Abstract
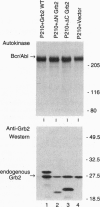
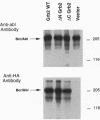
Growth factor-binding protein 2 (Grb2) is an adaptor protein that links tyrosine kinases to Ras. BCR-ABL is a tyrosine kinase oncoprotein that is implicated in the pathogenesis of Philadelphia chromosome (Ph1)-positive leukemias. Grb2 forms a complex with BCR-ABL and the nucleotide exchange factor Sos that leads to the activation of the Ras protooncogene. In this report we demonstrate that Grb2 mutant proteins lacking amino- or carboxyl-terminal src homology SH3 domains suppress BCR-ABL-induced Ras activation and reverse the oncogenic phenotype. The Grb2 SH3-deletion mutant proteins bind to BCR-ABL and do not impair tyrosine kinase activity. Expression of the Grb2 SH3-deletion mutant proteins in BCR-ABL-transformed Rat-1 fibroblasts and in the human Ph1-positive leukemic cell line K562 inhibits their ability to grow as foci in soft agar and form tumors in nude mice. Furthermore, expression of the Grb2 SH3-deletion mutants in K562 cells induced their differentiation. Because Ras plays an important role in signaling by receptor and nonreceptor tyrosine kinases, the use of interfering mutant Grb2 proteins may be applied to block the proliferation of other cancers that depend in part on activated tyrosine kinases for growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afar D. E., Goga A., McLaughlin J., Witte O. N., Sawyers C. L. Differential complementation of Bcr-Abl point mutants with c-Myc. Science. 1994 Apr 15;264(5157):424–426. doi: 10.1126/science.8153630. [DOI] [PubMed] [Google Scholar]
- Anafi M., Gazit A., Zehavi A., Ben-Neriah Y., Levitzki A. Tyrphostin-induced inhibition of p210bcr-abl tyrosine kinase activity induces K562 to differentiate. Blood. 1993 Dec 15;82(12):3524–3529. [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Rapp U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992 Apr;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992 Mar 26;356(6367):340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Cortez D., Kadlec L., Pendergast A. M. Structural and signaling requirements for BCR-ABL-mediated transformation and inhibition of apoptosis. Mol Cell Biol. 1995 Oct;15(10):5531–5541. doi: 10.1128/mcb.15.10.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac D., Frech M., Chardin P. Binding of the Grb2 SH2 domain to phosphotyrosine motifs does not change the affinity of its SH3 domains for Sos proline-rich motifs. EMBO J. 1994 Sep 1;13(17):4011–4021. doi: 10.1002/j.1460-2075.1994.tb06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B., Sprenger F., Morrison D., Hafen E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature. 1992 Dec 10;360(6404):600–603. doi: 10.1038/360600a0. [DOI] [PubMed] [Google Scholar]
- Fabian J. R., Morrison D. K., Daar I. O. Requirement for Raf and MAP kinase function during the meiotic maturation of Xenopus oocytes. J Cell Biol. 1993 Aug;122(3):645–652. doi: 10.1083/jcb.122.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath I., Schweighoffer F., Rey I., Multon M. C., Boiziau J., Duchesne M., Tocqué B. Cloning of a Grb2 isoform with apoptotic properties. Science. 1994 May 13;264(5161):971–974. doi: 10.1126/science.8178156. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988 Aug;8(8):3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein E., Gale R. P., Reed J., Canaani E. Expression of the normal p53 gene induces differentiation of K562 cells. Oncogene. 1992 Sep;7(9):1853–1857. [PubMed] [Google Scholar]
- Fukazawa H., Li P. M., Yamamoto C., Murakami Y., Mizuno S., Uehara Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem Pharmacol. 1991 Oct 9;42(9):1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- Han M., Golden A., Han Y., Sternberg P. W. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature. 1993 May 13;363(6425):133–140. doi: 10.1038/363133a0. [DOI] [PubMed] [Google Scholar]
- Kabarowski J. H., Allen P. B., Wiedemann L. M. A temperature sensitive p210 BCR-ABL mutant defines the primary consequences of BCR-ABL tyrosine kinase expression in growth factor dependent cells. EMBO J. 1994 Dec 15;13(24):5887–5895. doi: 10.1002/j.1460-2075.1994.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Lugo T. G., Witte O. N. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989 Mar;9(3):1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandanas R. A., Leibowitz D. S., Gharehbaghi K., Tauchi T., Burgess G. S., Miyazawa K., Jayaram H. N., Boswell H. S. Role of p21 RAS in p210 bcr-abl transformation of murine myeloid cells. Blood. 1993 Sep 15;82(6):1838–1847. [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Muller A. J., Young J. C., Pendergast A. M., Pondel M., Landau N. R., Littman D. R., Witte O. N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991 Apr;11(4):1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Quilliam L. A., Cripe L. D., Bassing C. H., Dai Z., Li N., Batzer A., Rabun K. M., Der C. J., Schlessinger J. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993 Oct 8;75(1):175–185. [PubMed] [Google Scholar]
- Puil L., Liu J., Gish G., Mbamalu G., Bowtell D., Pelicci P. G., Arlinghaus R., Pawson T. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J. 1994 Feb 15;13(4):764–773. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif K., Buday L., Downward J., Cantrell D. A. SH3 domains of the adapter molecule Grb2 complex with two proteins in T cells: the guanine nucleotide exchange protein Sos and a 75-kDa protein that is a substrate for T cell antigen receptor-activated tyrosine kinases. J Biol Chem. 1994 May 13;269(19):14081–14087. [PubMed] [Google Scholar]
- Renshaw M. W., McWhirter J. R., Wang J. Y. The human leukemia oncogene bcr-abl abrogates the anchorage requirement but not the growth factor requirement for proliferation. Mol Cell Biol. 1995 Mar;15(3):1286–1293. doi: 10.1128/mcb.15.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. The viral and cellular forms of the Abelson (abl) oncogene. Adv Virus Res. 1988;35:39–81. doi: 10.1016/s0065-3527(08)60708-3. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., McLaughlin J., Witte O. N. Genetic requirement for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J Exp Med. 1995 Jan 1;181(1):307–313. doi: 10.1084/jem.181.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife R., Gout I., Waterfield M. D., Margolis R. L. Growth factor-induced binding of dynamin to signal transduction proteins involves sorting to distinct and separate proline-rich dynamin sequences. EMBO J. 1994 Jun 1;13(11):2574–2582. doi: 10.1002/j.1460-2075.1994.tb06547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. How receptor tyrosine kinases activate Ras. Trends Biochem Sci. 1993 Aug;18(8):273–275. doi: 10.1016/0968-0004(93)90031-h. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Kostka G., Lammers R., Bashkin P., Daly R., Burgess W. H., van der Bliek A. M., Schlessinger J., Ullrich A. Dynamin binds to SH3 domains of phospholipase C gamma and GRB-2. J Biol Chem. 1994 Jun 10;269(23):16009–16014. [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Morishita T., Hashimoto Y., Hattori S., Nakamura S., Shibuya M., Matuoka K., Takenawa T., Kurata T., Nagashima K. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3443–3447. doi: 10.1073/pnas.91.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi T., Feng G. S., Shen R., Song H. Y., Donner D., Pawson T., Broxmeyer H. E. SH2-containing phosphotyrosine phosphatase Syp is a target of p210bcr-abl tyrosine kinase. J Biol Chem. 1994 May 27;269(21):15381–15387. [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B., Heidecker G., Huleihel M., Rapp U. R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989 May;9(5):2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993 Feb;67(2):205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]