Abstract
Background
Hypercortisolism leads to various physical, psychological and cognitive symptoms, which may partly persist after the treatment of Cushing's disease. The aim of the present study was to investigate abnormalities in white matter integrity in patients with long-term remission of Cushing's disease, and their relation with psychological symptoms, cognitive impairment and clinical characteristics.
Methods
In patients with long-term remission of Cushing's disease (n = 22) and matched healthy controls (n = 22) we examined fractional anisotropy (FA) values of white matter in a region-of-interest (ROI; bilateral cingulate cingulum, bilateral hippocampal cingulum, bilateral uncinate fasciculus and corpus callosum) and the whole brain, using 3 T diffusion tensor imaging (DTI) and a tract-based spatial statistics (TBSS) approach. Psychological and cognitive functioning were assessed with validated questionnaires and clinical severity was assessed using the Cushing's syndrome Severity Index.
Results
The ROI analysis showed FA reductions in all of the hypothesized regions, with the exception of the bilateral hippocampal cingulum, in patients when compared to controls. The exploratory whole brain analysis showed multiple regions with lower FA values throughout the brain. Patients reported more apathy (p = .003) and more depressive symptoms (p < .001), whereas depression symptom severity in the patient group was negatively associated with FA in the left uncinate fasciculus (p < 0.05). Post-hoc analyses showed increased radial and mean diffusivity in the patient group.
Conclusion
Patients with a history of endogenous hypercortisolism in present remission show widespread changes of white matter integrity in the brain, with abnormalities in the integrity of the uncinate fasciculus being related to the severity of depressive symptoms, suggesting persistent structural effects of hypercortisolism.
Keywords: Cushing's disease, Hypercortisolism, Diffusion tensor imaging, Cingulum, Corpus callosum, Uncinate fasciculus, Cortisol
Highlights
-
•
We study white matter integrity in patients in remission of Cushing's disease.
-
•
The patients were compared to matched controls on fractional anisotropy (FA) values.
-
•
We found widespread reductions of FA after exposure to hypercortisolism.
-
•
Depression severity was related to FA in the left uncinate fasciculus in patients.
1. Introduction
Cushing's disease is caused by an adrenocorticotropic hormone (ACTH) producing pituitary adenoma, which in turn causes hypercortisolism (Nieman and Ilias, 2005). Hypercortisolism is associated with severe physical and psychological symptoms and cognitive impairments (Forget et al., 2000; Leon-Carrion et al., 2009; Michaud et al., 2009; Newell-Price et al., 2006; Nieman and Ilias, 2005). Patients with Cushing's disease are treated by undergoing surgery, in some cases followed by postoperative radiotherapy and/or hydrocortisone substitution, dependent on the outcome of the surgery. Remission occurs with the re-instatement of normalized basal and ACTH-stimulated cortisol values. Although the clinical picture improves substantially after the successful treatment of hypercortisolism (Starkman et al., 1986), various symptoms remain present during long-term remission. Compared to healthy controls, patients treated for Cushing's disease demonstrated more cognitive impairment (Dorn and Cerrone, 2000; Forget et al., 2002; Merke et al., 2005; Resmini et al., 2012; Tiemensma et al., 2010b), more quality of life impairments (Tiemensma et al., 2011; van Aken et al., 2005), a higher prevalence of psychopathology (e.g. affective disorders and apathy; (Tiemensma et al., 2010a) and maladaptive personality traits (Tiemensma et al., 2010a)). These persistent symptoms following transient hypercortisolism are not well understood.
Neuroimaging studies investigating brain characteristics related to (a history of) Cushing's disease are scarce, and the few studies examining structural brain characteristics have mainly focused on gray matter volumes. Hypercortisolism was found to be associated with smaller hippocampal volumes and overall brain atrophy (Bourdeau et al., 2002; Simmons et al., 2000; Starkman et al., 1992). After an early successful abrogation of hypercortisolism, hippocampal volume increased and emotional and cognitive functioning improved (Bourdeau et al., 2002; Hook et al., 2007; Starkman et al., 1999, 2003; Toffanin et al., 2011). Patients with remission of Cushing's disease demonstrated no differences in hippocampal volume compared to controls (Andela et al., 2013; Resmini et al., 2012; Starkman et al., 1999). However, smaller gray matter volumes in the anterior cingulate cortex (ACC) and larger gray matter volumes of the left posterior lobe of the cerebellum have been shown in patients with remission of Cushing's disease (Andela et al., 2013). Interestingly, both the hippocampus and ACC are involved in the functional neurocircuitry of stress (Dedovic et al., 2009), with subregions of the ACC having an inhibitory effect on various limbic structures (Baumann and Turpin, 2010; Phelps et al., 2004). Disturbances in this inhibitory function resulted in the dysregulation of emotion and impaired cognition in affective disorders (Phan et al., 2005; Shin and Liberzon, 2010). In this light, studying the structural connectivity between these brain regions in patients with long-term remission of hypercortisolism could give further insight into the pathophysiology of persistent psychological symptoms and cognitive impairment.
White matter integrity has never been studied in Cushing's disease, and the relationship between white matter and elevated cortisol levels has only been studied in animal models (Alonso, 2000; Miyata et al., 2011; Willette et al., 2012). These studies showed an association between the prolonged exposure to elevated corticosteroid levels and the inhibition of the proliferation of oligodendrocyte precursors throughout the white matter. Oligodendrocytes play a major role in the process of remyelination, and thus white matter integrity (Alonso, 2000; Miyata et al., 2011). In addition, studies examining white matter in stress-related psychiatric disorders (depressive disorders, anxiety disorders and posttraumatic stress disorder) are of interest because these disorders are often accompanied by an unbalanced hypothalamic–pituitary–adrenal axis, resulting in increased levels of cortisol, as well as psychiatric symptoms similar to those reported by Cushing's disease patients. Stress-related psychiatric disorders have been related to reduced white matter integrity in mainly the corpus callosum, the cingulum and the uncinate fasciculus (Cullen et al., 2010; Eluvathingal et al., 2006; Jackowski et al., 2008; Kieseppa et al., 2010; Schuff et al., 2011; Sexton et al., 2009; Villarreal et al., 2004).
