.2 g), and fat mass (−15.6 ± 2.7 g), while espindolol treatment increased body weight (+8.0 ± 6.1 g, p < 0.05), particularly lean mass (+43.4 ± 3.5 g, p < 0.001), and reduced fat mass further (−38.6 ± 3.4 g, p < 0.001). Anabolic/catabolic signaling was assessed in gastrocnemius muscle. Espindolol decreased proteasome and caspase-3 proteolytic activities by approximately 50 % (all p < 0.05). Western blotting showed a reduced expression of key catabolic regulators, including NFκB, MuRF1, and LC-3 (all p < 0.01). The 50- and 26-kDa forms of myostatin were downregulated fivefold and 20-fold, respectively (both p < 0.001). Moreover, 4E-BP-1 was reduced fivefold (p < 0.01), while phospho-PI3K was upregulated fivefold (p < 0.001), although Akt expression and phosphorylation were lower compared to placebo (all p < 0.05). No regulation of p38 and expression of ERK1/2 were observed, while phosphorylation of p38 was reduced (−54 %, p < 0.001) and ERK1/2 was increased (115 and 83 %, respectively, both p < 0.01). Espindolol did not affect cardiac function (echocardiography) or clinical plasma parameters.
Conclusion
Espindolol reversed the effects of aging/sarcopenia, particularly loss of muscle mass and increased fat mass. Thus, espindolol is an attractive candidate drug for the treatment of sarcopenia patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-013-0125-7) contains supplementary material.
Keywords: Sarcopenia, Anabolic catabolic transforming agent (ACTA), Espindolol, Muscle mass, Fat mass
Introduction
Sarcopenia is a condition associated with loss of skeletal muscle mass and strength associated with aging [1]. A loss of 5 % of muscle mass per decade of life from the fourth decade onwards, potentially increasing after the age of 65 years, has been described [2, 3]. From a histological perspective, sarcopenia is characterized as a decrease in the number and size of muscle fibers. The prevalence of sarcopenia for those over 64 years of age has been shown to be 22.6 % in women and 26.8 % in men, rising to 31.0 and 52.9 %, respectively, in those over 80 years of age [4]. Thus, it can be estimated that over 3 % of the world’s population will be affected by sarcopenia by 2015. However, the exact mechanism causing sarcopenia in only a subpopulation of elderly is unclear.
Moreover, most people suffering from sarcopenia show signs of physical frailty and a slowing of movement [5, 6]. Furthermore, physical frailty is one of the commonest reasons why most old people have to give up independent living. Frailty also increases the prevalence of balance disorders, falls, fractures, and pain and, therefore, significantly reduces the quality of life [7, 8]. The sequelae that follow sarcopenia were responsible for approximately $18.5 billion in direct healthcare costs in the USA for the year 2000 alone [9]. With an overall aging population in the Western world, this figure is likely to rise further. Thus, it represents a major economic, social, and public health issue [10], but there are still no treatment options registered, which makes the development of novel medications imperative to reduce the great health and economic burden of sarcopenia. Current therapy strategies aim at preventing sarcopenia by exercise regiments, sometimes in combination with nutritional support [11] or the use of hormonal replacement therapy [12].
In this study, the novel small-molecule anabolic catabolic transforming agent (ACTA), espindolol, was used to treat 19-month-old rats over a period of 31 days. Espindolol is a nonspecific β-1 and β-2 adrenergic receptor blocker with intrinsic sympathomimetic activity (ISA) on the β-2 adrenergic receptor. In addition to its β-blocking and ISA activity, espindolol is a highly potent antagonist of 5-HT1A receptors and binds to 5-HT1A receptors in the brain [13]. In the context of sarcopenia, we hypothesized that espindolol treatment would lead to a reduction of catabolic/atrophic signaling caused by blocking the chronic activation of the β-1 adrenergic receptor, while inducing anabolic signaling by the ISA effect on the β-2 adrenergic receptor. We also analyzed cardiac function to ensure that espindolol had no negative effects on the heart.
Methods
Animals
Male Wistar Han rats (Charles River), aged 19 months, were kept under standard laboratory conditions in an SPF facility. Rats were randomized to treatment with placebo (sterilized water; n = 14) or 3 mg/kg/day espindolol (dissolved in sterilized water; n = 8) and treated for 31 days. Animals were housed in groups of three. Espindolol or placebo was administered per gavage (0.1 mL/100 g) once daily for 31 days. All phenotyping data were recorded before the start of treatment and at the end of the study. All procedures were approved by local animal ethics committees, and all personnel were blinded to treatment allocation.
Body composition
Body composition (fat and lean body mass) was analyzed with an NMR spectroscopy device EchoMRI-700TM (Echo Medical Systems, Houston, TX, USA) once weekly as previously described [14].
Quality of life indicators
Animals were housed individually, and spontaneous movement was recorded by an infrared monitoring system (Supermex, Muromachi, Tokyo, Japan) over a 24-h period as previously described [15]. Individual food and water intake was monitored during that period.
