Abstract
Objective:
Observational studies on the association among systemic/general and oral cavity indices, tooth loss, periodontal conditions, and socioeconomic inequalities are to be still performed in the population of Southern Europe. This study aims to determine the extent of this relationship among Italian healthy adults 50 years of age and above.
Materials and Methods:
Socioeconomic and lifestyle characteristics, cardiovascular indicators, and systemic indices were examined by contrasting the dental indices among adult people of Northern Italy. Data were processed through correlation analysis, and multivariate analysis was carried out using seemingly unrelated regressions.
Results:
A total of 118 adults 50 years of age and above, after anamnesis, underwent systemic and dental examination. Their socioeconomic status was found to be inversely associated only with smoking and dental parameters. Unexpected outcomes between lifestyle and risk factors were detected. The statistical analysis showed an uneven correlation among dental indices and between those indices and the socioeconomic status, such as, a periodontal condition, apparently free from influences, unusually became worse as the socioeconomic status enhanced.
Conclusions:
The study outcomes indicate a relationship between tooth loss and conservative endodontic therapy, but they result in alternative choices. Nevertheless, the socioeconomic status has an inverse relationship with tooth loss and conservative endodontic therapy, but a direct relation with worsening of the periodontal condition. This pilot study highlights a need for the public health administration to adopt a socioeconomic assessment not only based on the household income, but also to accordingly improve its therapeutic course.
Keywords: Adults, dental indices, health indices, lifestyle, periodontitis, socioeconomic status
INTRODUCTION
Periodontal disease is a chronic inflammatory disorder that affects the supporting tissue of the teeth with common clinical manifestations. The prevalence of periodontitis is known to be high in adult populations in both developing and developed countries. However, the prevalence of periodontitis, defined as clinical attachment level (CAL-loss) or loss of attachment (LOA) and/or probing pocket depth (PPD), was found to be 4-91%, considering the surveys performed in several countries among people over 50 years of age.[1,2,3,4] Some authors have used the community periodontal index of treatment needs (CPITN) to examine the periodontal status. The periodontal pathology was found to vary from 11% in Finnish,[5] 20-37% in Hong Kong,[6] to 70% in the UK elderly.[7] The periodontal pathology was found to be associated with smoking, lower socioeconomic status (SES), diabetes, gender, and dental care.[8,9,10,11,12,13,14,15]
The relative contributions of systemic and local risk factors to dental problems are unclear, particularly in the older population. Several risk factors and indicators have been associated with the occurrence of highly destructive forms of periodontal diseases.[16] The microbial destruction of teeth in caries and compound caries induces a local infective environment that could also negatively affect the periodontal status.[17,18] Several factors, including smoking, hyperglycemia, SES, nutritional status, and carious status, have been associated with periodontal diseases, but their role has not been substantiated.[8,16,19]
Caries and periodontal diseases were the most frequent reason for extraction for almost all tooth types, particularly in the community-dwelling elderly.[17,20,21] It must be stressed that some risk factors for periodontal disease were found in the pathogenesis of dental caries, but without a consistent relationship.[10] Therefore, it seems highly relevant to define the role of the above-mentioned risk factors in periodontal disease and caries, considering their significant role in tooth loss. Indices such as CPITN or periodontal screening and recording (PSR) scores were recommended by the American Dental Association and the American Academy of Periodontology to facilitate the early detection of periodontal disease, as a screening tool.[22,23,24] Additionally, the number of decayed and filled surfaces, and mixed teeth was largely reported to define the dental health and risk factors in dental studies.[25,26]
Several authors[9,10,11,12,13,14] documented inequalities in the association of health status to socioeconomic variables. However, all these studies did not involve the population of Southern Europe, which had a lifestyle clearly different from Northern Europe, USA, and the like. Generally, the indices of dental pathology seemed to decrease by getting a better SES, but this relationship was not completely defined.[15] Consequently, the statement of this relationship was particularly relevant in public health.
The objective of the present study was to determine whether an association existed between some systemic/lifestyle risk factors, oral cavity risk factors, loss of teeth, PSR scores, and SES, in healthy persons 50 years and older in Northern Italy.
MATERIALS AND METHODS
Study population and data collection
The target consisted of adults, of age 50 years and above, living in Northern Italy. Patients were recruited from among those seeking care for different dental problems at the Outpatient Hospital Clinic; data recorded during the study were the usual data and parameters needed during the first medical–dental examination to define the state of systemic and dental health of the patients. All patients signed their written informed consent in a form where all procedures were detailed, according to the Helsinki protocols.
Subjects with a history of severe, acute, or chronic systemic or oral diseases, pregnant or lactating women, subjects taking medications known to affect the oral status, and edentulous subjects were excluded. Patients with a history of myocardial or cerebrovascular ischemia, hypertension, or glucose intolerance (but not frank diabetes or a previous history of diabetes) were enrolled; they could not be taking medication to control these diseases. Moreover, the included subjects had to provide a laboratory glycemic assay not antecedent to three months. The participants’ history of myocardial or cerebrovascular ischemia, anthropometric measurements and nutritional assessment, and systolic (SBP) and diastolic blood pressures (DBP) were established by direct inquiry and clinical examinations.
Oral examination was performed with a flat rhodium-plating dental mirror, a dental probe, and a periodontal probe (UNC 15 HuFriedy, Chicago, IL, USA), with the patient sitting in a dentist's chair, to record the number of missing teeth (NMT), number of decayed surfaces (NDS), and number of filled surfaces (NFS). The PSR was assessed using the World Health Organization (WHO) periodontal probe. The PSR was measured in each sextant, but only the highest index value for each patient was considered in the study.
A pre-trial calibration session was performed on 37 healthy patients, to obtain the acceptable intra- and inter-examiner reproducibility in assessing the clinical periodontal parameters. Re-calibration was performed on the same patients some months later, to enhance the reproducibility.
Variables
BMI ― The body mass index was calculated as weight in kilograms divided by height in meters squared (kg/m2) and was used as an index of overall adiposity.[9]
NCD ― The number of cigarettes per day was auto reported.
NGT ― Normal glucose tolerance was defined as blood glucose up to 115 mg/dl after a fast of eight hours and no history of diabetes reported on medical history. Diabetes was defined as a previous diagnosis of diabetes by criteria or a fasting blood glucose value of >126 mg/dl, with no previous history of diabetes. Fast glycemic values (Gly) were recorded.[27]
SBP and DBP (mmHg) ― Systolic and diastolic blood pressures were measured at the forearm using an electronic sphygmomanometer, with the subject in the supine position, after a rest of five minutes.