In the present study we examined white matter integrity by measuring fractional anisotropy (FA) in patients with long-term remission of Cushing's disease and matched healthy controls. FA reflects the degree of diffusion directionality and is a sensitive marker of the tissue microstructural organization (Alexander et al., 2007; Hasan et al., 2004). Lower FA values are associated with decreased white matter integrity. However, as FA is a non-specific marker for white matter integrity, it gives no information about the structural properties underlying abnormalities in white matter tissue. In order to interpret the differences in FA, the mean diffusivity (MD) and the tensor regional eigenvalues were also assessed. Decreased diffusion along the principal direction of the fiber (axial diffusivity; AD) indicates axonal loss (Budde et al., 2009), while increased diffusion perpendicular to the principal direction of the fiber (Radial Diffusivity; RD) indicates demyelination (Alexander et al., 2007; Song et al., 2002, 2005). In addition, an increase in overall water diffusion in all directions (MD) is also an indication for demyelination (Horsfield and Jones, 2002), but could be caused by the presence of edema as well (Alexander et al., 2007).
Taking into account the previously identified gray matter abnormalities and the findings of studies on stress-related disorders, we hypothesized lower FA values in patients with long-term remission of Cushing's disease in a region-of-interest (ROI) including the corpus callosum, the bilateral cingulate cingulum, the bilateral hippocampal cingulum, and the bilateral uncinate fasciculus. In addition, we hypothesized that white matter abnormalities correlate with psychological and cognitive functioning, as well as duration of remission, disease duration and clinical severity. Furthermore, we performed an explorative whole brain analysis to detect possible changes in FA values in white matter outside our a priori defined regions of interest. Finally, to assess the nature of the white matter abnormalities indicated by differences in FA, post-hoc analyses are performed on the AD, RD, and MD.
2. Materials and methods
2.1. Subjects
All patients with remission of Cushing's disease of pituitary origin monitored at our institute (n = 49) and between 18 and 60 years of age, were invited by letter and those who did not respond were contacted by phone. The response rate was 96%, 31 patients were willing to participate and were screened for eligibility. 6 patients were excluded due to one of the following exclusion criteria: a (history of) drug- or alcohol abuse, neurological problems, contraindications for undergoing a magnetic resonance imaging (MRI) scan and left-handedness. Healthy controls were pair-wise matched to the patients on the variables gender, age, and education. They were recruited by advertisements in grocery stores and via the Internet. In addition to the general exclusion criteria of the study (a history of) psychiatric disorders was an exclusion criterion for the control group.
A total of 25 patients with remission of Cushing's disease and 25 matched healthy controls were included in this study. In the data analysis process, we decided to further exclude the data of 3 patients and their matched controls due to insufficient quality of the diffusion tensor imaging (DTI) data, resulting in a final sample of 22 patients and 22 matched controls.
The diagnosis of Cushing's disease had been confirmed in all patients, following previously described criteria (Tiemensma et al., 2010b). Some patients remained glucocorticoid dependent after surgery and were substituted with hydrocortisone (on average 20 mg/day, divided into three dosages). The estimated duration of disease was determined through patients' history by looking for the earliest physical/somatic signs. Duration of remission was calculated from the date of curative transsphenoidal surgery, or in case of persistent disease, from the date of normalization of biochemical tests after postoperative radiotherapy. Demographics and patient characteristics are reported in Table 1. Written informed consent was obtained from all participants prior to the clinical assessment and the MRI-scan session. The medical ethical committee of the Leiden University Medical Center approved the study protocol.
Table 1.
Demographics of the total sample and clinical characteristics of the patients with long-term remission of Cushing's disease.
| Characteristics | Patients with long-term remission of Cushing's disease (n = 22) | Matched healthy controls (n = 22) |
|---|---|---|
| Gender (male/female) | 4/18 | 4/18 |
| Age (years), mean (S.D.) | 44.42 (7.33) | 46.42 (7.30) |
| Education, n (%) | ||
| Low | 5 (22.7%) | 5 (22.7%) |
| Intermediate | 11 (50%) | 10 (45.5%) |
| High | 6 (27.3%) | 7 (31.8%) |
| Surgery, n (%) | ||
| Transsphenoidal adenomectomy | 22 (100%) | |
| Bilateral adrenalectomy | 2 (9.1%) | |
| Radiotherapy, n (%) | 5 (22.7%) | |
| Disease duration (years), mean (S.D.) | 6.73 (5.39) | |
| Duration of remission (years), mean (S.D.) | 11.87 (8.49) | |
| Estimated age on onset (years), mean (S.D.) | 25.81 (9.04) | |
| Hypopituitarism, n (%) | ||
| Any axis | 13 (59.1%) | |
| GH | 9 (40.9%) | |
| LH/FSH | 8 (36.4%) | |
| TSH | 9 (40.9%) | |
| ADH | 2 (9.1%) | |
| Hydrocortisone substitution | 12 (54.5%) | |
| Clinical Severity Index (CSI), mean (S.D.) | ||
| Active phase, total | 8.05 (1.96) | |
| Remission phase, total | 2.59 (1.50) |
2.2. Education
The education level was classified following the Dutch education system, which is comparable to the International Standard Classification of Education (ISCED). Low = primary education (elementary school) and lower secondary education (preparatory secondary education); intermediate = higher secondary education (higher general continued education, pre-university secondary education) and post-secondary education (intermediate vocational education); high = tertiary education (higher professional education, university).
2.3. Study procedure
We scheduled a single study visit that consisted of approximately two hours for MRI scanning (60 min), an interview for the evaluation of the clinical data and the assessment of psychological and cognitive functioning. After the scan session, participants were asked to complete several self-rating questionnaires at home for the assessment of psychopathology and cognitive functioning and to return them within a week.