Echocardiography
Echocardiography using the high-resolution Vevo770 system (Visual Sonics, Toronto, Canada) was performed as previously described [16]. Briefly, rats were anesthetized with 1.5 % isoflurane, laid in a supine position on a heated surface to maintain body temperature, and hair was removed from the left chest. Recordings were made in B-mode and M-mode to calculate functional parameters and measure cardiac function and dimensions.
Clinical chemistry parameters in plasma
At the end of the study, plasma levels of uric acid, urea, cholesterol (total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL)), triglycerides, albumin, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), creatinine kinase, creatinine, potassium, and sodium were measured by a validated laboratory (Labor28, Berlin, Germany).
Proteasome activity
Proteasome activity was analyzed as previously described [17]. Briefly, the gastrocnemius muscle was homogenized in ice-cold lysis buffer (10 mM Tris pH 7,5, 1 mM EDTA, 2 mM ATP, 20 % glycerin, and 4 mM dithiothreitol [DTT]), sonicated, and centrifuged at 13,000×g for 15 min. Forty micrograms protein was incubated with the fluorogenic substrates (benzyloxycarbonyl-Leu-Leu-Glu-7-amido-4-methylcoumarin [Z-LLE-AMC] for trypsin-like activity, succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin [LLVY-AMC] for chymotrypsin-like activity or benzoyl-Val-Gly-Arg-7-amidocoumarin [Bz-VGR-AMC] for peptidyl-glutamyl protein-hydrolyzing activity, Biomol, Hamburg, Germany). The change in fluorescence intensity was measured at 37 °C in 5 min intervals for 30 min. The activity, expressed as nanomoles per milligram protein per minute, was calculated by free AMC as working standard.
Caspase activity
Caspase-3 activity was analyzed as previously described [18]. Briefly, the gastrocnemius muscle was homogenized in ice-cold lysis buffer. The homogenate was frozen on dry ice/ethanol and heated to 37 °C for three cycles. After centrifugation (20,000×g for 30 min), 100 μg protein was used for caspase-3 activity measurement. The samples were preincubated in assay buffer (100 mM HEPES pH 7.5, 10 % sucrose, 0.1 % CHAPS, 2 % DMSO, and 10 mM DTT) with or without 50 μM caspase-3 inhibitor Ac-DEVD-CHO at 37 °C for 30 min. The caspase-3 specific fluorogenic substrate (50 μM) Ac-DEVD-AMC was added, and the change in fluorescence intensity was recorded. Assay conditions were identical to the proteasome assay.
Western blotting
Protein lysates were prepared from the gastrocnemius muscle according to standard protocols. Tissue from 13 placebo-treated and eight espindolol-treated (3 mg/kg/day) rats were used and 25 μg protein lysate was loaded per lane. We used primary antibodies against FOXO3a (2497), pFOXO3a (9466), MuRF-1 (4305), Akt (9272), pAkt (Ser473; 4051), pAkt (Thr308; 9275), 4E-BP1 (53H11; 9644), p4E-BP1 (Thr 37/47; 9459), p4E-BP1 (Ser65; 9451), and pPI3K p85 (Tyr458)/p55 (Tyr 199; 4228), all from Cell Signaling, Beverly, MA, USA; myostatin (AF788; R&D Systems, Minneapolis, MN, USA), LC-3 (NEB100-2220; Novus Biologicals, Littleton, CO, USA), and GAPDH (G9545; Sigma-Aldrich, St. Louis, MO, USA), as well as appropriate secondary antibodies. Immunoblots were developed using chemiluminescent detection with CDP-Star Reagent (New England BioLabs Inc., Ipswich, MA, USA). Signal intensities were quantified with ImageJ software.
Statistics
Data were analyzed using GraphPad PRISM 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Results are shown as the mean ± SEM. All data were tested for normal distribution using the D’Agostino and Pearson omnibus normality test. Between-group comparison was performed for data showing a normal distribution using Student’s t test; data showing a skewed distribution were analyzed using the Mann–Whitney U test. A paired test was used for comparison of baseline and end of study data. All statistical tests were two sided, and a p value <0.05 was considered significant.