CP-I ― Anamnestic cardiopathy/ischemia score, assigning value 1 to the positive and 0 to the negative CP-I.
NMT, NDS, NFS, and PSR ― An assortment of dental variables collected by examiners.
Socioeconomic status index
The study included 118 participants divided into three different groups by their household financial status. This index was ISEE (Indicatore Socio-Economico Equivalente – socioeconomic equivalent indicator), that is, the official parameter to define the socioeconomic status in Italy.[28] The ISEE index was constructed taking into account the data related to household income and real estate.[29] This index was also used to define the minimum guaranteed social-critical health care levels (SC-LEA).
Patients were split into three socioeconomic classes as follows:
Group 1 ― ISEE 0 – 7500 €. Indigent people, it authorizes all members of the household to dental treatments free of charge.
Group 2 ― ISEE 7500 – 12,500 €. Household group members receive the needed dental treatments with the charge of a moderate copayment.
Group 3 ― ISEE 12,500 – 15,000 €. Household group members receive the needed dental treatments with the charge of a substantial copayment.
An ISEE score greater than 15,000 € excludes household groups from the public dental care plan.
Statistical analysis
In the first step of the analysis, the data were summarized by descriptive statistics and the usual indices and forms: Cross-tables for qualitative variables in a bivariate or trivariate analysis, mean and standard deviation for the continuous variables in a univariate analysis or in tables and cross-tables when the continuous variables were illustrated with respect to one or two qualitative variables, and the Spearman correlation coefficient matrix determined by all the possible combinations of the couples of variables included in the set of interest. Gender was considered assigning the value 0 to males and 1 to females. CP-I was considered assigning the value 1 to positive anamnestic cardiopathy/ischemia and 0 to the negative one. Inter-rater agreement was measured using Cohen's K-coefficient, while intra-rater agreement was simply measured using the Spearman correlation coefficient.
In the second step of the analysis, the response or dependent variables, which were continuous, were identified and the multiple regression model was used to define the impact of some factors, assumed to be explanatory variables (covariates), on the dependent one. The ordinal variables were directly inserted into the regression model. The explanatory variables were selected by the backward method for each dependent variable. Analysis of covariance (ANCOVA) was applied to define interactions among the qualitative variables of the set, after explanatory identification of the variables.
In the third step the identified dependent variables and the corresponding explanatory variables were represented through a system of equations with correlated residuals, that is, the simultaneous-equation model. Therefore, the seemingly unrelated regression model was applied to simultaneously estimate the regression coefficients. In the system of a regression equation, the selected dependent variables were NMT in the first equation, NDS in the second equation, NFS in the third equation, and PSR in the fourth equation, while the others were considered to be covariates. Furthermore, in each equation, the dependents of the other equations were added to the set of covariates (BMI, NCD, etc.) because they were strictly correlated, but this implied the necessity to consider the four models jointly, as they became a simultaneous equation model. In each equation, the independent variables were selected by a backward procedure, and each one was eliminated if its P value was greater than 0.1. However, the dental variables appearing among the independent variables were always held in the explanatory set, apart from their P values. For each equation, the assumptions of the linear regression model were checked, analyzing the residuals: Linear form, homoscedasticity, non-autocorrelation, independence with respect to regressors, and normality. NDS showed residuals with a heavy long right tail, that is, skewed to the right, with cumulative probability distribution observed far from that expected in a normal P-P plot of regression standardized residuals, thus implying a violation of the assumptions. The data transformation, using the logarithms of NDS, ln (NDS), improved the behavior of the residuals, bringing their distribution closer to normality so that the assumption of normality held well. After four equation regression models were set up, that is, the explanatory variables for each equation were identified, the models were simultaneously estimated by the reg3 procedure of Stata.[30]
All statistical analyses were executed using the statistical package for social sciences,[31] except for the parameters’ estimation of the system equation regression, which was carried out by the reg3 procedure of Stata.[30] The null hypothesis of the independence of residuals of the equation system, H0, was rejected at a critical significance level of P < 0.05.
RESULTS
The inter-rater agreement, measured using the Cohen's K-coefficient in the two different steps, was good at 0.60-0.70, and the intra-rater agreement calculated by the Spearman test showed excellent concordance at 0.908-0.920 (concerning the examination of NMT, NDS, NFS, and PSR) for the two examiners.
Table 1 shows the frequencies of the tested parameters in the 118 examined patients. The patients’ results almost equally split into the three SES groups. CP-I events were almost equally distributed by gender, ranging from 21.1 to 23%.
Table 1.
Frequencies of tested parameters in the whole population and socioeconomic groups
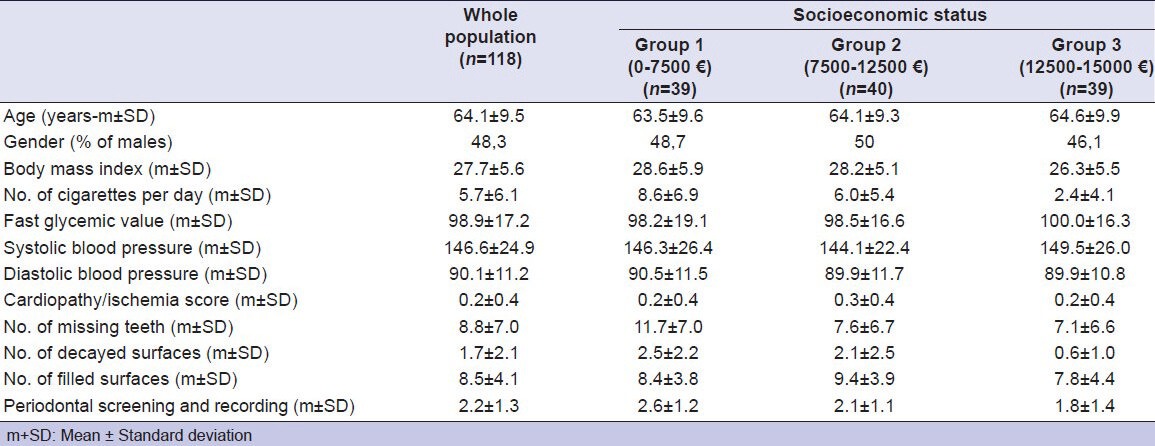
The statistical analysis of systemic/lifestyle indices showed a significant positive correlation of Gly with BMI (P < 0.001); SBP with age (P < 0.019), BMI (P < 0.001), and Gly (P < 0.001); DBP with age (P < 0.025), BMI (P < 0.001), Gly (P < 0.001), and SBP (P < 0.001); CP-I with SBP (P < 0.037) and DBP (P < 0.012). The analysis showed instead, a significant negative correlation of NCD with SES (P < 0.001) and age (P < 0.015), Gly with gender (P < 0.015) and NCD (P < 0.029); SBP with gender (P < 0.006); DBP with gender (P < 0.001) and NCD (P < 0.021).
