Abstract
Regulation of allergy responses by intestinal epithelial cells remain poorly understood. Using a model of oral allergen sensitization in the presence of cholera toxin as adjuvant and mice with cell-specific deletion of IKKβ in intestinal epithelial cells IECs (IKKβΔIEC), we addressed the contribution of IECs to allergic sensitization to ingested antigens and allergic manifestations at distant mucosal site of the airways. Cholera toxin induced higher proinflammatory responses and altered the profile of the gut microbiota in IKKβΔIEC mice. Antigen-specific IgE responses were unaltered in IKKβΔIEC mice, but their IgA Abs, Th1 and Th17 responses were enhanced. Upon nasal antigen challenge, these mice developed lower levels of allergic lung inflammation, which correlated with higher levels of IgA Abs in the airways. The IKKβΔIEC mice also recruited a higher number of gut-sensitized T cells in the airways after nasal antigen challenge and developed airway hyper-responsiveness, which were suppressed by treatment with anti-IL-17A. Fecal microbiota transplant during allergic sensitization reduced Th17 responses in IKKβΔIEC mice, but did not affect IgA Ab responses. In summary, we show that IKKβ in IECs shapes the gut microbiota and immune responses to ingested antigens and influences allergic responses in the airways via regulation of IgA Ab responses.
Introduction
Ingestion of food antigens generally fails to promote brisk immune responses but rather results in a state of immune tolerance. However, aberrant immune responses, including food allergy, can develop in individuals with a genetic predisposition. Clinical manifestations of food allergies include gastrointestinal, systemic (anaphylaxis), cutaneous (eczema) or respiratory (asthma) symptoms 1, 2 and are generally regarded as pathologic responses to food antigens mediated by excessive Th2 responses and antigen-specific IgE Ab responses. Past research on allergy and asthma focused on the role of cells and molecules involved in adaptive immunity. More recently, epithelial cells lining the sites of antigen entry and innate immune responses have emerged as important players in these pathologies 1, 3, 4. Studies in animal models have demonstrated that oral sensitization to allergens primes for adverse inflammatory responses at distant sites of the airways or the skin 5, 6. However, little is known about the mechanism(s) employed by intestinal epithelial cells (IECs) to shape immune responses to allergens and influence allergic manifestations in distant mucosal sites such as the airways.
The nuclear factor κB (NF-κB) pathway plays an important role in inflammatory responses 7. Its activation is regulated by the IkB kinase (IKKβ), the catalytic subunit of the IKK complex responsible for NF-κB translocation and transcription 7. Previous studies have shown that the IKKβ-NF-κB signaling controls a number of biological processes via tissue-specific regulation of inflammatory and anti-inflammatory responses and can mediate both pro- and anti-inflammatory effects 8, 9. These opposite effects were attributed to the nature of immune cells where the IKKβ-NF-κB signaling was affected 10. However, both attenuation of chronic and exacerbation of acute gut inflammatory diseases were reported in mice with alteration of IKKβ-dependent NF-κB activation in IECs 11. Others have shown that inhibition of IKKβ-dependent NF-κB activation limits the production of Th2-inducing cytokine by IECs and thus, impairs the development of protective immunity against the gut-dwelling parasite, Trichuris12.
The large intestine of mammals contains a huge community of commensal bacteria, which contributes to the digestive functions 13, prevent the development of inflammatory bowel diseases 14, and support the maturation of gut immune cells 15, 16. The gut microbiota can be perturbed by endogenous or exogenous factors and it is now established that microbial dysbiosis is associated with allergy 17, 18, obesity 19, and inflammatory diseases 20, 21. IECs sense changes in gut microbiota and transplantation of healthy infant gut microbiota could protect mice from developing allergy responses to food antigens 17,
Oral administration of food antigen with cholera toxin as adjuvant in experimental animals is a well-accepted model to study allergic sensitization to food antigens 5, 6, 22. To address the role of IECs in pathogenic immune responses to ingested food antigens, mice with targeted deletion of IKKβ in IECs (IKKβΔIEC) 23 were orally sensitized to a food antigen in the presence of cholera toxin. We show that a localized impairment of IKKβ in IECs alters the gut microbiota during oral allergic sensitization and regulates the profile of allergic inflammatory responses at the distant airways through IgA Abs and Th17 responses.
Results
IKKβ deletion in IECs enhances gut inflammatory responses to the adjuvant cholera toxin
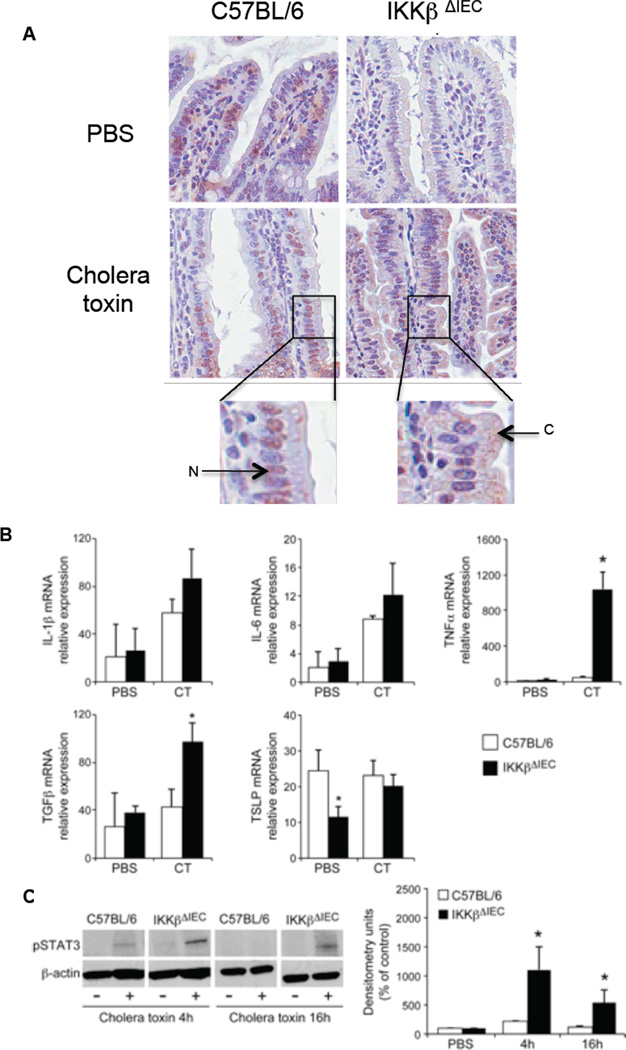
Sixteen hours after oral cholera toxin treatment, pNF-κB expression was enhanced in IECs of C57BL/6 mice and primarily found in the nucleus. Cholera toxin also increased pNF-κB expression in IECs of IKKβΔIEC mice, but the localization was primarily cytoplasmic (Fig. 1). Except for TSLP mRNA levels that were lower in the IKKβΔIEC group, we found no difference between control and IKKβΔIEC mice before treatment. Cholera toxin elevated mRNA levels of the pro-inflammatory cytokines in both IKKβΔIEC mice and control C57BL/6 mice, but TNF-α mRNA were increased by ~ 10-fold in IKKβΔIEC mice (Fig.1B). Cholera toxin also significantly increased TGFβ mRNA levels in the gut of IKKβΔIEC mice (Fig. 1B). At the time point examined, cholera toxin treatment did not change TSLP mRNA levels in the gut of control mice, but raised TSLP mRNA levels in IKKβΔIEC mice (Fig. 1B). The enhanced level of cytokine mRNA responses in the gut of IKKβΔIEC mice was associated with higher levels of pSTAT3 responses as determined by western blotting (Fig. 1C) and immuno-histochemistry (Fig. S1).