Results
Body weight and body composition
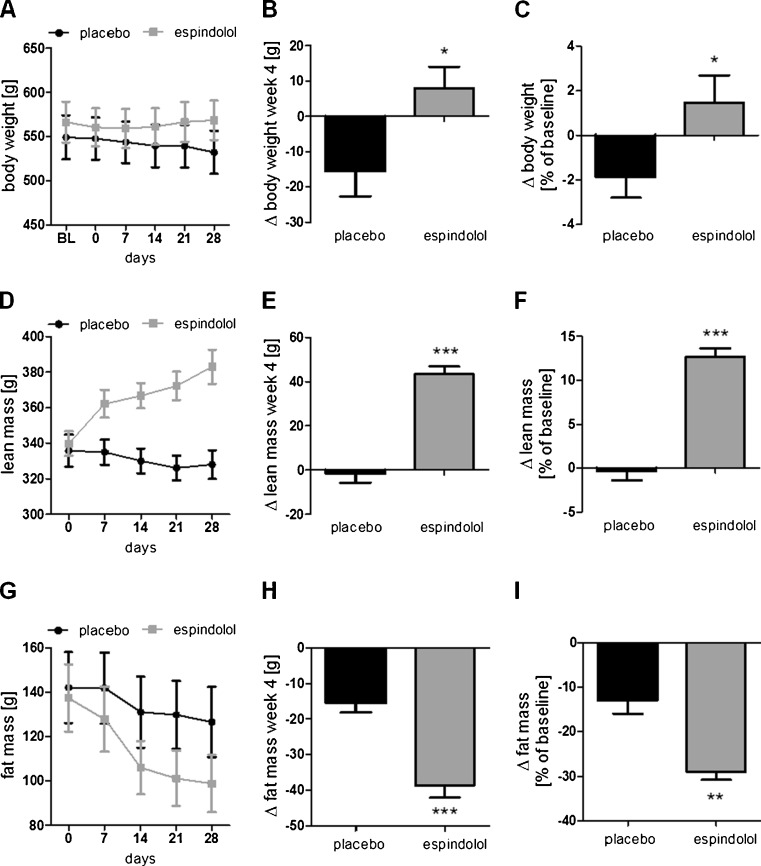
Baseline body weight and body composition (fat and lean mass) were similar in both groups (all p > 0.2; Supplemental Table 1). At the end of the study, body weight was increased in espindolol-treated animals, while it decreased in the placebo group (p < 0.05; Fig. 1a, b). However, the observed effect on overall body weight was small, a gain of 1.5 ± 1.2 % (espindolol) vs a loss of 1.8 ± 0.9 % (placebo, p < 0.05; Fig. 1c). In contrast, the change in body composition was profound, lean body mass progressively increased in the espindolol group, while a small, but progressive loss of lean mass was observed in the placebo group (p < 0.001; Fig. 1d, e). Both groups lost fat mass during the study, with a more pronounced loss of fat mass in the espindolol-treated group (p < 0.001; Fig. 1g, h). Lean body mass increased by 12.7 ± 0.9 % in the espindolol group vs baseline (p < 0.001), while the placebo group lost 0.3 ± 1.0 % (Fig. 1f). Fat mass decreased by 29.0 ± 1.8 % in the espindolol group vs 13.0 ± 3.0 % in the placebo group (p < 0.01 vs placebo; Fig. 1i).
Fig. 1.
Effect of espindolol treatment on body weight and body composition. Absolute change in body weight (a, b), lean body mass (d, e), and fat mass (g, h) and relative change (c, f, i, respectively) during the study. While placebo-treated rats lost weight, lean mass, and fat mass, espindolol-treated animals gained weight, increased lean mass, and lost more fat mass. Black bar placebo, gray bars 3 mg/kg/day espindolol. *p < 0.05, **p < 0.01, ***p < 0.001. Placebo: n = 14, espindolol: n = 8
A similar increase in lean mass was also observed in individual skeletal muscles after necropsy. The weight of the tibialis, gastrocnemius, and extensor digitorum longus (EDL) was higher (all p < 0.05), while the soleus weight was unchanged (Tables 1 and 2). To assure that the increased muscle weight was not due to edema, the tibialis was dried at 60 °C for 48 h. Dry mass was significantly higher in the treated group compared to placebo (p < 0.05; Table 2), whereas the percentage of dry mass and water content was unchanged between the groups (Table 2). The effects seem to be muscle specific as the weight of the liver, the heart, and the kidney was similar in both groups (Table 1). However, a nonsignificant reduction of epididymal fat was seen in espindolol-treated rats (white adipose tissue; Table 1), which is in accordance with the changes in overall body fat mass.
Table 1.
Tissue and organ weight at the end of the study
| Gastrocnemius [mg] | Soleus [mg] | EDL [mg] | Liver [g] | Kidney, left [g] | Heart [mg] | WAT [g] | |
|---|---|---|---|---|---|---|---|
| Placebo | 2,283 ± 78 | 181 ± 11 | 200 ± 9 | 13.68 ± 0.74 | 1.34 ± 0.06 | 1,315 ± 45 | 6.5 ± 0.7 |
| 3 mg/kg/day espindolol | 2,914 ± 133** | 173 ± 9 | 236 ± 5* | 13.98 ± 0.76 | 1.48 ± 0.06 | 1,383 ± 56 | 4.8 ± 0.7 |
WAT white adipose tissue
*p < 0.05, **p < 0.01
Table 2.