The correlative statistical analysis of systemic/lifestyle against dental indices showed a significant positive correlation of NMT with age (P < 0.001), NCD (P < 0.008), and SBP (P < 0.040); NDS with NCD (P < 0.001), Gly (P < 0.028), and DBP (P < 0.013); PSR with BMI (P < 0.022), NCD (P < 0.001), Gly (P < 0.001), SBP (P < 0.001), and DBP (P < 0.001). The correlative analysis showed instead a significant negative correlation of NMT with SES (P < 0.002); NDS with SES (P < 0.001); NFS with age (P < 0.031) and gender (P < 0.049); PSR with SES (P < 0.008).
The statistical analysis of dental indices showed a significant positive correlation of NFS with NDS (P < 0.001); PSR with NMT (P < 0.001); NDS (P < 0.001), and NFS (P < 0.001). The analysis showed instead a significant negative correlation of NFS with NMT (P < 0.047).
The system of regression equation of systemic/lifestyle indices [Table 2] highlighted:
Table 2.
Coefficients and P values for the four seemingly unrelated regressions
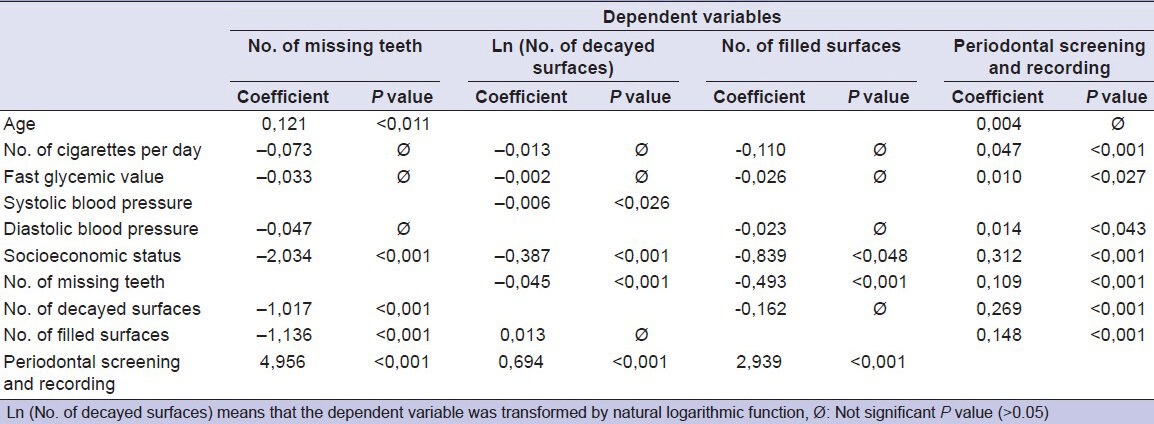
- 1 year increase of age produced a statistical decrease of about 1/9 dental element;
- 1 cigarette per day (NCD unit) increase produced about 1/20 PSR increase;
- 1 glycemic point (unit) increase produced about 1/100 PSR increase;
- 1 mmHg (SBP) increase produced about 0.6% NDS nonlinear decrease;
- 1 mmHg (DBP) increase produced about 1/70 PSR increase.
- 1 SES unit increase produced about 2 NMT decrease, 2/3 NDS decrease, 4/5 NFS decrease, and about 1/3 PSR increase;
The system of regression equation of dental indices [Table 2] highlighted:
- 1 missing tooth (NMT unit) produced 1/2 NFS decrease, NDS nonlinear decrease (about 4.4% for the first unit of NMT), and about 1/10 PSR increase;
- 1 decayed surface (NDS unit) increase produced about 1 NMT decrease and about 1/4 PSR increase;
- 1 filled surface (NFS unit) increase produced 1.14 NMT decrease and about 1/7 PSR increase;
- 1 PSR unit increase produced about 5 NMT increase, NDS nonlinear increase (about 200% for the first unit of PSR), and about 3 NFS increase.
DISCUSSION
Dental parameters
Baseline tooth loss is predictive of tooth loss and more attachment loss over time. However, NMT can also be influenced by caries, conservative dentistry treatments, endodontic treatments, and SES.[15,17,20,21,32,33,34,35,36,37,38,39,40]
In our study, the multivariate analysis of dental indices highlights that the increase in NMT has a weak effect on PSR increment and NDS decrement, and a moderate effect on NFS decrement. Previous studies have failed in exactly defining the correlations between NMT and the other dental parameters.[37] This result can be explained by the fact that NMT is significantly correlated to the periodontal status, as well as to other variables, just as the same NMT or decay status and the consequent conservative-endodontic therapy (NDS and NFS) may act as disturbing factors.[17,21,36] Moreover, studies show that these relationships are influenced by the position of teeth,[20] hygienic care taken by individuals, as well as the natural evolution of periodontitis.[37] We have just stated that different oral diseases can produce systemic inflammation and periodontal pockets can evolve in the adjacency of decay lesions, even if they are not an expression of periodontitis.
Our results reveal that an NDS increase corresponds to a moderate PSR increase, but an appreciable NMT decrease. Besides, the results show that an NFS increase has a weak increasing effect on PSR, but an appreciable effect on NMT decreases. The influence of NDS on NMT is a relation of equal terms, considering NDS is the independent variable. Thus, an incremental observation of tooth loss corresponds to a perceptible reduction in caries diagnosis, while an incremental observation of caries diagnosis corresponds to an effective reduction of tooth loss. Considering NFS as an independent variable, conservative dental therapy is extremely efficient in decreasing NMT. Therefore, it is conjecturable that tooth loss derived from causes other than periodontal disease in parallel with the diagnosis of caries may follow two different therapeutic management paths: Conservative-endodontic therapy or tooth loss and the conservative therapy of caries still further, to answer the decrease of tooth loss. Altogether, these data seem to point to the importance of promptly managing the conservative therapy to avoid dental extraction. The age,[21,40] lifestyle, spending power, and the degree of self-sufficiency may be significant[21] not only with regard to NMT, NDS, and NFS, but also with regard to the outcome of periodontal disease.[15,17,21,32,34] The literature data are conflicting and not conclusive.[15,17,32,38] Most of the authors have found better dental conditions in the strata of people characterized by better SES,[20,21,32,34] while other authors found SES or behavioral factors not significant.[15,17]
Our results show that by increasing the SES group (from 1 to 3), the NMT, NDS, and NFS decrease, but the PSR increases. Evaluating the data, the effects of SES on the PSR results are basically perceivable, but not fully expressed in our study. The SES impact on NDS is opposite to that on PSR, and of the same extent. The results show that SES moving from one group to the subsequent upper one corresponds to a decrease in about two tooth extractions. The SES impact on NMT is very substantial, while it is perceivable for NFS, but to a lower extent. Tooth loss apparently results from complex interactions among dental diseases, incident dental signs and symptoms, the tendency to use dental care in response to specific dental problems, dental attitudes, and the ability to afford non-extraction treatment alternatives.[20] In the low-income populations it could be a worry, with other daily issues, an attitude of waiting for a problem to occur before seeking dental care, and tooth extraction being the only solution or the only available treatment option.[39] However, PSR seems to be influenced by SES and the other dental parameters, although not in a very remarkable manner. Periodontitis probably has a largely independent pathogenic technique, which seems difficult to control, and hardly responds to other dental parameters or a non-specific therapy.