Figure 1. Innate responses to cholera toxin in intestinal tissues of IKKβΔIEC mice.
Mice were orally administered cholera toxin (10 µg) by gavage and small intestines were collected 4 or 16 h later. (A) pNFkB expression 16 h after administration of saline (PBS) or cholera toxin. Tissue sections were labeled with anti-pNF-κB p65 Ab and counter-stained with hematoxylin and eosin. Each image (x400) is representative of at least three independent experiments. Higher magnification show pNF-κB p65 expression in the nucleus (N) and cytoplasma (C) of epithelial cells (B) Real-time RT-PCR analysis of cytokine mRNA. Data are expressed as mean relative expression levels ± one SD. (C) Western blot analysis of phosphoSTAT3 (pSTAT3) expression and quantification of relative pSTAT3 expression as mean densitometry units. Data are from 4 separate experiments (*, p < 0.05)
Loss of IKKβ in IECs favors dysbiosis in cholera toxin–treated mice
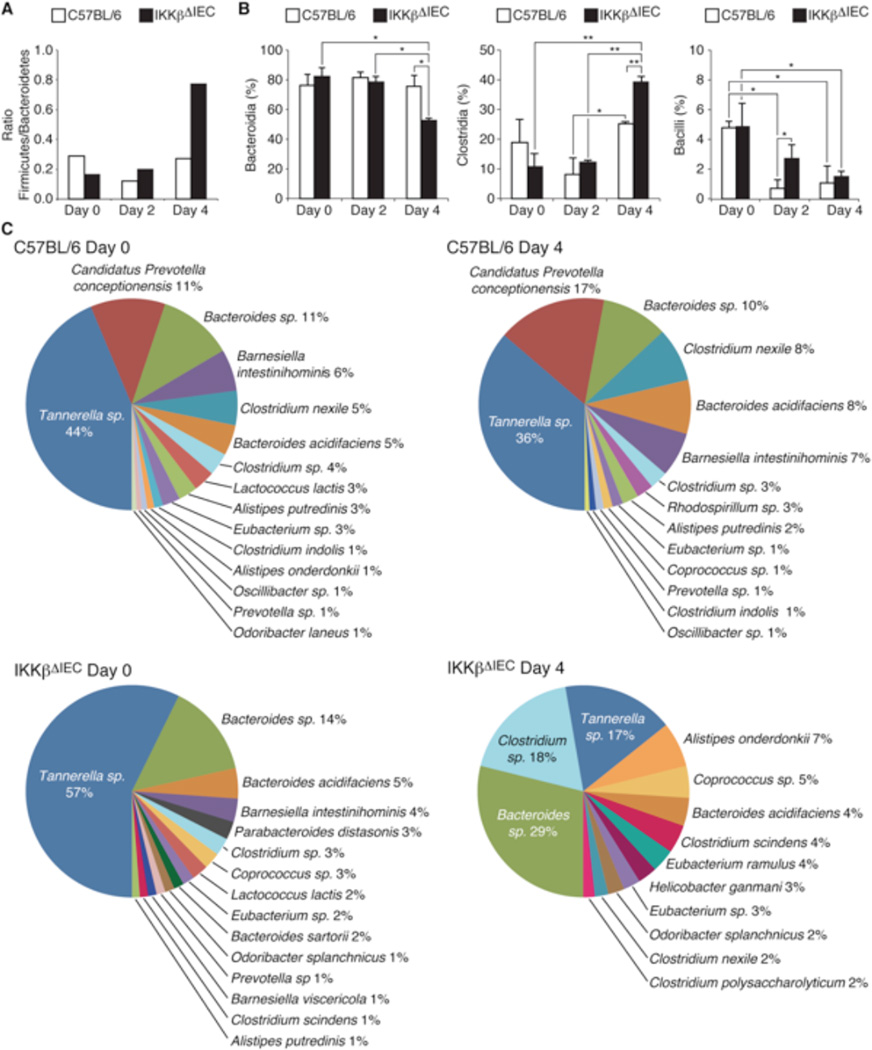
Unlike controls, IKKβΔIEC mice showed no signs of intestinal fluid accumulation 16 hrs after treatment with cholera toxin (Fig. S2). Analysis of bacterial 16S rRNA genes in fecal pellets showed no significant difference between the ration of Bacteroidetes and Firmicutes phyla in controls and IKKβΔIEC mice before cholera toxin treatment (Fig. 2A and Table S1). Four days after oral administration of cholera toxin, a dysbiosis affecting phyla (Fig. 2A), class (Fig. 2B) and species (Fig. 2B) was observed in the fecal pellets of IKKβΔIEC mice (Details in Fig. S3-S5 and Table S1-S6).
Figure 2. Cholera toxin alters the gut microbiota in IKKβΔIEC mice.
Bacterial flora was analyzed in fecal pellets collected before (day 0) and 2 and 4 days after administration of cholera toxin. (A) Ratio of main bacteria Phyla, (B) Percentage of main bacteria genus, (C) Pie-diagrams of the 15–17 main bacteria species, The results in the panel B are expressed as mean percentage ± one SD (*, p < 0.05; **, p < 0.01). All results were from 3–4 mice per groups
Loss of IKKβ in IECs alters antigen-specific CD4+ T cell and Ab responses to oral sensitization
Segmented Filamentous Bacteria (SFB) are noncultivable commensals Gram-positive anaerobic bacteria that share strong 16S rRNA similarities with the genus Clositridium 24–26 and regulate T helper cytokine responses 27, 28. Four days after oral administration of cholera toxin, the percentage of SFB was slightly increased in controls, but was significantly elevated in IKKβΔIEC mice (Fig. 3A).