2.4. Assessment of psychopathology and cognitive functioning
Presence and severity of depressive symptoms were evaluated using the Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979; Snaith et al., 1986), which was the only interviewer rated scale, and the Inventory of Depression Symptomatology (IDS; (Rush et al., 1996)). Anxiety was evaluated using the Beck Anxiety Inventory (BAI; Beck et al., 1988) and the Fear Questionnaire (FQ; Marks and Mathews, 1979). Apathy and irritability were assessed using the Apathy Scale (AS) and the Irritability Scale (IS), respectively (Chatterjee et al., 2005; Starkstein et al., 2001). The Cognitive Failures Questionnaire (CFQ) was used to assess failures in perception, memory, and motor function (Broadbent et al., 1982).
2.5. Cushing's syndrome Severity Index (CSI)
To assess clinical severity, the Cushing's syndrome Severity Index (CSI; (Sonino et al., 2000)) was used for current severity of symptoms and to retrospectively estimate (clinical) severity at the time of active disease. The CSI contains eight clinical features and can be scored on a 3-point scale, ranging from 0 to 2. A higher total score on the CSI indicates greater severity, with a range of 0–16. The information necessary for completing this index was derived from clinical history and medical files. Two raters, who reached consensus on each feature in case of discrepancy, scored the CSI. For the active phase, the CSI was scored retrospectively. The current score was evaluated based on the last yearly evaluation. The total score of the active phase and the total score of the remission phase were used in the analyses.
2.6. Data acquisition and preprocessing
DTI data were collected using a Philips 3.0 T Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands) with a 32-channel SENSE (sensitivity encoding) head coil. A single-shot echo-planar imaging sequence was used with the following scan parameters: repetition time = 6250 ms, echo time = 69 ms, flip angle = 90°, b-factor = 1000 s/mm2, voxel dimensions = 2 mm isotropic, number of slices = 60, and no slice gap. DTI data were acquired along 32 directions, together with a baseline image having no diffusion weighting (b = 0). Total DTI scanning time was ~ 8 min. Collected DTI data were preprocessed and analyzed using the Oxford Centre for Functional MRI of the Brain (FMRIB) software library (FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) version 5.0.2. First, DTI data were corrected for distortion and motion artifacts induced by eddy currents or by simple head motions, using affine registration of each diffusion weighted image to the b = 0 reference image. Next, non-brain tissue was removed using the Brain Extraction Tool. Following, in order to generate individual FA maps for each participant, the diffusion tensor model was fitted to each voxel using FMRIB's diffusion toolbox.
2.7. Whole brain TBSS
Tractal based spatial statistics (TBSS) version 1.2 was used for voxelwise analysis of the preprocessed FA data. First, individual FA images were aligned to the FMRIB58_FA standard-space image using nonlinear registration. Next, the mean FA image was generated and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the entire group. The mean FA skeleton was then thresholded at a FA value of ≥ 0.4 to exclude peripheral tracts and minimize partial voluming. Finally, each participant's aligned FA images were projected onto the mean FA skeleton, and the resulting data were fed into voxelwise permutation-based analysis.
2.8. Region of interest TBSS
To test for regional specific FA alterations, we first implemented a ROI-based TBSS. A binary mask encompassing the bilateral cingulate cingulum, the bilateral hippocampal cingulum, the corpus callosum, and the bilateral uncinate fasciculus was created as a ROI using the Johns Hopkins University (JHU) White Matter Atlas provided by FSL (Mori et al., 2005). The mask was then applied to the mean FA skeleton in order to include only voxels comprised in the mean FA skeleton. This confines the statistical analysis exclusively to voxels from the center of the tract, thereby minimizing anatomic inter-subject variability, registration errors, and partial voluming. The resulting study-specific ROI mask, consisting of 12,357 voxels, was used for voxelwise permutation-based ROI analysis.
2.9. Statistical analysis
Using FSL's Randomise Tool, permutation-based inferences with threshold-free cluster enhancement (TFCE) were carried out for voxelwise analysis of FA data (Smith and Nichols, 2009). In both the ROI analysis and the whole brain analysis 5000 random permutations were generated to build up the null distribution of the cluster size statistic, while testing the following contrasts: 1) controls < patients and 2) controls > patients. Age, gender and education (demeaned across groups) were included in the analysis as nuisance regressors to correct for between group variances. The resulting statistical maps were corrected for multiple comparisons (p < 0.05, TFCE corrected), and the JHU (John Hopkins University) White Matter and Juelich Histological atlases were used to label clusters with significant FA alterations.
2.10. Post-hoc analyses
In the patient group, the association between FA abnormalities and psychological and clinical characteristics was examined using a voxel-wise correlation approach. Clinical characteristics (disease duration in years, duration of remission in years, and CSI scores), and scores on psychological measures found to be significantly different between the patients and the controls (MADRS and AS scores), were fed into FSL's Randomise Tool along with the FA values of the voxels within regions of significant group differences resulting from the ROI analysis.
A mask was created of the voxels that were found to differ significantly between groups on FA resulting from the exploratory whole brain analysis. Along with this mask, information on each individuals' AD (the 1st eigenvalue), RD (the average of the 2nd and 3rd eigenvalues), and MD was fed into FSL's Randomise Tool using permutation-based inferences with TFCE.
3. Results
3.1. Psychometric data
At the day of the MRI scan, patients were asked about a lifetime history of psychiatric disorders. None of the patients reported any. Because of the subjective nature of this information, we also inspected their medical records and found no indications for the presence of psychiatric disorders prior to the onset of Cushing's disease.
The psychometric data are reported in Table 2. To correct for multiple comparisons we applied a Bonferroni correction and adjusted the level of significance to p < .005. Comparisons on the psychometric data between patients with long-term remission of Cushing's disease and the matched healthy controls showed significant differences on the MADRS (p < .001) and on the AS (p = .003). No further significant differences were found between patients and controls. Within the patient group we found no significant correlation between age and age of onset (p = .510), and age and disease duration (p = .057).
Table 2.