In our study, PSR increases all the dental indices NMT, NDS, and NFS, in a remarkable manner. Our data show a very close connection between PSR and tooth loss, when PSR is considered as an independent variable. However, NDS and NFS are also highly influenced by PSR. Besides, considering NDS and NFS as independent variables, PSR increases with them, and considering PSR as an independent variable, NDS and NFS remarkably increase with PSR. Consequently, it may be conjectured that decayed surfaces and filled surfaces negatively affect periodontal status. Considering NMT as an independent variable, PSR increases with NMT, and considering PSR as an independent variable, NMT strongly increases with PSR. Moreover, PSR may underestimate the periodontal pathological involvement.[41] Contrary to the previously stated effect of NFS/NDS on NMT, tooth loss cannot represent an alternative to periodontal deterioration, nor are conservative or dental extraction therapies able to resolve periodontal lesions. Within the limitations of this study, it is conjecturable that the lack of an effective and healthy periodontal strategy enables a group of patients to move inevitably toward losing their teeth.
Age and gender
Early evidence of the prevalence and severity of periodontitis suggests that an increase in age may be a marker for the loss of periodontal support and tooth loss.[42,43] Albandar[1] has observed that periodontal condition detriment increased with age in North American patients, but this relationship seems to be more influenced by the severity of disease and the decline may be due to loss of teeth, with the most severe disease in the older age group.
Our results show that NFS decreases and NMT increases with age. These findings are persistent only for NMT on the multivariate analysis. The age and other examined parameters, such as gender (G), are not always clearly related to the systemic and dental indices: The belief that periodontitis is a disease of the elderly has been challenged over time. Instead of increased susceptibility to periodontitis in elders, our results seem to highlight that age can conceivably represent a parameter within the true risk factors, and also may produce cumulative effects due to prolonged exposure.[23] Therefore, the increase of NMT may also be caused by the subject susceptibility level to periodontal disease and to the kind of dental treatments undergone in time.
Different results were published on the influence of gender on dental and systemic indices. In particular, Norderyd et al.[32] found a greater frequency of females with periodontal disease, while other authors[23,44] observed the same in males. Thus, it is not easy to establish inherent differences between men and women in their susceptibility to periodontitis and to the other dental indices considered.
In our study dental indices show that NFS increases in males. Our results are consistent with Machtei et al.[15] who found no relation between gender and periodontal disease.
Body mass index
Overweight and obesity, two of the most common nutritional disorders worldwide, with increasing prevalence over the past decades, are significant risk factors for numerous adult diseases (i.e., type 2 diabetes) and perhaps also periodontitis.[45]
Our results were consistent with Pischon et al.[46] Obese subjects had up to five times greater risk of hypertension[47] and of developing type-2 diabetes.[43] Our data showed a BMI correlation with PSR. Nutritional disorders probably represented a true risk factor for periodontal diseases, which could depend on many variables such as age,[48] fat distribution,[49] smoking status, diabetes, and the like,[33,50] to be clinically expressed. The correlation findings on dental variables were not persistent on the multivariate analysis in our study. This unexpected result did not contradict other studies, as the most evident correlation between the nutrition indices and periodontal disease was found in specific situations, for example, the absence of smoking, insulin resistance (IR), bad oral hygiene, upper body obesity, exclusively in youth, extreme obesity cases, and first stages of overweight or obesity onset.[48,50,51]
Besides, the decayed-filled score was found to be in direct correlation with the BMI in teens; and children having a correct nutrition showed a lower NDS.[52] Forslund et al.[53] reported an association of BMI with a decreased number of teeth in middle-aged women. Recent exhaustive studies on dental caries in the adult population and BMI are not available,[45,46] but our data for aged patients show no correlation between BMI and NMT, and BMI and NDS. Thus, it is possible to consider BMI as a periodontal complex risk factor, representing an objective facilitation for periodontitis. Recent studies indicate that adipose tissue is an important organ that secretes several bioactive substances known as adipocytokines,[11,51] which appear to be related to inflammation, diabetes, hypertension, CP-I, and periodontal disease.