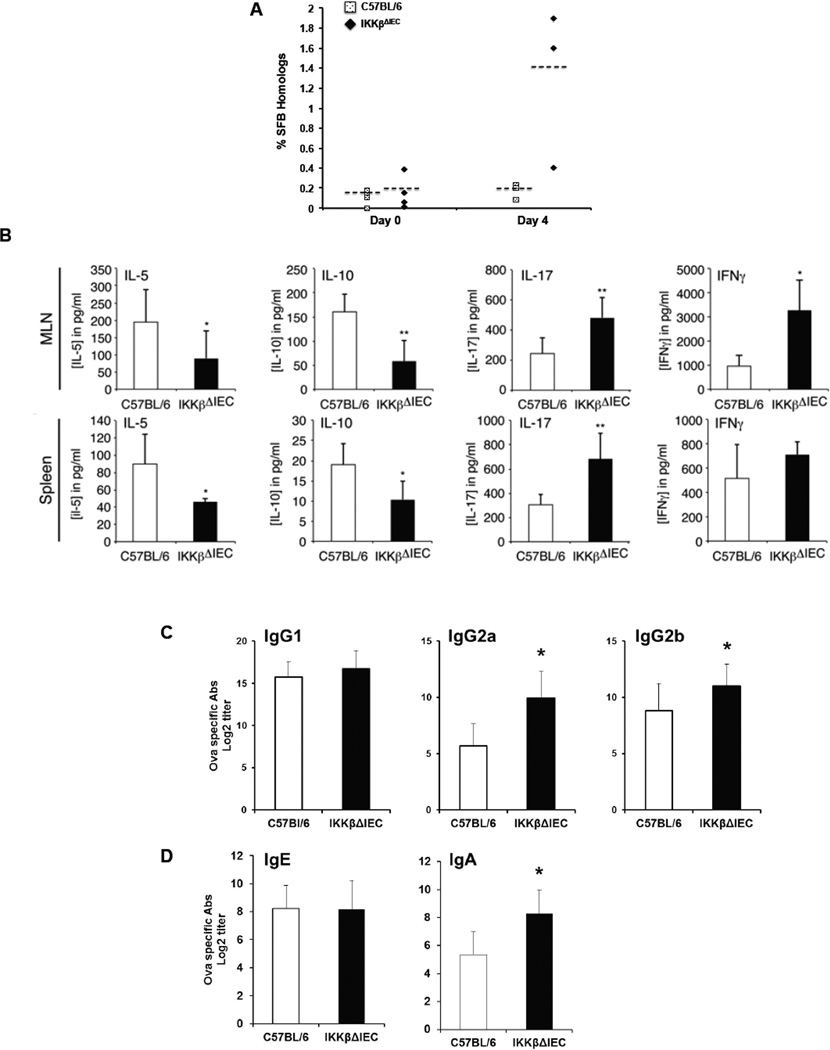
Figure 3. IKKβ-deficiency in intestinal epithelial cells alters the profile of antigen-specific CD4+ T cell and serum Ab responses in orally sensitized mice.
(A) Percentage of segmented filamentous bacteria homolog before (day 0) and 4 days after administration of cholera toxin. (B) Cytokine secretion by OVA-specific MLN and spleen T cells were analyzed by ELISA. Results are expressed as mean ± SD of three separate experiments, with 4 mice per group. (*, p < 0.05; **, p < 0.01 compared to control C57BL/6 mice). (C) OVA-specific IgG subclass and (D) IgE and IgA isotypes. Blood was collected on day 14, and Ab titers were analyzed by ELISA. The results are expressed as the log2 titers ± one SD and are from three experiments and five mice / group. (*, p < 0.05 compared to control C57BL/6 mice).
We next examined whether innate cytokine responses and dysbiosis in the gut of IKKβΔIEC mice were associated with changes in allergen-specific T helper cytokine and antibody responses. Mesenteric lymph node (MLN) and spleen T cells from control and IKKβΔIEC mice secreted the same level of IL-4 after in vitro restimulation with OVA (data not show). On the other hand, IL-5 and IL-10 secretion by cells from IKKβΔIEC mice was significantly decreased (Fig. 3B), while IL-17 and IFNγ responses were both enhanced (Fig. 3B). Th1-associated IgG2a (IgG2c) responses were significantly enhanced in IKKβΔIEC, while IgE and IgG1 Ab responses were unchanged (Fig.3C). In addition, IKKβΔIEC mice developed higher OVA-specific IgA Ab responses than control C57BL/6 mice.
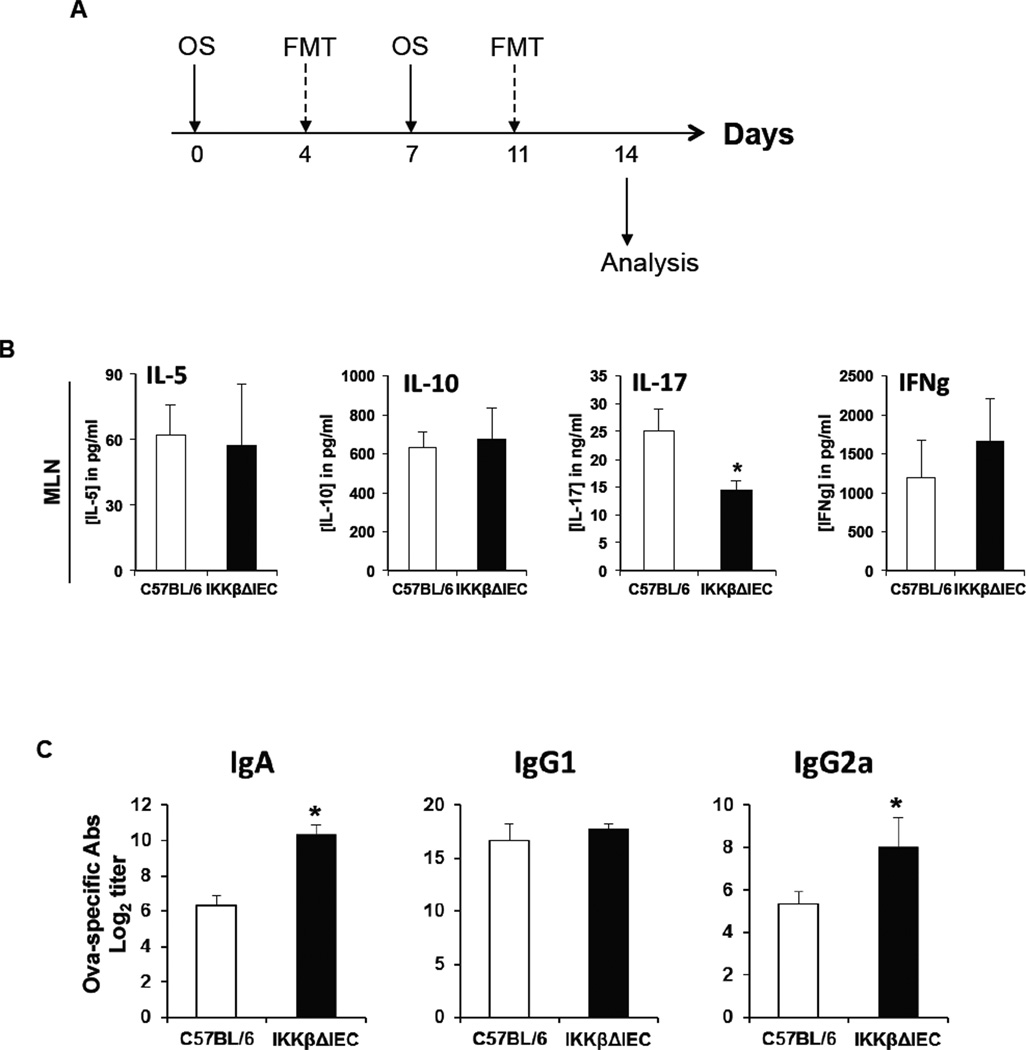
To clarify whether the changes in cytokine and antibody levels were the result of a changed microbiome or were a consequence of lack of IKKβ in epithelial cells, microbiome swap were performed by fecal microbiome transplant 4 days after each oral sensitization (Fig. 4A). The fecal microbiome swap affected antigen-specific T cell cytokine responses by mesenteric lymph node cells as it reduced IL-17A responses of IKKβΔIEC when compared to wild-type mice and eliminated the difference between the levels of IL-5, and IL-10 responses between these mice (Fig. 4B). Interestingly, IKKβΔIEC mice recipient of fecal microbiome transplant from orally sensitized wild-type mice retained higher levels of serum IgA responses (Fig. 4C). These results suggest that intestinal dysbiosis in IKKβΔIEC mice alters antigen-specific CD4+ T cell responses, while IKKβ in intestinal epithelial cells primarily regulates IgA Ab levels.