Wet and dry weight of the tibialis
| Tibialis wet [mg] | Tibialis dry [mg] | Water content [mg] | Dry mass [%] | Water content [%] | |
|---|---|---|---|---|---|
| Placebo | 818 ± 33 | 227 ± 9 | 591 ± 27 | 27.9 ± 0.8 | 72.1 ± 0.8 |
| 3 mg/kg/day espindolol | 1,015 ± 40** | 276 ± 13* | 739 ± 28** | 27.1 ± 0.5 | 72.9 ± 0.5 |
Weight increase by treatment is due to a true gain in mass and not caused by edema
*p < 0.05, **p < 0.01
Activity and food intake
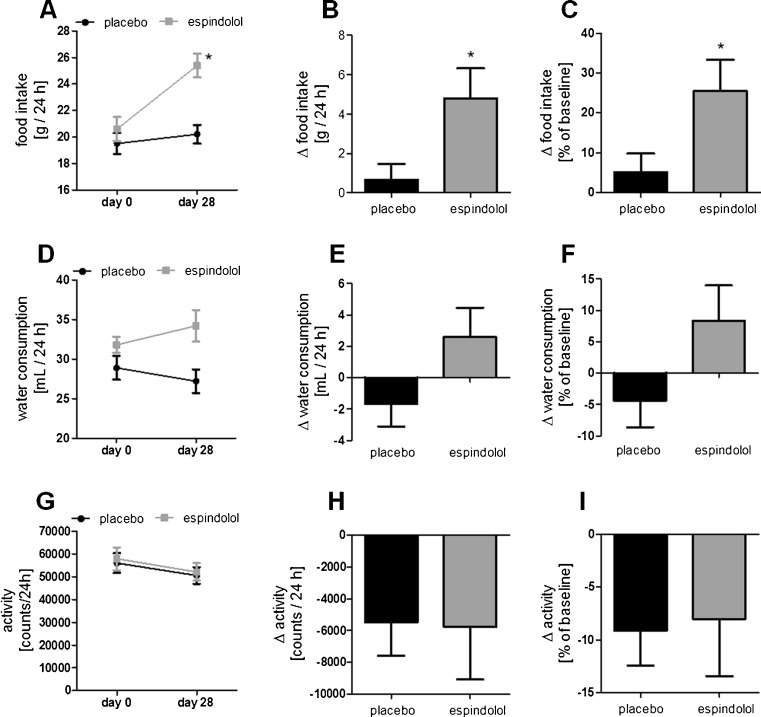
Baseline food intake, water consumption, and activity were similar in both groups (Supplemental Table 1). Treatment with espindolol increased food intake compared to baseline and placebo (both p < 0.05; Fig. 2a–c), but had no significant effect on water consumption (Fig. 2d–f). Placebo-treated rats showed no significant differences in food intake and water consumption compared to baseline (Fig. 2a–f). Spontaneous locomotor activity was somewhat decreased in both groups, independent of treatment allocation (Fig. 2g–i).
Fig. 2.
Spontaneous activity (a), food intake (b), and water intake (c) over 24 h on day 28. Activity was not affected by treatment, while food and water intake were increased by espindolol. Black bar placebo, gray bars: 3 mg/kg/day espindolol. *p < 0.05, **p < 0.01. Placebo n = 14, espindolol n = 8
Cardiac function and clinical blood parameters
Cardiac function and clinical blood parameters were assessed to ensure tolerability of espindolol therapy. Baseline echocardiography showed no differences between the two groups (all p > 0.2; Supplemental Table 2). Treatment with espindolol had no detectable hypertrophic effect on the heart (Table 1) during the study period as cardiac function and heart geometry did not differ between the espindolol-treated group and the placebo group (Table 3). Lipids, albumin, liver enzymes, creatinine kinase, creatinine, electrolytes, urea, potassium, and sodium were similar in both groups, with the exception of a significantly lower triglyceride content in the plasma of espindolol-treated rats (Table 4).
Table 3.
Cardiac dimensions and function were similar at the end of the study, indicating that espindolol is a well-tolerated drug
| Placebo (n = 14) | 3 mg/kg/day espindolol (n = 8) | |
|---|---|---|
| LVEF [%] | 82 ± 1 | 82 ± 1 |
| LVFS [%] | 47 ± 1 | 50 ± 2 |
| LVEDD [mm] | 8.2 ± 0.2 | 8.2 ± 0.2 |
| LVESD [mm] | 4.3 ± 0.2 | 4.1 ± 0.2 |
| LVEDV [μL) | 433 ± 11 | 443 ± 11 |
| LVESV [μL] | 78 ± 3 | 81 ± 5 |
| LVSV [μL] | 355 ± 11 | 363 ± 11 |
| HR [bpm] | 339 ± 4 | 348 ± 10 |
| CO [mL/min] | 209 ± 38 | 237 ± 41 |
| Septum thickness d [mm] | 1.46 ± 0.06 | 1.55 ± 0.08 |
| Septum thickness s [mm] | 3.01 ± 0.11 | 3.18 ± 0.12 |
Table 4.