Symptom severity scores patients with long-term remission of Cushing's disease versus matched healthy controls.
| Patients with long-term remission of Cushing's disease (n = 22) | Matched healthy controls (n = 22) | p | |
|---|---|---|---|
| Montgomery–Åsberg Depression Rating Scale (MADRS), mean (S.D.) | 6.09 (5.71) | 1.45 (1.84) | .000a |
| Inventory Depression Scale (IDS), mean (S.D.) | 45.67 (12.99) | 36.14 (6.10) | .020a |
| Beck Anxiety Inventory (BAI), mean (S.D.) | 27.95 (5.65) | 24.18 (3.20) | .020a |
| Fear Questionnaire (FQ), mean (S.D.) | 23.76 (16.60) | 14.36 (10.04) | .033b |
| Agoraphobia subscale, mean (S.D.) | 4.90 (6.26) | 2.82 (3.43) | .495a |
| Blood injury phobia subscale, mean (S.D.) | 6.71 (8.75) | 3.55 (4.28) | .334a |
| Social phobia subscale, mean (S.D.) | 12.14 (7.88) | 8.00 (4.97) | .048b |
| Irritability Scale (IS), mean (S.D.) | 11.76 (9.17) | 8.73 (6.22) | .342a |
| Apathy Scale (AS), mean (S.D.) | 13.62 (6.70) | 8.23 (3.77) | .003b |
| Cognitive Failure Questionnaire, mean (S.D.) | 35.19 (14.57) | 29.36 (8.68) | .123b |
| Motion parameters in millimeters | |||
| Absolute displacement, mean (S.D.) | 1.67 (.367) | 1.54 (.345) | .078a |
| Relative displacement, mean (S.D.) | .640 (.082) | .597 (.111) | .146b |
S.D. = standard deviation. Due to multiple comparisons, level of significance was adjusted to p < .005 using Bonferroni correction. Displayed in bold are the p-values that are considered significant.
Mann–Whitney U test.
Independent sample t-test.
3.2. TBSS analysis
Motion parameters (Table 2) did not differ significantly between groups for both absolute displacement (p = .078) and relative displacement (p = .146). The TBSS ROI analysis showed significant reductions (p < .05, TFCE corrected) of FA values in patients compared to controls in 8394 voxels of the 12,357 voxels that comprised the ROI study specific mask (67.9%). Regions that were found to have smaller FA values in patients are the corpus callosum, the bilateral cingulate cingulum, and the bilateral uncinate fasciculus (Fig. 1). No significant differences in FA values were observed in the bilateral hippocampal cingulum.
Fig. 1.
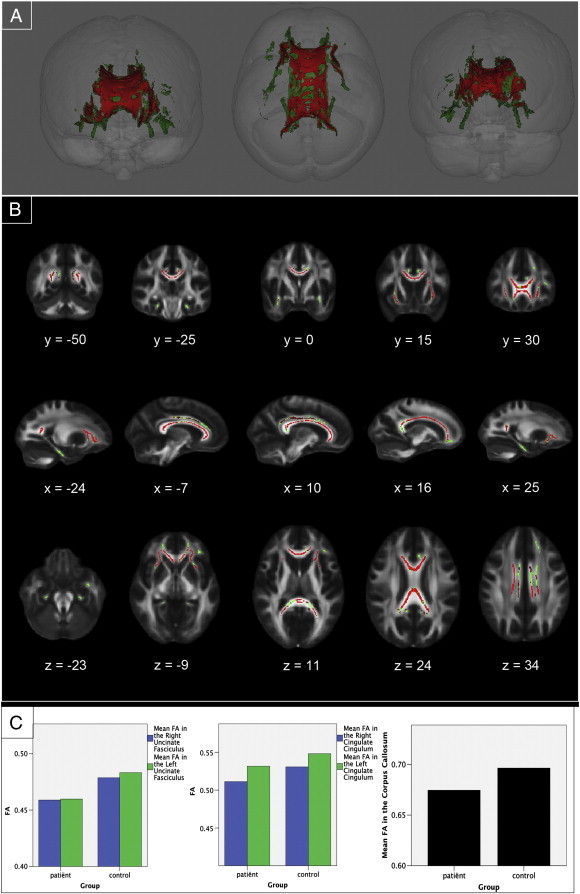
Region-of-interest analysis results. (A) Three-dimensional renderings showing (from left to right) anterior, top, and posterior views of the white matter skeleton (green) of the bilateral cingulate cingulum, the bilateral hippocampal cingulum, the corpus callosum, and the bilateral uncinate fasciculus. Superimposed in red are the regions in which FA values are significantly smaller in patients with long-term remission of Cushing's disease compared to matched healthy controls. (B) Coronal, sagittal and transversal axial sections of the white matter skeleton (green; 12,357 voxels) superimposed on the FMRIB58_FA_1mm standard brain (gray). Depicted in red are the regions in which FA values are significantly smaller in patients with long-term remission of Cushing's disease compared to matched healthy controls (8394 voxels). All TBSS results are corrected for multiple comparisons (p < 0.05, TFCE corrected), and the axial images are in radiological convention (the right side of the image corresponds with the left hemisphere of the brain and vice-versa). (C) Plots depicting the mean FA value per group in the significant ROIs. From left to right, the first plot shows FA values in the right uncinate fasciculus (blue) and left uncinate fasciculus (green). The second plot shows FA values in the right cingulate cingulum (blue) and left cingulate cingulum (green). The third plot shows the mean FA values in the corpus callosum.
Using a voxel-wise correlation approach, we examined the association between the observed smaller FA values, and psychological measurements and clinical characteristics in the patient group. This was conducted within the patient group by correlating the FA values of the voxels that were observed in the between-group difference with the scores on psychological measures. Because patients scored significantly higher on the MADRS and the AS, these were tested in addition to disease duration, duration of remission, and CSI scores (active phase and remission phase). To correct for multiple comparisons we applied a Bonferroni correction and adjusted the significance level to p < .0083. MADRS scores in the patient group correlated negatively with FA values in the left uncinate fasciculus (Fig. 2). We found no significant correlations in the patient group between FA values and AS scores, disease duration, duration of remission, and CSI scores.
Fig. 2.