Figure 4. Fecal microbiota transfer modulates antigen-specific CD4+ T cell cytokines, but not serum IgA responses orally sensitized IKKβΔIEC mice.
(A) Schedule of oral sensitization (OS) and fecal microbiota transfer (FMT). (B) Cytokine secretion by OVA-specific MLN T cells were analyzed by ELISA. (C) OVA-specific IgG subclass and IgA responses. Blood was collected on day 14, and Ab titers were analyzed by ELISA. The results are expressed as the log2 titers ± one SD and are from three experiments and five mice / group. (*, p < 0.05 compared to control C57BL/6 mice).
To further establish how local (gut) versus systemic (circulating myeloid cells) alteration of IKKβ affected allergen-specific Ab responses, we analyzed these responses in mice lacking IKKβ in myeloid cells (IKKβΔMye) throughout the body8. These mice showed higher pSTAT3 responses in he small intestine than C57BL/6 mice 16 hours after oral administration of cholera toxin and developed higher levels of antigen-specific Th1-associated IgG2a (IgG2c) responses (Fig. S6). The OVA-specific IgE and IgG1 Ab responses were unchanged in IKKβΔMye mice, while IgA responses were enhanced although not to the same extent as in IKKβΔIEC mice (Fig. S6). Thus, IKKβ signaling is epithelial cells or in circulating myeloid cells regulate Ab responses to ingested allergens.
Alteration of IKK signaling in IECs limits allergic inflammation in the airways
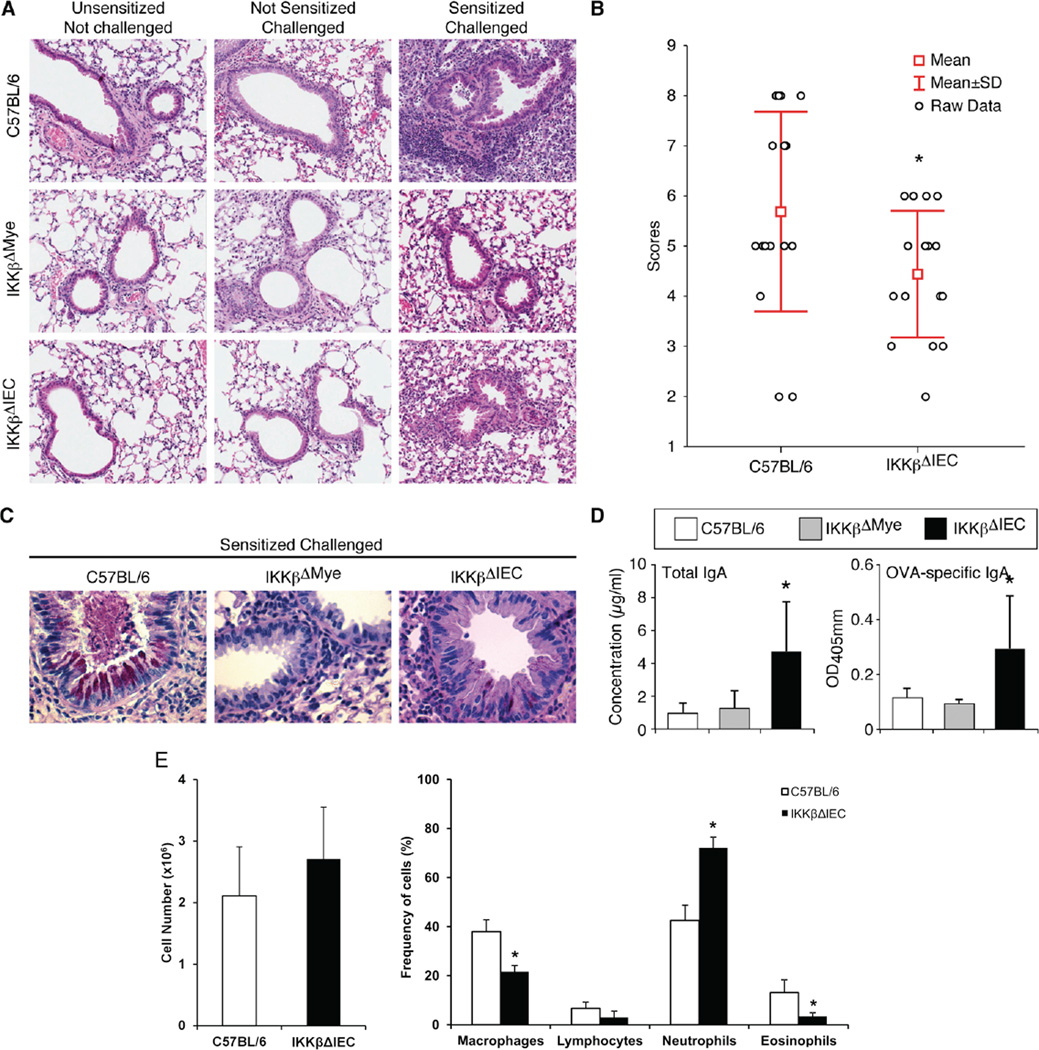
Allergic inflammation can develop in the airways following nasal allergen challenge of mice sensitized by the oral route 5, 6. Other reports have indicated that intestinal microbes influence susceptibility to food allergy 29 and regulate immunity to respiratory influenza virus infection 30. Lung inflammation was not seen in naïve (non-sensitized and not challenged), or challenged but not sensitized C57BL/6, IKKβΔMye and IKKβΔIEC mice. Orally sensitized control C57BL/6 mice developed lung inflammation with cell recruitment in the lung parenchyma and the perialveolar and perivascular space after nasal antigen challenge (Fig. 4A-4B). Interestingly lung inflammation was significantly reduced in IKKβΔIEC mice (Fig. 4A-4B), and in IKKβΔMye mice (Fig. 4A). Only minimal mucus formation was seen in the lungs of IKKβΔIEC mice after antigen challenge and none in the lungs of IKKβΔMye mice (Fig. 4C). In contrast with C57B/6 mice or IKKβΔMye mice, BAL of IKKβΔIEC mice contained higher levels of IgA (Fig. 4D), and their lungs showed more IgA secreting cells (Fig. S7). IgA Abs are believed to suppress allergic inflammation 31. The presence of IgA in BAL of IKKβΔIEC mice was associated with a reduction of eosinophils and macrophages, but the number of neutrophils was increased (Fig. 4E). On the other hand, other mechanisms are likely involved in the protection of IKKβΔMye mice, which exhibited overall lower number of cells in the lung after allergen challenges. In this regard, the proportion of F4/80+CD11c+ alveolar macrophages after nasal challenge was reduced in IKKβΔIEC when compared to control C57BL/6 mice (20±2.2% versus 28±2.5%) (Fig. S8A). Nasal challenge of orally sensitized control C57BL/6 mice recruited CD11c+F4/80-CD103+ cells in the lungs, an effect significantly reduced in mice with impaired IKKβ (Fig. S8B). We also found a higher frequency of CD3+ T cells in the lungs of IKKβΔIEC mice than in control C57BL/6 or IKKβΔMye mice after nasal challenge of orally sensitized mice (Fig. S8C).