Clinical blood parameters were similar in both groups at the end of the study
| Placebo (n = 14) | 3 mg/kg/day espindolol (n = 8) | |
|---|---|---|
| Total cholesterol [mg/dL] | 82.6 ± 4.8 | 84.8 ± 6.1 |
| HDL [mg/dL] | 55.5 ± 2.9 | 59.3 ± 3.8 |
| LDL [mg/dL] | 7.4 ± 0.9 | 7.1 ± 0.9 |
| Triglycerides [mg/dL] | 94.3 ± 10.4 | 59.4 ± 4.7* |
| Albumin [g/L] | 9.6 ± 0.2 | 10.1 ± 0.2 |
| GOT | 135.4 ± 18.3 | 148.0 ± 17.6 |
| GPT | 59.2 ± 12.3 | 69.0 ± 8.9 |
| Creatinine kinase [U/L] | 983 ± 233 | 1,502 ± 439 |
| Creatinine [mg/dL] | 0.32 ± 0.01 | 0.32 ± 0.01 |
| Potassium [mmol/L] | 6.4 ± 0.5 | 5.8 ± 0.4 |
| Sodium [mmol/L] | 185.7 ± 6.6 | 173.4 ± 3.8 |
| Urea [mg/dL] | 29.9 ± 1.6 | 32.6 ± 0.7 |
*p < 0.05
Catabolic signaling is downregulated in the gastrocnemius muscle
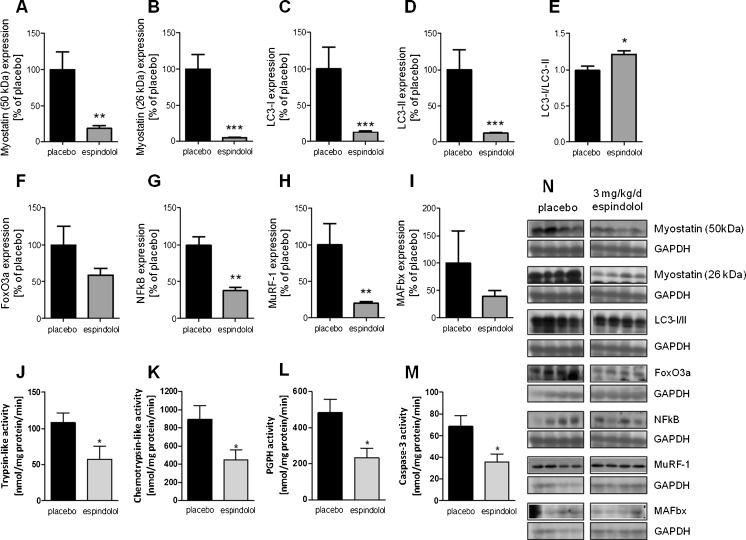
The negative regulator of muscle growth, myostatin, was downregulated in both the preform and the mature protein (both p < 0.01; Fig. 3a, b). The marker for autophagy, LC-3, was downregulated in both forms as well (both p < 0.0001; Fig. 3c, d), while the LC-1/LC-II ratio was increased in the espindolol group (p < 0.05; Fig. 3e). Espindolol treatment did not reduce the expression of FoxO3 (p = 0.15) and NFκB significantly (p < 0.01) in muscle (Fig. 3f, g), which drive the expression of key E3 ligases of the ubiquitin proteasome pathway. Indeed, MuRF-1 (p < 0.01) was downregulated (Fig. 3h), while the effect on MAFbx expression did not reach significance (Fig. 3i). As a result, a reduced proteasomal protein degradation activity was observed (all p < 0.05; Fig. 3j–l). Moreover, a reduction of caspase-3 activity was observed (p < 0.05; Fig. 3m).
Fig. 3.