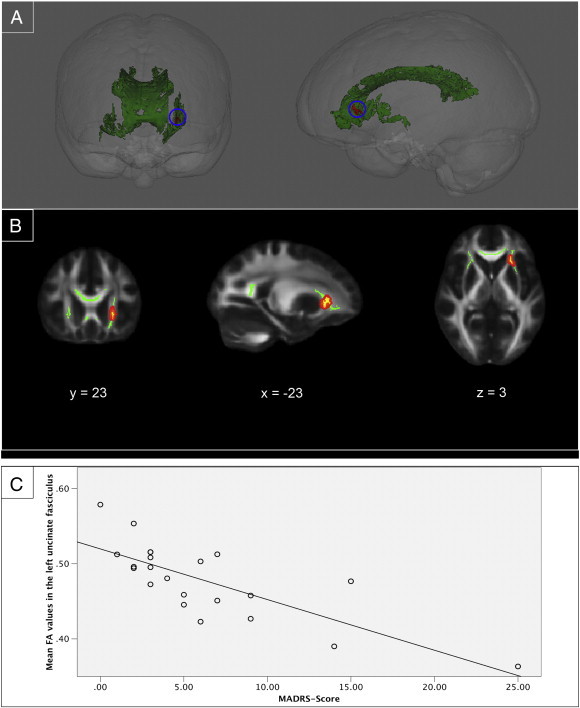
Voxel-wise correlation between MADRS scores and FA values in patients with long-term remission of Cushing's disease. Voxel-wise correlation between MADRS scores and FA values in patients with long-term remission of Cushing's disease. (A) Three-dimensional renderings showing (from left to right) anterior and left side views of the white matter skeleton (green) of areas that differed between groups in the ROI analysis. FA values in the left uncinate fasciculus correlated negatively with MADRS scores (p < .0083) in the patients with long-term remission of Cushing's disease (red). (B) Coronal, sagittal and transversal axial sections of the white matter skeleton (green) superimposed on the FMRIB58_FA_1mm standard brain (gray). FA values in the left uncinate fasciculus correlated negatively with MADRS scores (p < .0083) in the patients with long-term remission of Cushing's disease (yellow). For better visibility, the results are thickened using the “tbss-fill” command (red). The axial images are in radiological convention (the right side of the image corresponds with the left hemisphere of the brain and vice-versa). (C) A scatterplot showing the negative correlation between the mean FA in the left uncinate fasciculus and the scores on the MADRS in the patient group.
The exploratory whole brain analysis showed multiple reductions of FA values (p < .05, TFCE corrected) in various white matter tracts throughout the brain in patients compared to controls (Fig. 3). 31,343 voxels of the 56,976 voxels that comprised the study-specific whole brain mask were found to have significantly smaller FA values in patients (55%). Lowering the threshold (p < .1, TFCE corrected) resulted in the same pattern of reductions although slightly expanded. Tracts that were not found to differ significantly include: the inferior parts of the brainstem, the white matter in the bilateral cerebellum, the bilateral hippocampal cingulum, the left inferior fronto-occipital fasciculus, and parts of the bilateral superior longitudinal fasciculus.
Fig. 3.
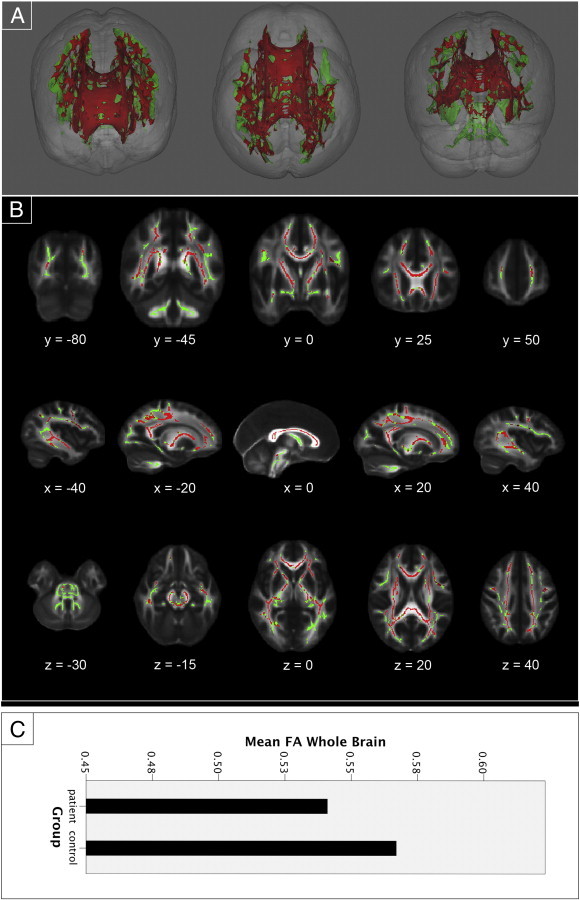
Exploratory whole brain analysis results. Whole brain TBSS results. (A) Three-dimensional renderings showing (from left to right) anterior, top, and posterior views of the white matter skeleton (green). Superimposed in red are the regions in which FA values are significantly smaller in patients with long-term remission of Cushing's disease compared to matched healthy controls. (B) Coronal, sagittal and transversal axial sections of the white matter skeleton (green; 56,976 voxels) superimposed on the FMRIB58_FA_1mm standard brain (gray). Depicted in red are the regions in which FA values are significantly smaller in patients with long-term remission of Cushing's disease compared to matched healthy controls (31,343 voxels). All TBSS results are corrected for multiple comparisons (p < 0.05, TFCE corrected), and the axial images are in radiological convention (the right side of the image corresponds with the left hemisphere of the brain and vice-versa). (C) A plot of the mean FA value per group in the significant effect found in the whole brain analysis.
Post-hoc analyses of the AD, RD, and MD in the voxels that showed decreased FA between groups revealed a significant increase (p < .05, TFCE corrected) of RD and MD in the patient group compared to controls. No significant differences were found between groups in AD.
4. Discussion
Using a ROI approach, we found reduced white matter integrity in patients compared to controls in the corpus callosum, the bilateral cingulate cingulum, and the bilateral uncinate fasciculus, but not in the bilateral hippocampal cingulum. The explorative whole brain analysis showed widespread reductions of white matter integrity throughout the brain. In the examined regions, we observed increased RD and MD, as well as no differences in AD. Furthermore, we found that FA values in the left uncinate fasciculus correlated with the severity of depressive symptoms in the patients.