Protection by intestinal epithelial cell IKKβ requires oral sensitization
To further establish the role played by IECs in protection of IKKβΔIEC mice against allergic airway inflammation, mice were sensitized by intraperitoneal injection of OVA plus cholera toxin. Although IgG2a Abs were lower in IKKβΔIEC mice, the rest of Ig isotype and IgG subclass responses were similar to control C57BL/6 mice (Fig. S9A). No difference was seen in lung inflammation after nasal antigen challenge, although IKKβΔIEC mice exhibited higher mucus production than control mice (Fig. S9B). These results underline the influence of the route of antigen sensitization on Ab responses and airway responses to subsequent antigen exposure or challenge.
Gut-sensitized IL-17A producing CD4+ T cells limit protection by intestinal epithelial cell IKKβ
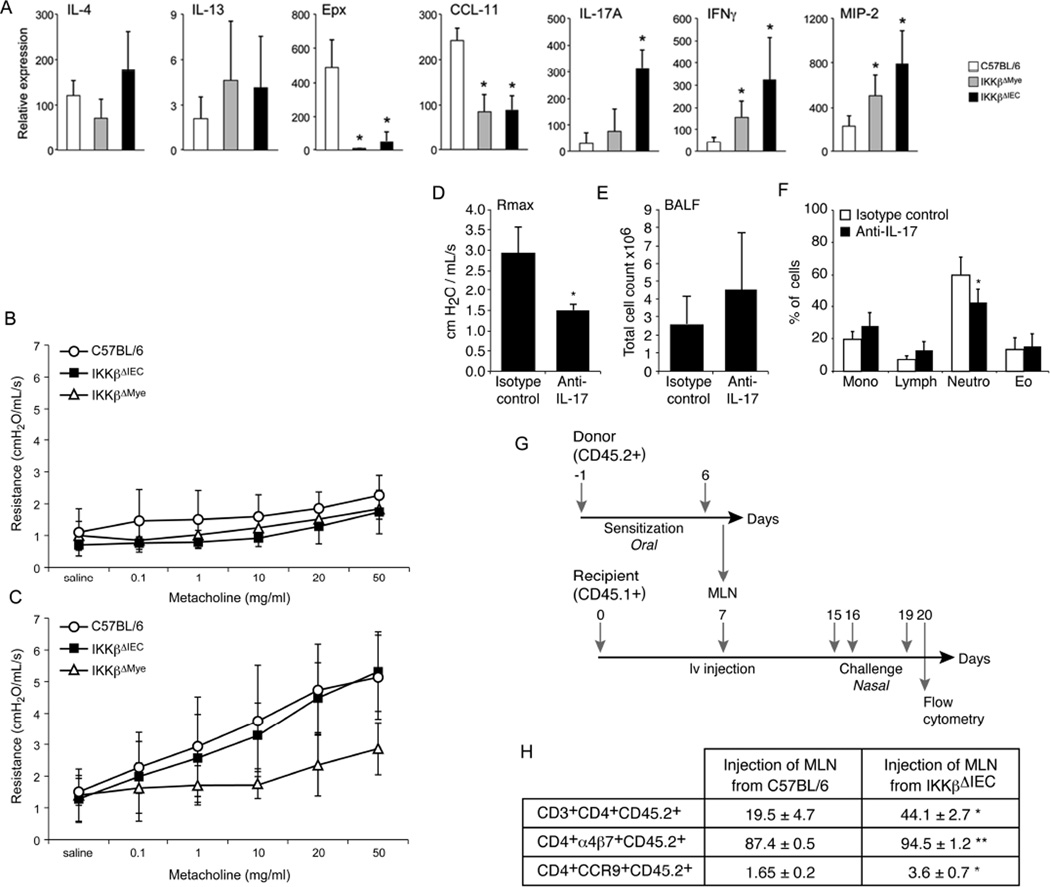
We next examined airway cytokine responses and lung functions after allergen challenges. IKKβΔIEC and IKKβΔMye mice exhibited lower lung eotaxin (CCL11) and eosinophil peroxidase (Epx) mRNA responses, but enhanced IFN-γ, IL-17A and MIP2 mRNA responses, with highest levels of response in IKKβΔIEC mice, after nasal antigen challenge (Fig. 6A). Furthermore, low or no IFN-γ and IL-17 responses were measured in BAL of control mice after antigen challenge, while their levels were elevated in IKKβΔIEC mice (Fig. S10). No mice developed signs of airway hyper-responsiveness without prior oral sensitization (Fig.6B) and sensitized IKKβΔMye mice were completely protected against airway hyper-responsiveness. Upon challenge, IKKβΔIEC mice developed airway hyper-responsiveness responses, which were not statistically different from control C57BL/6 mice (Fig. 6C). Interestingly, nasal treatment with anti-IL-17A mAbs reduced airway hyper-responsiveness in IKKβΔIEC mice (Fig.6D), and the percentage of neutrophils in BALs (Fig. 6E-6F).
Figure 6. Gut-sensitized IL-17A producing CD4+ T cells limit the protective effect of IgA Abs in the airways of IKKβΔIEC mice.
(A-C) Mice were orally sensitized on day 0 and 14 and nasally challenged on days 15, 16, and 19 (A) Cytokines, chemokines and neutrophil peroxidase mRNA responses in lung tissues on day 20. Results are expressed as mean ± SD of three separate experiments, with 4 mice per group. (*, p < 0.05 compared to control C57BL/6 mice). (B). Airway hyper-reactivity after nasal challenge of naive mice. (C) Airway hyper-reactivity after nasal challenge (day 20) of orally sensitized mice. (D-F) Responses to anti-IL-17A treatment. (D) Airway hyper-reactivity, (E) Total number of cells and (F) immune cell populations in BAL. Results are expressed as mean ± SD of 5 mice per group. (*, p< 0.05). (G-H) Adoptive transfers of MLN. (G) Timeline of adoptive transfer experiments. (H) Frequency of donor (CD45.2) CD4+ T cells expressing α4β7 and CCR9. Results are expressed as the mean ± one SD and are from four separate experiments. (*, p< 0.05; **, p< 0.01).