Catabolic signaling in the gastrocnemius muscle. Expression is shown as relative to the mean of placebo. Expression of the atrophy driving transcription factors FoxO3a (a) and NFκB (b) were lower in espindolol-treated rats, resulting in a reduced expression of the E3 ubiquitin ligases MuRF-1 (c) and MAFbx (d). This led to a reduced activity of the proteasome (e–g). Moreover, the activity of caspase-3 was lowered by treatment (h). Both the preform (i) and the mature form (j) of myostatin were strongly reduced, as well as the autophagy marker LC-3 (k, l). Black bar placebo, gray bars 3 mg/kg/day espindolol. PGPH peptidyl-glutamyl protein-hydrolyzing. *p < 0.05, **p < 0.01, ***p < 0.001. All proteins: placebo n = 13, espindolol n = 8
Anabolic signaling in the gastrocnemius muscle
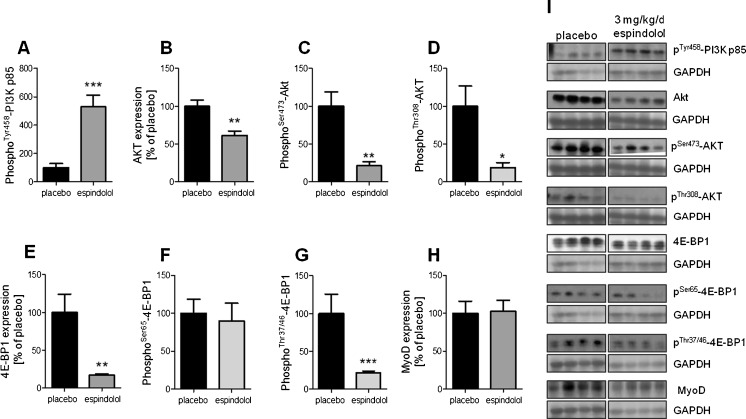
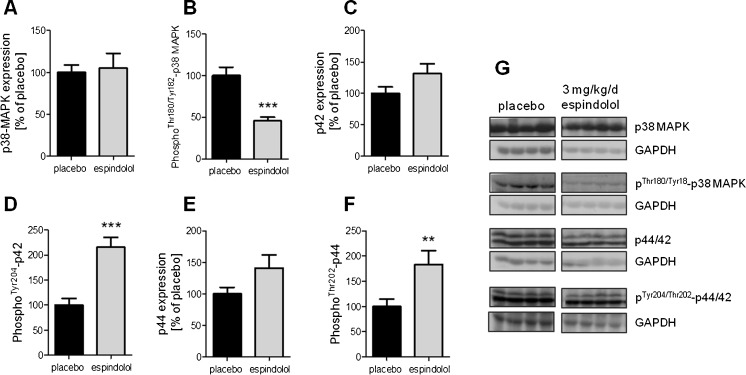
The effect of espindolol treatment on anabolic signaling is somewhat less clear. While the phosphorylation and activation of PI3K was increased fivefold (p < 0.001; Fig. 4a), its downstream target Akt showed a reduced overall expression as well as decreased phosphorylation and activation (all p < 0.05; Fig. 4b–d). The expression of the negative regulator of protein synthesis, 4E-EP1, was reduced fivefold (p < 0.01; Fig. 4e). 4E-BP1 is inactivated by phosphorylation and, while the Ser65 site was similarly phosphorylated (Fig. 4f), the Thr37 and Thr47 sites were less phosphorylated compared to placebo-treated animals (p < 0.001; Fig. 4g). The muscle-specific transcription factor MyoD, which is involved in the expression of sarcomeric proteins, was not regulated by espindolol (Fig. 4h). The expression of p38 mitogen-activated protein kinase (MAPK) and p42/44 extracellular signal-regulated kinase (ERK1/2) was not significantly different in espindolol-treated animals (Fig. 5a, c, e). However, the phosphorylation and activation of p38 MAPK was decreased (p < 0.001; Fig. 5b), while it was increased for ERK1/2 (both p < 0.01; Fig. 5d, f).
Fig. 4.
Anabolic signaling in the gastrocnemius muscle. Expression is shown as relative to the mean of placebo. Increased phosphorylation of PI3K (a) by espindolol did not result in higher phosphorylation of Akt (b–d). However, the inhibitor of protein synthesis 4E-BP1 was downregulated (e–g) and MyoD was unchanged (h). Black bar placebo, gray bars: 3 mg/kg/day espindolol. *p < 0.05, **p < 0.01, ***p < 0.001. All proteins: placebo n = 13, espindolol n = 8
Fig. 5.
Expression and phosphorylation of p38 MAPK and p42/44 ERK1/2. Expression is shown as relative to the mean of placebo. Espindolol reduced activation of p38 MAPK (a, b), while it increased activation of p42 (c, d) and p44 (e, f). Black bar placebo, gray bars 3 mg/kg/day espindolol. **p < 0.01, ***p < 0.001. All proteins: placebo n = 13, espindolol n = 8
Discussion
Sarcopenia is a multifactorial disease that is characterized by an age-related extensive remodeling of skeletal muscle and a loss of muscle fibers, which contributes to loss of muscle strength and, ultimately, frailty [19]. Sarcopenia does not necessarily lead to a net loss of body weight because lipids are deposited within the muscle fibers [12] and fat tissue, especially intra-abdominal fat is increased [20].
The main finding of our study is that espindolol given at a daily dose of 3 mg/kg/day to 19-month-old rats increased muscle mass, while decreasing fat mass at the same time, hence reversing the effects of sarcopenia/aging. So far, the treatment of sarcopenia has been challenging and is mostly concentrated on prevention by exercise with or without nutritional support and the use of anabolic medication, like testosterone, estrogens, growth hormone, and angiotensin-converting enzyme inhibition [21, 22].