Our ROI analysis showed decreased FA in most of the hypothesized regions in patients. However, the exploratory whole brain analysis showed reductions of FA values in nearly all white matter tracts throughout the brain. This leads to the idea that a general, white matter-affecting mechanism underlies the observed differences between patients and controls. Due to the cross-sectional design of this study no causal conclusions can be drawn about the relation between FA reductions and the reported psychopathology (i.e. depressive symptoms, apathy). However, since all patients had a history of hypercortisolism, we suggest that prolonged exposure to high levels of cortisol has directly or indirectly affected the white matter tissue in patients with Cushing's disease. Data from animal studies seem to support this suggestion. White matter mRNA expression (likely reflecting oligodendrocytes responses) responds strongly and acutely to corticosterone treatment (van Gemert et al., 2006). Prolonged exposure to corticosteroids was associated with the inhibition of proliferation of oligodendrocyte precursors throughout the white matter (Alonso, 2000). A more direct link with the lower FA values that we observed in patients, is the finding that corticosterone in mice can lead to an increased distance between nerve fibers in fiber tracts as a consequence of direct glucocorticoid receptor activation in oligodendrocytes leading to increased branching of these cells and perhaps lower levels of myelination. This is in line with our findings of increases in RD, which has been related to demyelination and dysmyelination (Alexander et al., 2007; Song et al., 2005). The increased MD we found in the patient group is also an indication for decreased demyelination (Horsfield and Jones, 2002), but could also indicate the presence of edema (Alexander et al., 2007).
In humans, direct effects of exposure to extreme levels of cortisol on white matter integrity have never been studied. However, Johansson et al. (2012) found that long-standing psychological distress in midlife increases the risk of white matter lesions (Johansson et al., 2012). Additionally, it has been found that a specific genotype (ER22/23EK) related to glucocorticoid resistance is associated with lower presence and progression of white matter lesions in dementia (van Rossum et al., 2008). These results and the findings from our study, support the hypothesis of prolonged elevated cortisol levels affecting white matter in the brain in a non-localized manner. Longitudinal research should elucidate the mechanisms of the lasting effects of extreme levels of cortisol on white matter integrity.
Our data may also point at a neural substrate for the persistence of affective symptomatology in patients with Cushing's disease after treatment for hypercortisolism (Tiemensma et al., 2010a). Using a voxel-wise correlation approach, we found an association between depressive symptom severity, as measured on the MADRS, and decreases in FA values in the left uncinate fasciculus in patients. Recent studies in patients with depressive disorder also found decreased FA values in the uncinate fasciculus (Carballedo et al., 2012; Taylor et al., 2007). The uncinate fasciculus passes from the rostral part of the temporal lobe (lateral to the amygdala) and the insula, through the orbital and medial frontal cortices (Kier et al., 2004; Schmahmann et al., 2008). This white matter bundle effectively connects the limbic system with the frontal regions of the brain and is an important connection in the emotion regulation and stress network (Ghashghaei and Barbas, 2002).
In a previous study from our group, it was demonstrated that patients with long-term remission of Cushing's disease demonstrate subtle cognitive impairments (Tiemensma et al., 2010b). In the present study no differences were found using the CFQ. Although the CFQ is a validated and commonly used questionnaire, it is not equal to extensive neuropsychological testing that would provide a more accurate assessment of cognitive functioning. It might be that the CFQ was not sensitive enough to detect these subtle impairments. 12 patients remained hydrocortisone dependent after treatment for Cushing's disease. We did not find differences in FA values between patient with and without hydrocortisone substitution (data not shown), suggesting that our results were not driven by hydrocortisone dependence. Due to the gradual onset of the a-specific symptoms of Cushing's disease, and the high prevalence of psychiatric symptomatology in Cushing's disease it is difficult to make a clear distinction between Cushing's related psychiatric symptoms and independently developed psychiatric symptoms. Therefore, it is possible that psychiatric symptomatology in the patients developed independently or prior to the onset of Cushing's disease.
The data presented in this study provide a further perspective towards a detailed phenotyping of patients after the treatment of Cushing's disease, who often report persisting psychological symptomatology and cognitive impairment. It is tempting to speculate that our findings, to a certain extent, could also apply to patients with chronic or recurrent forms of highly prevalent stress-related disorders such as depression, and, in addition, to patients treated with exogenous corticosteroids that are commonly prescribed to suppress the immune system (Brown and Suppes, 1998).
This study is the first to demonstrate that white matter integrity is affected in patients treated for Cushing's disease, despite long-term remission of cortisol excess. More research is needed to elucidate the role of cortisol in affecting white matter tissue integrity and the cellular mechanisms that underlie this process. Furthermore, it should be clarified as to how abnormalities in structural connectivity interact with potential differences in functional connectivity and how this interaction relates to the psychological symptomatology observed in patients with remission of Cushing's disease.
Acknowledgments
S.J.A. van der Werff was supported through the Netherlands Organization for Scientific Research — National Initiative Brain and Cognition project (NWO-NIHC, project no. 056-25-010). N.R. Biermasz was supported through the Netherlands Organization for Scientific Research — (NWO-VENI, project no. 016136125). S.A.R.B. Rombouts was supported through the Netherlands Organization for Scientific Research — (NWO-VICI, project no. 016.130.677).