Finally, we adoptively transferred MLN cells of orally sensitized control C57BL/6 and IKKβΔIEC mice into naïve CD45.1+ congenic recipient mice (Fig. 6G). Analysis of lung cells after nasal antigen challenge showed a higher number of donor CD3+CD4+CD45.2+ cells in recipients of cells from IKKβΔIEC mice (Fig. 6H). A larger number of these donor CD4+ T cells also expressed α4β7 and CCR9, suggesting they were sensitized in the gut (Fig. 6H).
Discussion
Immune homeostasis is crucial at the mucosal surface of the gastrointestinal tract, which is constantly exposed to ingested antigens and commensal flora. Alteration of this homeostasis can lead to inflammatory bowel disease or food allergy. The higher incidence of asthma in inflammatory bowel diseases patients has suggested that these pathologies could share common etiological factors 32. Furthermore, growing evidence suggest that gut commensal microbiota can affect distant mucosal sites such as those of the airways, and regulate innate and adaptive immune responses to respiratory virus infection 30, 33 and allergic airway inflammation 34. Using genetically modified mice with cell-specific alteration of IKKβ, we found that depletion of IKKβ-NFκB signaling in IECs alters the microbial community in the gut, reshapes immune responses to food allergens, and regulates allergic responses in the airways via its effect on IgA Ab and Th17 cell responses.
Allergic sensitization to food antigens can be modeled by oral administration of antigen with cholera toxin as adjuvant 5, 6, 22. Cholera toxin can break oral tolerance and promote adaptive immunity by binding to IECs and stimulating inflammatory cytokines 35, 36. The NF-κB pathway can mediate both pro- and anti-inflammatory effects 8, 9 and alteration of the IKKβ-NF-κB in IECs could either attenuate chronic or exacerbated acute gut inflammatory diseases 11. We clearly show that lack of IKKβ in IECs does not prevent these cells from developing proinflammatory and pSTAT3 responses, but also increased TGFβ mRNA in IKKβΔIEC mice. The latter finding is significant since bone marrow stromal cells were reported to suppress allergic responses via induction of TGFβ 37. Intestinal bacteria play a role in the maturation of gut immune cells 15, 27, 28 and selected commensal bacteria support allergic responses 38. The Clostridium-related bacterium SFB promotes Th17 responses 27, 28, but is also known to support IgA Abs 39, 40. It is important to indicate that immunoglobulin switch to the IgA isotype requires IL-6 and TGFβ 41, which also support the differentiation of Th17 cells 42. Our results clearly show that alteration of IKKβ signaling in IEC can reorganize the gut microbiota, and increase the proportion of Clostridium species and SFB during allergic sensitization in our experimental model. Our findings are consistent with the reported role of SFB and suggest that this bacterium and other Clostridium sp helped enhance Th17 and IgA Ab responses in IKKβΔIEC mice.
Nasal exposure to antigen after allergic sensitization promotes asthma-like pathology with increased airway hyper-responsiveness, airway inflammation, eosinophilia, and mucus secretion 2, 43. Despite similar levels of IgE Ab responses than control wild-type mice, IKKβΔIEC mice only developed limited lung inflammation upon antigen challenge. The fact that only IKKβΔIEC mice showed high level of IgA Abs and IgA secreting cells in the airways, support the notion that IgA Abs suppress allergic inflammation 31, possibly by neutralizing allergens in mucosal tissues.
Consistent with the reported role of alveolar macrophages in asthma-induced inflammation 44, their percentage was lower in IKKβΔIEC mice than in control mice. Airway hyper-responsiveness and eosinophilia in mice were reported to be associated with a high number of CD103+ dendritic cells45. Our study suggests that these cells also play a role in pathologies associated with priming to food antigens in the GI tract. IL-17A is a mucogenic cytokine 46, 47, which regulates airway inflammation 48 and double negative T cells, producing IFNγ and IL-17A, were reported to be the major responders in the lungs of mice during pulmonary infection with a live Francisella tularensis vaccine 49. We show that CD4+ T cells primed in mucosal tissues represent an important fraction of effector cells recruited in the lungs of IKKβΔIEC mice upon antigen challenge and that they limits the protective affect of IgA Abs on allergic airway symptoms in this model via production of IL-17A.
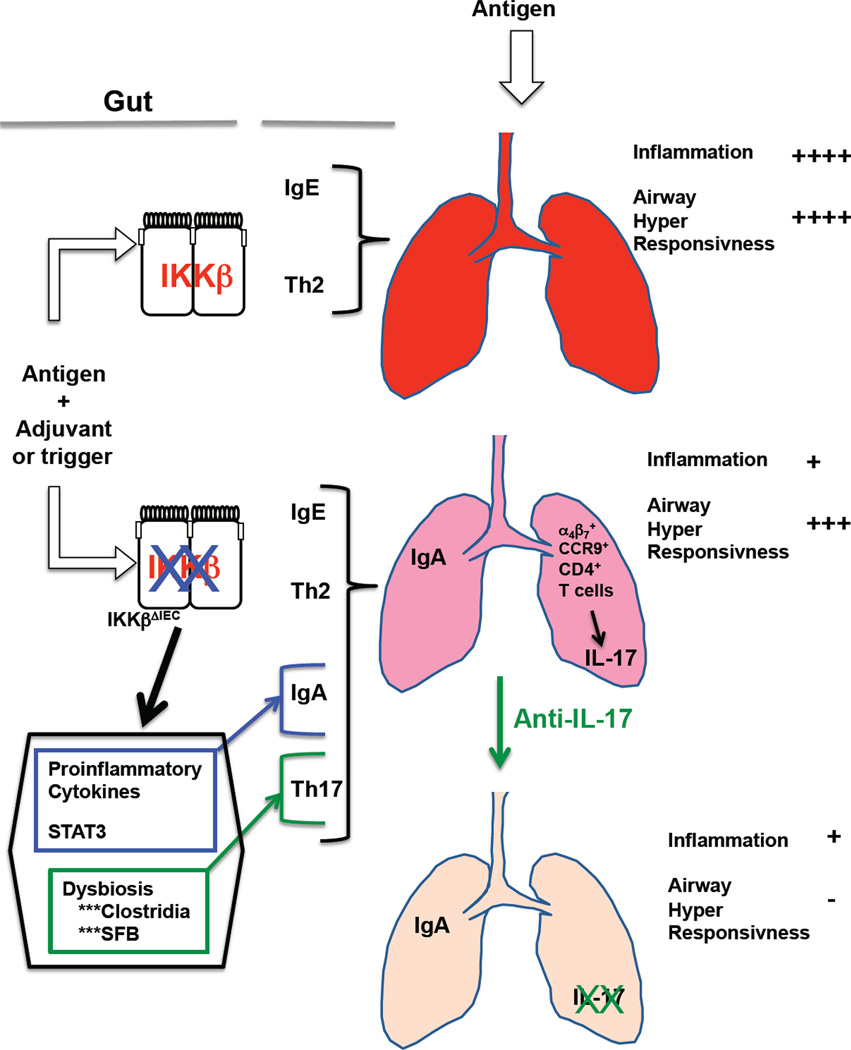
We have shown that IKKβ deficiency in intestinal epithelial cells promote a cascade of events that ultimately protect the airways against the development of allergic inflammation (Fig. 7). Our results suggest that future efforts for controlling allergic responses could include strategies that promote IgA Ab responses and prevent or reduce IL-17 responses.