Number of cigarettes per day
The proportion of current smokers in our study was 55%, 60% in women and 50% in men, without a statistical significance in gender and SES. Nevertheless, a significant decreasing trend in the number of smokers was found from the first SES group to the third. Cigarette smoking has been often, but not always, associated with both the prevalence and severity of periodontitis, bone loss, and NMT.[15,48,50,54,55,56]
In our study, the results show a significant relationship between NCD and PSR, NCD and NMT, and NCD and NDS. This evidence is consistent with those of some authors,[34,35,57,58] but some important studies limit this correlation to only periodontal indices[59] and adults.[60] The correlation findings on dental variables do not completely persist at the multivariate analysis. Only the positive relationship of NCD and PSR has been confirmed: One unit of PSR increase is produced by the additional consumption of a whole packet of cigarettes per day. These results point to the risk of tooth loss by the smoking status, even if no statistical difference is found between smokers and non-smokers for NMT, NDS, NFS, and tooth mobility, at the baseline.[59] Moreover, the association between smoking and periodontal pocket controlling is particularly significant in the elderly.[60] Thus, we consider that smoking can be an effective risk factor for periodontitis, in ways that not are completely well known.[61] Other factors, such as Gly, educational level, dental care, NMT, and NDS, may play a role in enhancing periodontitis and periodontal damage in smokers.[34,35]
Fast glycemic value
Impaired glucose tolerance (IGT) is defined as excessive levels of blood glucose developed after a carbohydrate-rich meal or glucose test, and it is not necessarily diagnostic of diabetes mellitus. The impaired fast glucose IFG is an intermediate status between NGT and IGT.[62]
Our study shows that Gly has a positive correlation with SBP, DBP, and BMI. Type-2 diabetes may be preceded by the metabolic syndrome (MeS ― consisting of IR, obesity, dyslipidemia, high blood pressure, and a pro-inflammatory and pro-thrombotic status in adulthood[63]). Augmented BMI has been associated with an increase in the number and size of adipocytes, which have a high metabolic activity that produces large quantities of inflammatory mediators that could also increase inflammation and IR, raising plasma glucose levels.[19]
Irrespective of the diabetes type, periodontal disease[19,64] and NMT[19,34] are significantly greater in diabetics. However, the considerable heterogeneity of the literature on study populations, sample sizes, onset and duration of diabetes, level of glycemic control, and diagnosis of periodontal disease does not allow any easy discrimination.[27,64,65,66,67] Chronic hyperglycemia is one of the key features of diabetes mellitus, but its effect on periodontal disease is much less known than the effects of diabetes,[19,68] particularly on the influence of IGT or IGF on periodontitis. Periodontal diseases can also play a role in cytokine mediation as an inflammatory condition.[68] D’Aiuto et al.[69] have found that an intensive periodontal treatment reduces some systemic inflammatory markers and SBP, and improves the lipid profiles, with changes in cardiovascular risk, in comparison with standard periodontal therapy. A chronic activation of the acute-phase response, characteristic of severe periodontitis, is believed to decrease the action of insulin, with a consequent increase in the circulating glucose levels.[70] A control of the inflammatory parameters can improve other pathological conditions influenced by the same factors.
In our study, Gly showed a positive correlation with NDS and PSR. On the multivariate analysis, the Spearman correlations on dental variables were not persistent for NDS. Gly influenced PSR in a bad but not extreme manner. In our sample, Gly and high BMI could be related to lifestyle habits, and metabolic and inflammatory parameters. It could be conjectured that Gly affects the periodontal status and tooth preservation in a subtle way. As this relation is not circumstantiated in the literature at all, it is not elucidated to patients.
Systolic and diastolic blood pressure
High blood pressure[71] was prevalent (52.5%) in our studied population. No correlation was found between blood pressure and the SES of our patients. Steptoe et al.[72] studied socioeconomic disparities in participants, anamnestically, in health. The SES of participants resulted as inversely related to SBP, financial strain, and the hostility score, whereas, it resulted in being directly related to the lifestyle. However, the SES considered using the British civil service as a reference,[72] and based on the grade of employment, was strongly associated with other SES markers (educational attainment, personal and household income).
Machtei et al.,[15] showed that in patients with little or no periodontitis, patients with high blood pressure lost twice as many teeth as did normotensives, but without statistical significance. Sometimes, the greater NMT might also be explained by the dentist, considering the likelihood of treating subjects with complex systemic conditions, preferring extraction over an elaborate treatment plan that might be needed to preserve these teeth.[73] However, Taguchi et al.[74] in a study on non-smoking, healthy women, found a significant association between the incidence of hypertension and NMT.
Our study shows an increase of NMT with SBP, of NDS with DBP, and of PSR with both SBP and DBP. On the multivariate analysis, when controlling for behavioral, biological, and socioeconomic factors, SBP has a negative coefficient for NDS, whereas, DBP has a positive coefficient for PSR. This result highlights both SBP and DBP as having a significant, but moderate influence on the dental parameters.
The relationship between the periodontal condition and blood pressure is debated.[55,56,75] However, our data highlight a complex relationship between blood pressure and the dental parameters, considering that decays may also influence the NMT values. It may be that infections of the periodontal structure could accelerate atherosclerosis by promoting a systemic inflammatory status through the release of endotoxins, shock proteins, or acute-phase reactants, involving mechanism that may implicate the pressure overload.[9,75]
Cardiopathy/ischemia score
Some periodontitis and cardiovascular disease (CVD) components have a multifactorial etiology, are associated with infectious agents, and have a characteristic inflammatory component.[15,69,74,75] In some disease states, the systemic challenge of the bacterial toxin derived from periodontal lesions represents an important link between periodontitis, monocytic inflammatory response, and metabolic dysregulation.[76,77] The cytokine pathogenetic role will be particularly remarkable following an infectious challenge or trauma.[78]
Our study shows that CP-I increased with SBP and DBP, but did not show a correlation with the dental parameters, and therefore, it was not included in the explanatory variables of the seemingly unrelated regressions.
A positive, but complex correlation between NMT, blood-inflammation markers, and other risk factors for CVD was found.[69,74,76] Moreover, tooth eradication might reduce the systemic inflammatory burden of individuals with severe periodontitis.[77] The relationship between SES and periodontitis, and the impact of these factors on CHD, atherosclerosis or stroke were not specifically studied.[77] Actually, tooth extractions could improbably resolve the inflammatory condition inherent to periodontitis. However, oral diseases different from periodontitis, such as, periapical granuloma or diffuse dental and root caries could produce systemic inflammation. The definition of periodontal and heart diseases differ in studies and constitute a major problem.
Socioeconomic status
Several studies documented differences in dental health by socioeconomic indicators,[9,44] economic indices,[79] income and education,[15,32,34,80] economic indices and education,[8,33,35,55,81] and education,[38] and social classes and conditions.[17,21,40,51,80,82] However, considering the SES indicators, some authors found that the relationship between SES and dental indices was not persistent, in an in-depth statistical analysis.[14,33,51,80] In particular, our SES rating was founded on the ISEE index, and it was aimed at defining disadvantaged socioeconomic conditions of families and at identifying the unified criteria in evaluating people asking welfare services, reserved only for subjects having the requisites defined by law.
In our study, an increase in SES condition corresponded to an increase in PSR and a decrease in NDS, NFS, and mostly, NMT. Thus, it could be conjectured that a lower SES class showed many more teeth extracted, decayed, and filled, than did a higher class. We assumed that tooth loss represented a sort of therapeutic alternative to the conservative in our sample.