References
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31(3):219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Andela C.D., van der Werff S.J., Pannekoek J.N., van den Berg S.M., Meijer O.C., van Buchem M.A., Pereira A.M. Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing's disease: a case–control study. Eur. J. Endocrinol. 2013 doi: 10.1530/EJE-13-0471. [DOI] [PubMed] [Google Scholar]
- Baumann N., Turpin J.C. Neurochemistry of stress. An overview. Neurochem. Res. 2010;35(12):1875–1879. doi: 10.1007/s11064-010-0298-9. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bourdeau I., Bard C., Noel B., Leclerc I., Cordeau M.P., Belair M., Lacroix A. Loss of brain volume in endogenous Cushing's syndrome and its reversibility after correction of hypercortisolism. J. Clin. Endocrinol. Metab. 2002;87(5):1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Brown E.S., Suppes T. Mood symptoms during corticosteroid therapy: a review. Harv. Rev. Psychiatry. 1998;5(5):239–246. doi: 10.3109/10673229809000307. [DOI] [PubMed] [Google Scholar]
- Budde M.D., Xie M., Cross A.H., Song S.K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballedo A., Amico F., Ugwu I., Fagan A.J., Fahey C., Morris D., Frodl T. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B(5):537–548. doi: 10.1002/ajmg.b.32060. [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Anderson K.E., Moskowitz C.B., Hauser W.A., Marder K.S. A comparison of self-report and caregiver assessment of depression, apathy, and irritability in Huntington's disease. J. Neuropsychiatry Clin. Neurosci. 2005;17(3):378–383. doi: 10.1176/jnp.17.3.378. [DOI] [PubMed] [Google Scholar]
- Cullen K.R., Klimes-Dougan B., Muetzel R., Mueller B.A., Camchong J., Houri A., Lim K.O. Altered white matter microstructure in adolescents with major depression: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(2):173–183. doi: 10.1097/00004583-201002000-00011. (e171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K., D'Aguiar C., Pruessner J.C. What stress does to your brain: a review of neuroimaging studies. Can. J. Psychiatry. 2009;54(1):6–15. doi: 10.1177/070674370905400104. [DOI] [PubMed] [Google Scholar]
- Dorn L.D., Cerrone P. Cognitive function in patients with Cushing syndrome: a longitudinal perspective. Clin. Nurs. Res. 2000;9(4):420–440. doi: 10.1177/10547730022158672. [DOI] [PubMed] [Google Scholar]
- Eluvathingal T.J., Chugani H.T., Behen M.E., Juhasz C., Muzik O., Maqbool M., Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Forget H., Lacroix A., Somma M., Cohen H. Cognitive decline in patients with Cushing's syndrome. J. Int. Neuropsychol. Soc. 2000;6(1):20–29. doi: 10.1017/s1355617700611037. [DOI] [PubMed] [Google Scholar]
- Forget H., Lacroix A., Cohen H. Persistent cognitive impairment following surgical treatment of Cushing's syndrome. Psychoneuroendocrinology. 2002;27(3):367–383. doi: 10.1016/s0306-4530(01)00059-2. (doi: S0306453001000592 [pii]) [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Hasan K.M., Alexander A.L., Narayana P.A. Does fractional anisotropy have better noise immunity characteristics than relative anisotropy in diffusion tensor MRI? An analytical approach. Magn. Reson. Med. 2004;51(2):413–417. doi: 10.1002/mrm.10682. [DOI] [PubMed] [Google Scholar]
- Hook J.N., Giordani B., Schteingart D.E., Guire K., Giles J., Ryan K., Starkman M.N. Patterns of cognitive change over time and relationship to age following successful treatment of Cushing's disease. J. Int. Neuropsychol. Soc. 2007;13(1):21–29. doi: 10.1017/S1355617707070051. [DOI] [PubMed] [Google Scholar]
- Horsfield M.A., Jones D.K. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases — a review. NMR Biomed. 2002;15(7–8):570–577. doi: 10.1002/nbm.787. [DOI] [PubMed] [Google Scholar]
- Jackowski A.P., Douglas-Palumberi H., Jackowski M., Win L., Schultz R.T., Staib L.W., Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162(3):256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L., Skoog I., Gustafson D.R., Olesen P.J., Waern M., Bengtsson C., Guo X. Midlife psychological distress associated with late-life brain atrophy and white matter lesions: a 32-year population study of women. Psychosom. Med. 2012;74(2):120–125. doi: 10.1097/PSY.0b013e318246eb10. [DOI] [PubMed] [Google Scholar]
- Kier E.L., Staib L.H., Davis L.M., Bronen R.A. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am. J. Neuroradiol. 2004;25(5):677–691. [PMC free article] [PubMed] [Google Scholar]
- Kieseppa T., Eerola M., Mantyla R., Neuvonen T., Poutanen V.P., Luoma K., Isometsa E. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J. Affect. Disord. 2010;120(1–3):240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J., Atutxa A.M., Mangas M.A., Soto-Moreno A., Pumar A., Leon-Justel A., Leal-Cerro A. A clinical profile of memory impairment in humans due to endogenous glucocorticoid excess. Clin. Endocrinol. 2009;70(2):192–200. doi: 10.1111/j.1365-2265.2008.03355.x. [DOI] [PubMed] [Google Scholar]
- Marks I.M., Mathews A.M. Brief standard self-rating for phobic patients. Behav. Res. Ther. 1979;17(3):263–267. doi: 10.1016/0005-7967(79)90041-x. [DOI] [PubMed] [Google Scholar]
- Merke D.P., Giedd J.N., Keil M.F., Mehlinger S.L., Wiggs E.A., Holzer S., Chrousos G.P. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J. Clin. Endocrinol. Metab. 2005;90(5):2531–2536. doi: 10.1210/jc.2004-2488. (jc.2004-2488 [pii]) [DOI] [PubMed] [Google Scholar]
- Michaud K., Forget H., Cohen H. Chronic glucocorticoid hypersecretion in Cushing's syndrome exacerbates cognitive aging. Brain Cogn. 2009;71(1):1–8. doi: 10.1016/j.bandc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Miyata S., Koyama Y., Takemoto K., Yoshikawa K., Ishikawa T., Taniguchi M., Tohyama M. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS One. 2011;6(5):e19859. doi: 10.1371/journal.pone.0019859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Nagae-Poetscher L.M., van Zijl P.C.M. Elsevier; Amsterdam, The Netherlands: 2005. MRI Atlas of Human White Matter. [Google Scholar]