Figure 7. Regulation of pathogenic airway responses to allergens by IKKβ in intestinal epithelial cells.
Allergic sensitization in the gut promotes antigen-specific IgE and Th2 responses, which will later induce allergic airway inflammation and airway hyper-responsiveness support in the event of airway exposure to the same antigen. Loss of IKKβ in IEC does not alter immune homeostasis in the gut at the basal level. However, during allergic sensitization, lack of IKKβ signaling in IEC could enhance gut proinflammatory responses and promote dysbiosis. The change in cytokine milieu could support IgA Abs, while alteration of the gut microbiota would support Th17 responses. Subsequent exposure of the airways to allergen will result in the accumulation of IgA Abs, which will protect against allergic inflammation. CD4+ cells expressing gut the homing receptors α4β7 and CCR9 and producing IL-17 are also recruited in the airways of IKKβΔIEC mice upon antigen exposure. These cells more likely support hyper-responsivess responses of the airways to antigen exposure, which could be suppressed by treatment with anti-IL-17A Ab.
Materials and Methods
Mice
Mice in which IKKβ-dependent NFκB signaling was selectively eliminated in the intestinal epithelial cells (IKKβΔIEC) or myeloid cells (IKKβΔmye) were generated as previously described 8, 23 and breed in our facility. Control C57BL/6 mice were obtained from the NCI-Frederick, and housed for 3–4 weeks with IKKβ deficient mice. Studies were performed on mice 10–12 weeks of age, in accordance with NIH and OSU IACUC guidelines.
Quantification of mRNA by real-time RT-PCR
Real-time RT-PCR was performed as previously described 50, and mRNA responses were expressed as mRNA relative expression= (1/2^ΔCt)*100*1000) where ΔCt= CPunknown-CPβ-actin.
Histology
Five 5 µm paraffin sections were stained with hematoxylin and eosin, alone or with anti-pSTAT3 (Cell-Signaling), anti-pNF-κB p65 (Santa Cruz Biotech) or subjected to PAS staining.
Immunoblotting
Intestines were lysed and proteins were separated by SDS-PAGE. After transfer, PVDF membranes were probed with anti-pSTAT3 (Cell Signaling) and an HRP-conjugated secondary antibody. The membranes were re-probed with β-actin-specific antibody (Santa Cruz Biotech) and relative ratios of pSTAT3/β-actin were determined using Image J software
Oral treatment, oral sensitization and nasal challenge of mice
For oral treatment, mice were deprived of food for 2 hours and given 250 µl of sodium bicarbonate 30 min before intragastric gavage of 10 µg cholera toxin in 250 µl of PBS. Oral sensitization was performed on days 0 and 7 by intragastric gavage of 250 µl of PBS containing 1 mg of ovalbumin (OVA) and 10 µg cholera toxin as adjuvant. Blood samples were collected on days 7 and 14. Nasal antigen challenges were performed on days 15, 16 and 19. For this purpose, mice were anesthetized by intraperitoneal injection of ketamine/xylazine and administered 200 µg of OVA in PBS. In selected experiments, mice were nasally treated (10 µg/dose) with an anti-IL-17A mAb or isotype control Ab (R&D Systems).
Analysis of gut microbiota
Bacterial tag-encoded FLX amplicon pyrosequencing (Roche Titanium 454 FLX pyrosequencing) was used for detection and identification of the primary populations of microbes in fecal pellet samples. For identification of segmented filamentous bacteria, blast search was performed with the fasta sequences against Candidatus Arthromitus (taxid:49082) genome sequences (Candidatus Arthromitus sp. SFB-mouse-Yit and Candidatus Arthromitus sp. SFB-mouse-Japan), using Megablast (optimized for highly similar sequences; ≥ 95 %) and E-values below 1e-15.
Fecal microbiota transplantation
Fecal material for microbiota transfer was prepared by using a modification of a previously described method 51. Briefly, freshly emitted fecal pellets were homogenized by vortexing in sterile PBS (1 ml per 0.1g of fecal material). After filtration of particulate matter, mice were gavage with 0.2 ml of the suspension.
Antigen-specific CD4+ T cell cytokine responses
Spleens and MLN were collected one week after the last immunization and mononuclear cells were restimulated in vitro with OVA (1 mg/ml) as previously reported 52. After 5 days of culture, levels of Th1 (IFN-γ), Th2 (IL-4, IL-5, IL-10) or Th17 (IL-17A) cytokines in supernatants were determined by ELISA using mAb pairs and cytokine standards (BD Biosciences or R&D Systems).
Antigen-specific Ab responses and total IgA levels
OVA-specific Ab responses were measured by ELISA as previously described 52. Total IgA levels, were determined by ELISA and IgA standards. The frequency of IgA secreting cells were evaluated by ELISPOT 53.
Analysis of lung functions and airway responses to metacholine challenge
Mechanical properties of the mouse lung were assessed using the forced-oscillation technique 54 and a flexiVent computer controlled piston ventilator (SCIREQ®, Montreal, Canada). Mice were exposed to increasing doses of metacholine (0.1, 1, 10, 20 and 50 mg/ml) in sterile normal saline 54 and total lung resistance were recorded.
Adoptive transfer
Cells (8x106) from control C57BL/6 (CD45.2) or IKKβΔIEC (CD45.2) were injected i.v. into congenic CD45.1 mice. Donor and recipient cells were discriminated in tissues of recipient mice by flow cytometry using CD45.1− and CD45.2− specific mAbs (BD Biosciences).
Bronchoalevolar lavages
Bronchoalveolar lavage fluids were obtained via cannulation of the exposed trachea, by infusion of 600 µl of sterile PBS through a 22-gauge catheter into the lungs 5.
Analysis of cell populations in lung tissues
Whole-lung tissue was dissociated in 0.5 mg/ml collagenase type V (Sigma) 15 min at 37°C. Single cell preparations were stained with one or a combination of the fluorescent anti-mouse Abs: anti-CD11b, anti-CD11c, anti-F4/80 anti-B220, anti-CD3, anti-CD4, anti-CD8, anti-CD103, anti-α4β7, and anti-CCR9 (BD Biosciences and eBiosciences) and analyzed by flow cytometry (Accuri Cytometers, Inc).
Statistics
Results are expressed as the mean ± 1 SD. Statistical significance was determined by Student’s T test or by ANOVA followed by the Fisher Least Significant Difference Test. For analysis of mRNA responses, we used one-way ANOVA, followed by Duncan’s Multiple Range Test.. All statistical analyses were performed with the Statistica 9.0 software package (StatSoft, Inc., Tulsa, OK).