SES, in our country, enables economic assistance in inverse relation to the SES class, and the lower class is entitled to free dental care. It is likely that a primary barrier to the correct therapy being performed in low-income patients is their belief that tooth extraction is the solution, or an attitude of waiting for a problem to occur before seeking dental care, or the attachment of scarce importance to regular dental visits and preventive treatment. It is also possible that not everybody has become acquainted with the SES regulations, while the patients’ preoccupation with other daily issues or the consequence of medical choices seems less believable. Moreover, educational attainment, another factor of SES, may be a more reliable marker of oral health than the economics in some studies.[32] However, SES classification seems to merge fairly different classes of therapeutic requirements with the SES of our sample. More care must be taken to improve alternative dental treatment to tooth extraction in the lower class. It must be noted that periodontal conditions remain substantially unvaried or are even made worse with increasing SES.
Our study highlights an increase of dental, but not of periodontal care, moving from the lower to the higher SES group. The SES index does not fully meet the social needs of dental care health planning. It identifies different socioeconomic groups, but it does not allow the public health administration to implement therapies that will be able to standardize the needs of different SES groups.
Footnotes
Source of Support: The study was funded by the authors’ own institutions.
Conflict of Interest: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Albandar J. Periodontal diseases in North America. Periodontol 2000. 2002;29:31–69. doi: 10.1034/j.1600-0757.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- 2.Borrell L, Burt B, Neighbors H, Taylor G. Social factors and periodontitis in an older population. Am J Publ Health. 2004;94:748–54. doi: 10.2105/ajph.94.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mack F, Mojon P, Budtz-Jørgensen E, Kocher T, Splieth C, Schwahn C, et al. Caries and periodontal disease of the elderly in pomerania, germany: Results of the study of health in pomerania. Gerodontology. 2004;21:27–36. doi: 10.1046/j.1741-2358.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Baelum V, Pisuithanakan S, Teanpaisan R, Pithpornchaiyakul W, Pongpaisal S, Papapanou PN, et al. Periodontal conditions among adults in southern Thailand. J Periodontal Res. 2003;38:156–63. doi: 10.1034/j.1600-0765.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 5.Ajwani S, Tervonen T, Närhi T, Ainamo A. Periodontal health status and treatment needs among the elderly. Spec Care Dent. 2001;21:98–103. doi: 10.1111/j.1754-4505.2001.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 6.Lo E, Luo Y, Dyson J. Oral health status of institutionalised elderly in Hong Kong. Community Dent Health. 2004;21:224–6. [PubMed] [Google Scholar]
- 7.Morris A, Steele J, White D. The oral cleanliness and periodontal health of UK adults in 1998. Br Dent J. 2001;191:186–92. doi: 10.1038/sj.bdj.4801135. [DOI] [PubMed] [Google Scholar]
- 8.Susin C, Dalla Vecchia CF, Oppermann R, Haugejorden O, Albandar J. Periodontal attachment loss in an urban population of Brazilian adults: Effect of demographic, behavioral, and environmental risk indicators. J Periodontol. 2004;75:1033–41. doi: 10.1902/jop.2004.75.7.1033. [DOI] [PubMed] [Google Scholar]
- 9.Borges-Yáñez S, Irigoyen-Camacho M, Maupome G. Risk factors and prevalence of periodontitis in community-dwelling elders in mexico. J Clin Periodontol. 2006;33:184–94. doi: 10.1111/j.1600-051X.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor GW, Manz MC, Borgnakke WS. Diabetes, periodontal diseases, dental caries, and tooth loss: A review of the literature. Compend Contin Educ Dent. 2004;25:179–84. [PubMed] [Google Scholar]
- 11.Genco R, Grossi S, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–84. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 12.Kunst A, Bos V, Lahelma E, Bartley M, Lissau I, Regidor E, et al. Trends in socioeconomic inequalities in self-assessed health in 10 European countries. Int J Epidemiol. 2005;34:295–305. doi: 10.1093/ije/dyh342. [DOI] [PubMed] [Google Scholar]
- 13.Mackenbach J, Stirbu I, Roskam A, Schaap M, Menvielle G, Leinsalu M, et al. European union working group on socioeconomic inequalities in health socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–81. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 14.Sabbah W, Tsakos G, Chandola T, Newton T, Kawachi I, Sheiham A, et al. The relationship between social network, social support and periodontal disease among older Americans. J Clin Periodontol. 2011;38:547–52. doi: 10.1111/j.1600-051X.2011.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machtei E, Hausmann E, Dunford R, Grossi S, Ho A, Davis G, et al. Longitudinal study of predictive factors for periodontal disease and tooth loss. J Clin Periodontol. 1999;26:374–80. doi: 10.1034/j.1600-051x.1999.260607.x. [DOI] [PubMed] [Google Scholar]
- 16.Albandar J. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 17.Nuttall N, Nugent Z. Indicators of dental extractions and full mouth clearances: A longitudinal analysis. Community Dent Oral Epidemiol. 1997;25:181–3. doi: 10.1111/j.1600-0528.1997.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 18.Saotome Y, Tada A, Hanada N, Yoshihara A, Uematsu H, Miyazaki H, et al. Relationship of cariogenic bacteria levels with periodontal status and root surface caries in elderly Japanese. Gerodontology. 2006;23:219–25. doi: 10.1111/j.1741-2358.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 19.Salvi G, Carollo-Bittel B, Lang N. Effects of diabetes mellitus on periodontal and peri-implant conditions update on associations and risks. J Clin Periodontol. 2008;35:398–409. doi: 10.1111/j.1600-051X.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert G, Miller M, Duncan R, Ringelberg M, Dolan T, Foerster U. Tooth-specific and person-level predictors of 24-month tooth loss among older adults. Community Dent Oral Epidemiol. 1999;27:372–85. doi: 10.1111/j.1600-0528.1999.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins R, Main P, Locker D. The normative need for tooth extractions in older adults in Ontario, Canada. Gerodontology. 1997;14:75–5. doi: 10.1111/j.1741-2358.1997.00075.x. [DOI] [PubMed] [Google Scholar]
- 22.Khocht A, Zohn H, Deasy M, Chang K. Assessment of periodontal status with PSR and traditional clinical periodontal examination. J Am Dent Assoc. 1995;126:1658–65. doi: 10.14219/jada.archive.1995.0115. [DOI] [PubMed] [Google Scholar]