Myostatin has been implicated in several wasting conditions including sarcopenia and cachexia [23, 24]. In contrast, a recent study reported no change in myostatin expression and its interacting proteins in sarcopenic elderly men compared to young men [25]. However, inhibition of myostatin has been shown to improve muscle mass in aging mice [26] and in mice with cancer cachexia [27]. Treatment of aged rats with espindolol reduced the expression of myostatin in skeletal muscle, which may have contributed to the increase in muscle mass observed in this study. This may have been mediated by the ISA effect on the β-2 adrenoreceptor, as its activation by formoterol has been shown to reduce myostatin expression [28]. Also, further catabolic mechanisms were inhibited by espindolol. Proteasomal protein degradation was reduced, which was consistent with a reduced expression of the E3 ubiquitin ligases MAFbx and MuRF-1, as well as FoxO3 and NFκB. An upregulation of MAFbx and MuRF-1has been described in muscle of old rats [29] and elderly women [30]. A crucial involvement of the ubiquitin proteasome system in the loss of muscle mass has been described [11]. In our study, the activity of caspase-3, an inducer of apoptosis, was lower in treated rats. An upregulation of caspase activity and apoptosis was described in old rats [31] and in a single human study, comparing biopsies from young and elderly persons [32]. The marker of autophagy, LC-3, was also downregulated by espindolol, as its expression is partly driven by FoxO3 [33]. Overall, espindolol targeted several wasting mechanisms simultaneously, which allowed a rapid gain of muscle mass of 12.7 ± 0.9 % in just 4 weeks. At the same time, rats lost fat mass. This might have reduced the expression of pro-inflammatory cytokines, which have been shown to be expressed and released from visceral fat [34]. These cytokines can cause a low-level chronic inflammation including oxidative stress, which in turn contributes to the activation of protein degradation in muscle and the development of insulin resistance [35].
The gain of muscle mass was observed in the fast twitch EDL and the mixed fiber type muscle gastrocnemius and tibialis, but not in the slow twitch soleus. Even without a detailed histological analysis, this suggests a possible buildup of fast twitch type II or mixed fibers by espindolol. In sarcopenia, type II fibers are lost or transformed into slow twitch type I fibers, resulting in a loss of muscle power necessary for everyday living [19].
While a robust induction of PI3K phosphorylation by espindolol was seen, its downstream target Akt was reduced and less phosphorylated. Low levels of phospho-Akt suggest a reduced protein expression/translation because they would lead to a reduced phosphorylation of mTOR and mTORs’ subsequent target 4E-BP1. 4E-BP1 is a negative regulator of protein expression and is inactivated by phosphorylation [36]. Interestingly, espindolol reduced the expression of 4E-BP1 by fivefold, which may reduce its inhibitory action of protein synthesis. Also, the ratio of 4E-BP-1 phosphorylation was increased for the Ser65 site, again suggesting a more permissive state for translation. Moreover, ERK1/2 MAPK phosphorylation was increased by espindolol. ERK1/2 are important regulators of gene transcription via activation of nuclear transcription factors and have been associated with adaptations in skeletal muscle during exercise training [37]. Therefore, upregulation of ERK1/2 phosphorylation by espindolol is likely enhancing muscle-specific gene expression. While the p38 MAPK has been implicated in the promotion of early myogenesis and seems to be important for myotube formation [38], it decreases the expression of muscle-specific genes by inhibition of MRF4 in terminal myogenic differentiation [39]. An upregulation of p38 phosphorylation was described in branchial muscles of aged rats, suggesting reduced muscle-specific gene expression [40]. Espindolol treatment reduced p38 phosphorylation, which likely contributed to the increased muscle mass seen in our study.
The present study also addressed drug safety issues. While an increase of skeletal muscle mass is obviously the aim of any sarcopenia study, hypertrophy of the heart must be prevented. Cardiac hypertrophy very often progresses to dilated cardiomyopathy and congestive heart failure at a later stage [41]. Heart rate reduction is considered an important feature of β-blockers in cardiovascular indications [42]. However, in sarcopenia, this is primarily an unwanted attribute, as the heart rate is not elevated per se. Importantly, treatment with espindolol had no effect on heart rate, which is likely due to the ISA effect of espindolol. A weaker response on heart rate reduction has been described for several β-blockers with ISA, and this is considered to be a disadvantage post infarction or in patients with heart failure [42]. Moreover, differences in heart rate regulation by β-blockers, ranging from lowering to increasing the heart rate, have been described in a pig migraine model [43]. In our model, espindolol also had no effect on heart weight, cardiac dimensions, or cardiac function and, therefore, appears well tolerated from a cardiological standpoint. Furthermore, no effect on metabolic markers such as cholesterol, liver enzymes, electrolytes, creatinine, urea, and albumin was seen by espindolol treatment, suggesting that espindolol is a well-tolerated drug to prevent or treat sarcopenia.
The main limitations of this study are the missing histology and muscle strength measurements, which leaves us to only speculate on fiber type composition/switching of the skeletal muscle. However, the gain in muscle mass, as assessed by total lean body mass, muscle wet weight, and muscle dry weight, does point to a strong anabolic effect of espindolol and implicates a possibly improved muscle function. Based on the rat data shown here, espindolol can be considered a well-tolerated and effective option to prevent or treat age-related sarcopenia and clinical trials using espindolol seem merited.
Electronic supplementary material
(DOCX 11 kb)
(DOCX 11 kb)
Acknowledgments
The study was supported by PsiOxus Therapeutics Ltd. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ, and Anker SD).