- Newell-Price J., Bertagna X., Grossman A.B., Nieman L.K. Cushing's syndrome. Lancet. 2006;367(9522):1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- Nieman L.K., Ilias I. Evaluation and treatment of Cushing's syndrome. Am. J. Med. 2005;118(12):1340–1346. doi: 10.1016/j.amjmed.2005.01.059. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., LeDoux J.E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Resmini E., Santos A., Gomez-Anson B., Vives Y., Pires P., Crespo I., Webb S.M. Verbal and visual memory performance and hippocampal volumes, measured by 3-Tesla magnetic resonance imaging, in patients with Cushing's syndrome. J. Clin. Endocrinol. Metab. 2012;97(2):663–671. doi: 10.1210/jc.2011-2231. (c.2011-2231 [pii]) [DOI] [PubMed] [Google Scholar]
- Rush A.J., Gullion C.M., Basco M.R., Jarrett R.B., Trivedi M.H. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol. Med. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Smith E.E., Eichler F.S., Filley C.M. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. N. Y. Acad. Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N., Zhang Y., Zhan W., Lenoci M., Ching C., Boreta L., Neylan T.C. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54(Suppl. 1):S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C.E., Mackay C.E., Ebmeier K.P. A systematic review of diffusion tensor imaging studies in affective disorders. Biol. Psychiatry. 2009;66(9):814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N.E., Do H.M., Lipper M.H., Laws E.R., Jr. Cerebral atrophy in Cushing's disease. Surg. Neurol. 2000;53(1):72–76. doi: 10.1016/s0090-3019(99)00197-4. (S0090-3019(99)00197-4 [pii]) [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Snaith R.P., Harrop F.M., Newby D.A., Teale C. Grade scores of the Montgomery–Asberg Depression and the Clinical Anxiety Scales. Br. J. Psychiatry. 1986;148:599–601. doi: 10.1192/bjp.148.5.599. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sonino N., Boscaro M., Fallo F., Fava G.A. A clinical index for rating severity in Cushing's syndrome. Psychother. Psychosom. 2000;69(4):216–220. doi: 10.1159/000012396. (doi: 12396) [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Schteingart D.E., Schork M.A. Cushing's syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19(3):177–188. doi: 10.1016/0165-1781(86)90096-x. [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Gebarski S.S., Berent S., Schteingart D.E. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol. Psychiatry. 1992;32(9):756–765. doi: 10.1016/0006-3223(92)90079-f. (0006-3223(92)90079-F [pii]) [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Giordani B., Gebarski S.S., Berent S., Schork M.A., Schteingart D.E. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol. Psychiatry. 1999;46(12):1595–1602. doi: 10.1016/s0006-3223(99)00203-6. (S0006-3223(99)00203-6 [pii]) [DOI] [PubMed] [Google Scholar]
- Starkman M.N., Giordani B., Gebarski S.S., Schteingart D.E. Improvement in learning associated with increase in hippocampal formation volume. Biol. Psychiatry. 2003;53(3):233–238. doi: 10.1016/s0006-3223(02)01750-x. (S000632230201750X [pii]) [DOI] [PubMed] [Google Scholar]
- Starkstein S.E., Petracca G., Chemerinski E., Kremer J. Syndromic validity of apathy in Alzheimer's disease. Am. J. Psychiatry. 2001;158(6):872–877. doi: 10.1176/appi.ajp.158.6.872. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., MacFall J.R., Gerig G., Krishnan R.R. Structural integrity of the uncinate fasciculus in geriatric depression: relationship with age of onset. Neuropsychiatr. Dis. Treat. 2007;3(5):669–674. [PMC free article] [PubMed] [Google Scholar]
- Tiemensma J., Biermasz N.R., Middelkoop H.A., van der Mast R.C., Romijn J.A., Pereira A.M. Increased prevalence of psychopathology and maladaptive personality traits after long-term cure of Cushing's disease. J. Clin. Endocrinol. Metab. 2010;95(10):E129–E141. doi: 10.1210/jc.2010-0512. (jc.2010-0512 [pii]) [DOI] [PubMed] [Google Scholar]
- Tiemensma J., Kokshoorn N.E., Biermasz N.R., Keijser B.J., Wassenaar M.J., Middelkoop H.A., Romijn J.A. Subtle cognitive impairments in patients with long-term cure of Cushing's disease. J. Clin. Endocrinol. Metab. 2010;95(6):2699–2714. doi: 10.1210/jc.2009-2032. (jc.2009-2032 [pii]) [DOI] [PubMed] [Google Scholar]
- Tiemensma J., Kaptein A.A., Pereira A.M., Smit J.W., Romijn J.A., Biermasz N.R. Negative illness perceptions are associated with impaired quality of life in patients after long-term remission of Cushing's syndrome. Eur. J. Endocrinol. 2011;165(4):527–535. doi: 10.1530/EJE-11-0307. [DOI] [PubMed] [Google Scholar]
- Toffanin T., Nifosi F., Follador H., Passamani A., Zonta F., Ferri G., Perini G.I. Volumetric MRI analysis of hippocampal subregions in Cushing's disease: a model for glucocorticoid neural modulation. Eur. Psychiatry. 2011;26(1):64–67. doi: 10.1016/j.eurpsy.2010.09.003. [DOI] [PubMed] [Google Scholar]
- van Aken M.O., Pereira A.M., Biermasz N.R., van Thiel S.W., Hoftijzer H.C., Smit J.W., Romijn J.A. Quality of life in patients after long-term biochemical cure of Cushing's disease. J. Clin. Endocrinol. Metab. 2005;90(6):3279–3286. doi: 10.1210/jc.2004-1375. [DOI] [PubMed] [Google Scholar]
- van Gemert N.G., Meijer O.C., Morsink M.C., Joels M. Effect of brief corticosterone administration on SGK1 and RGS4 mRNA expression in rat hippocampus. Stress. 2006;9(3):165–170. doi: 10.1080/10253890600966169. [DOI] [PubMed] [Google Scholar]
- van Rossum E.F., de Jong F.J., Koper J.W., Uitterlinden A.G., Prins N.D., van Dijk E.J., Breteler M.M. Glucocorticoid receptor variant and risk of dementia and white matter lesions. Neurobiol. Aging. 2008;29(5):716–723. doi: 10.1016/j.neurobiolaging.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Villarreal G., Hamilton D.A., Graham D.P., Driscoll I., Qualls C., Petropoulos H., Brooks W.M. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131(3):227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Willette A.A., Coe C.L., Colman R.J., Bendlin B.B., Kastman E.K., Field A.S., Johnson S.C. Calorie restriction reduces psychological stress reactivity and its association with brain volume and microstructure in aged rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):903–916. doi: 10.1016/j.psyneuen.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