- 23.Wolf HF, Rateitschak-Plüss EM, Rateitschak KH. Indici parodontali-PSR. In: Wolf HF, Rateitschak-Plüss EM, Rateitschak KH, editors. Parodontologia. Milano: Masson Elsevier; 2005. p. 73. [Google Scholar]
- 24.Ghiabi E, Weerasinghe S. The periodontal examination profile of general dentists in Nova Scotia, Canada. J Periodontol. 2011;75:33–40. doi: 10.1902/jop.2010.100348. [DOI] [PubMed] [Google Scholar]
- 25.Bratthall D. Introducing the significant caries index together with a proposal for a new global oral health goal for 12-year-olds. Int Dent J. 2000;50:378–84. doi: 10.1111/j.1875-595x.2000.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 26.Solinas G, Campus G, Maida C, Sotgiu G, Cagetti MG, Lesaffre E, et al. What statistical method should be used to evaluate risk factors associated with DMFS index? Evidence from the national pathfinder survey of 4-year-old Italian children. Community Dent Oral Epidemiol. 2009;37:539–46. doi: 10.1111/j.1600-0528.2009.00500.x. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28:S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 28.DL-130. Disposizioni correttive ed integrative del decreto legislativo 31 marzo 1998, n 109, in materia di criteri unificati di valutazione della situazione economica dei soggetti che richiedono prestazioni sociali agevolate.: GU (Italian Official Gazette), Serie generale, n 118 del 23/5/2000. 2000:4–12. [Google Scholar]
- 29.Tangorra P, Sestito R. Rapporto ISEE. Roma: Ministero del lavoro e delle politiche sociali; 2006. Implementazione, popolazione e selettività dell’indicatore della situazione economica. Available from: http://www.lavorogovit/NR/rdonlyres/EC8735DE-6C60-48B9-976B-DA916221613E/0/rapportoisee2006.pdf, 2006 . [Google Scholar]
- 30.StataCorp . 1-4. College Station, TX: StataCorp LP; 2005. Stata statistical software, Release 9. [Google Scholar]
- 31.Chicago IL: SPSS Inc; 2006. SPSS. Spss base 150 user's guide. [Google Scholar]
- 32.Norderyd O, Hugoson A, Grusovin G. Risk of severe periodontal disease in a Swedish adult population a longitudinal study. J Clin Periodontol. 1999;26:608–15. doi: 10.1034/j.1600-051x.1999.260908.x. [DOI] [PubMed] [Google Scholar]
- 33.Dalla Vecchia CF, Susin C, Kuchenbecker RC, Oppermann R, Albandar J. Overweight and obesity as risk indicators for periodontitis in adults. J Periodontol. 2005;76:1721–8. doi: 10.1902/jop.2005.76.10.1721. [DOI] [PubMed] [Google Scholar]
- 34.Dolan T, Gilbert G, Ringelberg M, Legler D, Antonson D, Foerster U, et al. Behavioral risk indicators of attachment loss in adult Floridians. J Clin Periodontol. 1997;24:223–32. doi: 10.1111/j.1600-051x.1997.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 35.Susin C, Haas A, Opermann R, Albandar J. Tooth loss in a young population from south brazil. J Public Health Dent. 2006;66:110–5. doi: 10.1111/j.1752-7325.2006.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 36.Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. 2004;31:749–57. doi: 10.1111/j.1600-051X.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 37.Neely A, Holford T, Löe H, Anerud A, Boysen H. The natural history of periodontal disease in humans: Risk factors for tooth loss in caries-free subjects receiving no oral health care. J Clin Periodontol. 2005;32:984–93. doi: 10.1111/j.1600-051X.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 38.Akhter R, Hassan N, Aida J, Zaman K, Morita M. Risk indicators for tooth loss due to caries and periodontal disease in recipients of free dental treatment in an adult population in Bangladesh. Oral Health Prev Dent. 2008;6:199–207. [PubMed] [Google Scholar]
- 39.Hanson W, Persson G. Periodontal conditions and service utilization behaviors in a low income adult population. Oral Health Prev Dent. 2003;1:99–109. [PubMed] [Google Scholar]
- 40.Thorstensson H, Johansson B. Why do some people lose teeth across their lifespan whereas others retain a functional dentition into very old age? Gerodontology. 2010;27:19–25. doi: 10.1111/j.1741-2358.2009.00297.x. [DOI] [PubMed] [Google Scholar]
- 41.Dhingra K, Vandana KL. Indices for measuring periodontitis: A literature review. Int Dent J. 2011;61:76–84. doi: 10.1111/j.1875-595X.2011.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence W, Smith S, Baker T, Fiore M. Does over-the-counter nicotine replacement therapy improve smokers’ life expectancy? Tob Control. 1998;7:364–8. doi: 10.1136/tc.7.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Field A, Coakley E, Must A, Spadano J, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–6. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 44.Borrell L, Papapanou P. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32(Suppl 6):132–58. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 45.Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol 2000. 2007;43:254–66. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 46.Pischon N, Heng N, Bernimoulin J, Kleber B, Willich S, Pichon T. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86:400–9. doi: 10.1177/154405910708600503. [DOI] [PubMed] [Google Scholar]
- 47.Wolf H, Tuomilehto J, Kuulasmaa K, Domarkiene S, Cepaitis Z, Molarius A, et al. pressure levels in the 41 populations of the WHO MONICA project. J Hum Hypertension. 1997;11:733–42. doi: 10.1038/sj.jhh.1000531. [DOI] [PubMed] [Google Scholar]
- 48.Al-Zahrani M, Bissada N, Borawskit E. Obesity and periodontal disease in young, middle-aged, and older adults. J Periodontol. 2003;74:610–5. doi: 10.1902/jop.2003.74.5.610. [DOI] [PubMed] [Google Scholar]
- 49.Wood N, Johnson R, Streckfus C. Comparison of body composition and periodontal disease using nutritional assessment techniques: Third national health and nutrition examination survey (NHANES III) J Clin Periodontol. 2003;30:321–7. doi: 10.1034/j.1600-051x.2003.00353.x. [DOI] [PubMed] [Google Scholar]
- 50.Nishida N, Tanaka M, Hayashi N, Nagata H, Takeshita T, Nakayama K, et al. Determination of smoking and obesity as periodontitis risks using the classification and regression tree method. J Periodontol. 2005;76:923–8. doi: 10.1902/jop.2005.76.6.923. [DOI] [PubMed] [Google Scholar]
- 51.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Lida M, et al. Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: The Hisayama study. J Periodontal Res. 2005;40:346–53. doi: 10.1111/j.1600-0765.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 52.Willershausen B, Haas G, Krummenauer F, Hohenfellner K. Relationship between high weight and caries frequency in German elementary school children. Eur J Med Res. 2004;9:400–4. [PubMed] [Google Scholar]