Conflict of interest
Stefan D. Anker is a shareholder of, received support from, and is a consultant for PsiOxus Therapeutics Ltd. Andrew J.S. Coats is a shareholder of, received support from, and is a consultant for PsiOxus Therapeutics Ltd. and receives honoraria from CSL Biotherapies. Jochen Springer received support from and is a consultant for PsiOxus Therapeutics Ltd. John Beadle is a shareholder, employee, and board director of PsiOxus Therapeutics Ltd. Stefan D. Anker, John Beadle, Andrew J.S. Coats, and Jochen Springer have filed a patent on the use of espindolol in sarcopenia (WO002010125348A1). Anika Tschirner, Sandra Palus, Stephan von Haehling, and Wolfram Doehner report no conflict of interest.
References
- 1.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol. 1977;43:1001. doi: 10.1152/jappl.1977.43.6.1001. [DOI] [PubMed] [Google Scholar]
- 3.Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- 4.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772. doi: 10.1093/gerona/57.12.M772. [DOI] [PubMed] [Google Scholar]
- 5.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 6.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432. doi: 10.1249/00005768-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 10.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart Coats AJ, et al. The ACT-ONE trial, a multicentre, randomised, double-blind, placebo-controlled, dose-finding study of the anabolic/catabolic transforming agent, MT-102 in subjects with cachexia related to stage III and IV non-small cell lung cancer and colorectal cancer: study design. J Cachexia Sarcopenia Muscle. 2011;2:201. doi: 10.1007/s13539-011-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palus S, Akashi Y, von Haehling S, Anker SD, Springer J. The influence of age and sex on disease development in a novel animal model of cardiac cachexia. Int J Cardiol. 2009;133:388. doi: 10.1016/j.ijcard.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt K, et al. IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2011;2:105. doi: 10.1007/s13539-011-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akashi YJ, et al. No effects of human ghrelin on cardiac function despite profound effects on body composition in a rat model of heart failure. Int J Cardiol. 2009;137:267. doi: 10.1016/j.ijcard.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 17.Baumgarten A, et al. TWIST1 regulates the activity of ubiquitin proteasome system via the miR-199/214 cluster in human end-stage dilated cardiomyopathy. Int J Cardiol. 2013;168:1447. doi: 10.1016/j.ijcard.2012.12.094. [DOI] [PubMed] [Google Scholar]
- 18.Springer J, et al. Inhibition of xanthine oxidase reduces wasting and improves outcome in a rat model of cancer cachexia. International journal of cancer. Journal international du cancer. 2012;131:2187. doi: 10.1002/ijc.27494. [DOI] [PubMed] [Google Scholar]
- 19.Lang T, et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54(Suppl 3):S48. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 22.Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;5:217. doi: 10.2147/cia.s11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han HQ, Mitch WE. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr Opin Support Palliat Care. 2011;5:334. doi: 10.1097/SPC.0b013e32834bddf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2:143. doi: 10.1007/s13539-011-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratkevicius A, et al. Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol A Biol Sci Med Sci. 2011;66:620. doi: 10.1093/gerona/glr025. [DOI] [PubMed] [Google Scholar]
- 26.Murphy KT, et al. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. Faseb J. 2010;24:4433. doi: 10.1096/fj.10-159608. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Busquets S, et al. Formoterol treatment downregulates the myostatin system in skeletal muscle of cachectic tumour-bearing rats. Oncology letters. 2012;3:185. doi: 10.3892/ol.2011.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clavel S, et al. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev. 2006;127:794. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 31.Chung L, Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762:103. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin–proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch. 2005;450:437. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- 33.Teng BT, Pei XM, Tam EW, Benzie IF, Siu PM. Opposing responses of apoptosis and autophagy to moderate compression in skeletal muscle. Acta Physiol (Oxf). 2011;201(239). [DOI] [PubMed]
- 34.Kim DH, et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 35.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 36.Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 37.Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. 2004;97:243. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- 38.Simone C, et al. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 39.Suelves M, Lluis F, Ruiz V, Nebreda AR, Munoz-Canoves P. Phosphorylation of MRF4 transactivation domain by p38 mediates repression of specific myogenic genes. Embo J. 2004;23:365. doi: 10.1038/sj.emboj.7600056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahnert JA, Luo Q, Balog EM, Sokoloff AJ, Burkholder TJ. Changes in growth-related kinases in head, neck and limb muscles with age. Exp Gerontol. 2011;46:282. doi: 10.1016/j.exger.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiter MJ. Cardiovascular drug class specificity: beta-blockers. Prog Cardiovasc Dis. 2004;47:11. doi: 10.1016/j.pcad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Saxena PR, Heiligers JP, Villalon CM, Ferrari MD. Effects of tertatolol, a beta-adrenoceptor antagonist with agonist affinity at 5-HT1A receptors, in an animal model of migraine: comparison with propranolol and pindolol. Eur J Pharmacol. 1992;220:79. doi: 10.1016/0014-2999(92)90014-U. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11 kb)
(DOCX 11 kb)