Supplementary Material
Figure 5. IKKβ-deficiency in intestinal epithelial cells limits lung allergic inflammation in orally sensitized mice.
(A) Hematoxylin and eosin staining of lung sections (x40). (B) Inflammation scores in lungs of C57BL/6, and IKKβΔIEC mice. (C) Tissues were stained with PAS and counterstained with hematoxylin and eosin to visualize mucus (x400). Each picture in (A) and (C) is representative of three separate experiments, with 3–4 mice per group. (D). Total and OVA-specific IgA Ab levels, and (E) cell subsets in BAL fluids . The results are expressed as the mean ± one SD and are from three experiments and five mice / group. (*, p < 0.05 compared to control C57BL/6 mice).
Acknowledgments
Supported by grants from the National Institute of Health (R01 AI043197), the OSU Food Innovation Center, and a fellowship from Fondation Lelous, France (to A.BB). The authors thank Dr. Kate Hayes-Ozello for editorial assistance, Dr. Jessica Grieves for assistance with scoring of histology, Tim Vojt for help with graphic design, and Dr. J Delton Hanson for review of the metagenomic data.
References
- 1.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011;128(3):495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S116–S125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, et al. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc Natl Acad Sci U S A. 2005;102(49):17723–17728. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway epithelial NF-kappaB activation promotes allergic sensitization to an innocuous inhaled antigen. Am J Respir Cell Mol Biol. 2011;44(5):631–638. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer R, McGhee JR, Vu HL, Atkinson TP, Jackson RJ, Tome D, et al. Oral and nasal sensitization promote distinct immune responses and lung reactivity in a mouse model of peanut allergy. Am J Pathol. 2005;167(6):1621–1630. doi: 10.1016/S0002-9440(10)61246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oyoshi MK, Elkhal A, Scott JE, Wurbel MA, Hornick JL, Campbell JJ, et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest. 2011;121(6):2210–2220. doi: 10.1172/JCI43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 8.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130(5):918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7(12):1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 10.Fong CH, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, et al. An antiinflammatory role for IKKbeta through the inhibition of "classical" macrophage activation. J Exp Med. 2008;205(6):1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105(39):15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446(7135):552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 13.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 14.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 15.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(6):478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez B, Prioult G, Hacini-Rachinel F, Moine D, Bruttin A, Ngom-Bru C, et al. Infant gut microbiota is protective against cow's milk allergy in mice despite immature ileal T-cell response. FEMS Microbiol Ecol. 2012;79(1):192–202. doi: 10.1111/j.1574-6941.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- 18.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108(4):516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2012;109(2):594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9(5):575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 24.Snel J, Heinen PP, Blok HJ, Carman RJ, Duncan AJ, Allen PC, et al. Comparison of 16S rRNA sequences of segmented filamentous bacteria isolated from mice, rats, and chickens and proposal of "Candidatus Arthromitus". Int J Syst Bacteriol. 1995;45(4):780–782. doi: 10.1099/00207713-45-4-780. [DOI] [PubMed] [Google Scholar]
- 25.Umesaki Y, Okada Y, Imaoka A, Setoyama H, Matsumoto S. Interactions between epithelial cells and bacteria, normal and pathogenic. Science. 1997;276(5314):964–965. doi: 10.1126/science.276.5314.964. [DOI] [PubMed] [Google Scholar]
- 26.Prakash T, Oshima K, Morita H, Fukuda S, Imaoka A, Kumar N, et al. Complete genome sequences of rat and mouse segmented filamentous bacteria, a potent inducer of th17 cell differentiation. Cell Host Microbe. 2011;10(3):273–284. doi: 10.1016/j.chom.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172(11):6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 30.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits HH, Gloudemans AK, van Nimwegen M, Willart MA, Soullie T, Muskens F, et al. Cholera toxin B suppresses allergic inflammation through induction of secretory IgA. Mucosal Immunol. 2009;2(4):331–339. doi: 10.1038/mi.2009.16. [DOI] [PubMed] [Google Scholar]
- 32.Weng X, Liu L, Barcellos LF, Allison JE, Herrinton LJ. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern california-managed care organization. Am J Gastroenterol. 2007;102(7):1429–1435. doi: 10.1111/j.1572-0241.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 33.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteriaderived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18(4):538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bromander AK, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37(4):452–458. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 36.Bandyopadhaya A, Sarkar M, Chaudhuri K. Transcriptional upregulation of inflammatory cytokines in human intestinal epithelial cells following Vibrio cholerae infection. FEBS J. 2007;274(17):4631–4642. doi: 10.1111/j.1742-4658.2007.05991.x. [DOI] [PubMed] [Google Scholar]
- 37.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107(12):5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–652. e641–e645. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 39.Klaasen HL, Van der Heijden PJ, Stok W, Poelma FG, Koopman JP, Van den Brink ME, et al. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect Immun. 1993;61(1):303–306. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67(4):1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2(6):468–471. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- 42.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 43.Salvatori N, Reccardini F, Convento M, Purinan A, Colle R, De Carli S, et al. Asthma induced by inhalation of flour in adults with food allergy to wheat. Clin Exp Allergy. 2008;38(8):1349–1356. doi: 10.1111/j.1365-2222.2008.03023.x. [DOI] [PubMed] [Google Scholar]
- 44.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181(9):6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 45.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)- beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176(4):2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278(19):17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 47.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virusinduced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185(4):2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durrant DM, Gaffen SL, Riesenfeld EP, Irvin CG, Metzger DW. Development of allergen-induced airway inflammation in the absence of T-bet regulation is dependent on IL-17. J Immunol. 2009;183(8):5293–5300. doi: 10.4049/jimmunol.0803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowley SC, Meierovics AI, Frelinger JA, Iwakura Y, Elkins KL. Lung CD4-CD8- double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. J Immunol. 2010;184(10):5791–5801. doi: 10.4049/jimmunol.1000362. [DOI] [PubMed] [Google Scholar]
- 50.Duverger A, Carre JM, Jee J, Leppla SH, Cormet-Boyaka E, Tang WJ, et al. Contributions of edema factor and protective antigen to the induction of protective immunity by Bacillus anthracis edema toxin as an intranasal adjuvant. J Immunol. 2010;185(10):5943–5952. doi: 10.4049/jimmunol.0902795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duverger A, Jackson RJ, van Ginkel FW, Fischer R, Tafaro A, Leppla SH, et al. Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens. J Immunol. 2006;176(3):1776–1783. doi: 10.4049/jimmunol.176.3.1776. [DOI] [PubMed] [Google Scholar]
- 53.Boyaka PN, Ohmura M, Fujihashi K, Koga T, Yamamoto M, Kweon MN, et al. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J Immunol. 2003;170(1):454–462. doi: 10.4049/jimmunol.170.1.454. [DOI] [PubMed] [Google Scholar]
- 54.Aeffner F, Traylor ZP, Yu EN, Davis IC. Double-stranded RNA induces similar pulmonary dysfunction to respiratory syncytial virus in BALB/c mice. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L99–L109. doi: 10.1152/ajplung.00398.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.