- 53.Forslund H, Lindroos A, Blomkvist K, Hakeberg M, Berggren U, Jontell M, et al. Number of teeth, body mass index, and dental anxiety in middle-aged Swedish women. Acta Odontol Scand. 2002;60:346–52. doi: 10.1080/000163502762667379. [DOI] [PubMed] [Google Scholar]
- 54.Albandar J, Streckfus C, Adesanya M, Winn D. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71:1874–81. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- 55.Tsai C, Hayes C, Taylor G. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:175–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa H, Yoshihara A, Hirotomi T, Ando Y, Miyazaki H. Risk factors for periodontal disease progression among elderly people. J Clin Periodontol. 2002;29:592–7. doi: 10.1034/j.1600-051x.2002.290702.x. [DOI] [PubMed] [Google Scholar]
- 57.Susin C, Valle P, Oppermann R, Haugejorden O, Albandar JM. Occurrence and risk indicators of increased probing depth in an adult Brazilian population. J Clin Periodontol. 2005;32:123–9. doi: 10.1111/j.1600-051X.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 58.Ojima M, Hanioka T, Tanaka K, Aoyama H. Cigarette smoking and tooth loss experience among young adults: A national record linkage study. BMC Public Health. 2007;7:313. doi: 10.1186/1471-2458-7-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krall E, Garvey A, Garcia R. Alveolar bone loss and tooth loss in male cigar and pipe smokers. J Am Dent Assoc. 1999;130:57–64. doi: 10.14219/jada.archive.1999.0029. [DOI] [PubMed] [Google Scholar]
- 60.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27:61–8. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 61.Johnson G, Hill M. Cigarette smoking and the periodontal patient. J Periodontol. 2004;75:196–209. doi: 10.1902/jop.2004.75.2.196. [DOI] [PubMed] [Google Scholar]
- 62.Marugame T, Hayasaki H, Lee K, Eguchi H, Matsumoto S. Alveolar bone loss associated with glucose tolerance in Japanese men. Diabet Med. 2003;20:746–51. doi: 10.1046/j.1464-5491.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 63.Nibali L, D’Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inlammation and a dysmetabolic status: A case-control study. J Clin Periodontol. 2007;34:931–7. doi: 10.1111/j.1600-051X.2007.01133.x. [DOI] [PubMed] [Google Scholar]
- 64.Khader Y, Dauod A, El-Qaderi SS, Alkafajei A, Batayha W. Periodontal status of diabetics compared with nondiabetics: A meta-analysis. J Diabetes Compl. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Page R, Eke P. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(Suppl 7):1387–99. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 66.Bertoldi C, Bencivenni D, Lucchi A, Consolo U. Augmentation of keratinized gingiva through bilaminar connective tissue grafts: A comparison between two techniques. Minerva Stomatol. 2007;56:3–20. [PubMed] [Google Scholar]
- 67.Bertoldi C, Pellacani C, Lalla M, Consolo U, Pinti M, Cortellini P, et al. Herpes simplex I virus impairs regenerative outcomes of periodontal regenerative therapy in intrabony defects: A pilot study. J Clin Periodontol. 2012;39:385–92. doi: 10.1111/j.1600-051X.2012.01850.x. [DOI] [PubMed] [Google Scholar]
- 68.Nishimura F, Murayama Y. Periodontal inflammation and insulin resistance-lessons from obesity. J Dent Res. 2001;80:1690–4. doi: 10.1177/00220345010800080201. [DOI] [PubMed] [Google Scholar]
- 69.D’Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: Results from a randomized controlled clinical trial. Am Heart J. 2006;151:977–84. doi: 10.1016/j.ahj.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 70.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Lida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: The Hisayama study. J Dent Res. 2004;83:485–90. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 71.WHO. World Health Organization-International Society of hypertension guidelines for the management of hypertension guidelines subcommittee. J Hypertens. 1999;17:151–83. [PubMed] [Google Scholar]
- 72.Steptoe A, Hamer M, O’Donnell K, Venuraju S, Marmot M, Lahiri A. Socioeconomic status and subclinical coronary disease in the Whitehall II epidemiological study. PLoS One. 2010;5:e8874. doi: 10.1371/journal.pone.0008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genco R. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:S1041–9. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 74.Taguchi A, Sanada M, Suei Y, Ohtsuka M, Lee K, Tanimoto K, et al. Tooth loss is associated with an increased risk of hypertension in postmenopausal women. Hypertension. 2004;43:1297–300. doi: 10.1161/01.HYP.0000128335.45571.ce. [DOI] [PubMed] [Google Scholar]
- 75.Angeli F, Verdecchia P, Pellegrino C, Pellegrino R, Pellegrino G, Prosciutti L, et al. Association between periodontal disease and left ventricle mass in essential hypertension. Hypertension. 2003;41:488–92. doi: 10.1161/01.HYP.0000056525.17476.D7. [DOI] [PubMed] [Google Scholar]
- 76.Mustapha IZ, Debrey S, Oladubu M, Ugarte R. Markers of systemic bacterial exposure in periodontal disease and cardiovascular disease risk: A systematic review and meta-analysis. J Periodontol. 2007;78:2289–302. doi: 10.1902/jop.2007.070140. [DOI] [PubMed] [Google Scholar]
- 77.Persson GR, Persson RE. Cardiovascular disease and periodontitis: An update on the associations and risk. J Clin Periodontol. 2008;35(Suppl 8):362–79. doi: 10.1111/j.1600-051X.2008.01281.x. [DOI] [PubMed] [Google Scholar]
- 78.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–34. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 79.Parker E, Jamieson L. Oral health comparisons between children attending an Aboriginal health service and a Government school dental service in a regional location. Rural Remote Health. 2007;7:625. [PubMed] [Google Scholar]
- 80.Silva-Boghossian C, Luiz R, Colombo A. Periodontal status, sociodemographic, and behavioral indicators in subjects attending a public dental school in Brazil: Analysis of clinical attachment loss. J Periodontol. 2009;80:1945–54. doi: 10.1902/jop.2009.090242. [DOI] [PubMed] [Google Scholar]
- 81.Villalobos-Rodelo J, Medina-Solís C, Maupomé G, Vallejos-Sánchez A, Lau-Rojo L, de León-Viedas MV. Socioeconomic and sociodemographic variables associated with oral hygiene status in Mexican schoolchildren aged 6 to 12 years. J Periodontol. 2007;78:816–52. doi: 10.1902/jop.2007.060324. [DOI] [PubMed] [Google Scholar]
- 82.Bernabé E, Suominen AL, Nordblad A, Vehkalahti MM, Hausen H, Knuuttila M, et al. Education level and oral health in Finnish adults: Evidence from different lifecourse models. J Clin Periodontol. 2011;38:25–32. doi: 10.1111/j.1600-051X.2010.01647.x. [DOI] [PubMed] [Google Scholar]


