Abstract
Neuroimaging studies investigating the neural profile of anorexia nervosa (AN) have revealed a predominant imbalance between the reward and inhibition systems of the brain, which are also hallmark characteristics of the disorder. However, little is known whether these changes can also be determined independent of task condition, using resting-state functional magnetic resonance imaging, in currently ill AN patients.
Therefore the aim of our study was to investigate resting-state connectivity in AN patients (n = 12) compared to healthy athlete (n = 12) and non-athlete (n = 14) controls. For this purpose, we used degree centrality to investigate functional connectivity of the whole-brain network and then Granger causality to analyze effective connectivity (EC), to understand directional aspects of potential alterations.
We were able to show that the bilateral inferior frontal gyrus (IFG) is a region of special functional importance within the whole-brain network, in AN patients, revealing reduced functional connectivity compared to both healthy control groups. Furthermore, we found decreased EC from the right IFG to the midcingulum and increased EC from the bilateral orbitofrontal gyrus to the right IFG. For the left IFG, we only observed increased EC from the bilateral insula to the left IFG.
These results suggest that AN patients have reduced connectivity within the cognitive control system of the brain and increased connectivity within regions important for salience processing. Due to its fundamental role in inhibitory behavior, including motor response, altered integrity of the inferior frontal cortex could contribute to hyperactivity in AN.
Keywords: Anorexia nervosa, Functional connectivity, Effective connectivity, Resting-state fMRI
Highlights
-
•
We evaluate resting-state functional (FC) and effective (EC) connectivity.
-
•
We compare anorexia nervosa (AN) patients with healthy controls.
-
•
AN patients show reduced FC in the inferior frontal gyrus (IFG).
-
•
AN patients show reduced EC from the IFG and increased EC to the IFG.
-
•
Altered FC patterns correlate with physical activity.
1. Introduction
Anorexia nervosa (AN) is an eating disorder that tends to begin during adolescence in women and is characterized by relentless pursuit to lose weight, mostly by self-starvation, and distorted body image (APA, 2000), as well as high mortality rates (Zipfel et al., 2000). Nonetheless, a large number of AN patients benefit from treatments offered in specialized eating disorder centers (Zipfel et al., 2013b). Besides food restriction, physical hyperactivity is another frequent puzzling symptom in AN patients that is poorly understood, but plays a central role in the pathogenesis and progression of the disorder (Hebebrand and Bulik, 2011). AN patients with hyperactivity show poorer recovery rates, higher rates of relapse, longer periods of hospitalization (Carter et al., 2004; Casper and Jasbine, 1996; Strober et al., 1997) and increased energy requirements (Zipfel et al., 2013a). Thus, it has been recommended to include hyperactivity as part of the core psychopathology of AN (Hebebrand and Bulik, 2011).
Until today, the etiology of AN is still largely unknown and mechanisms that maintain the disorder remain poorly understood (Kaye et al., 2013). Hence advances in neuroimaging techniques have become increasingly important for understanding the pathophysiology of AN. These studies revealed a predominant imbalance between the reward and inhibition systems of the brain, which are hallmark characteristics of the disorder. Recovered AN patients show increased dopamine receptor availability (Frank et al., 2005) and also functional magnetic resonance imaging (fMRI) studies point to dopamine dysfunction by discovering hypoactivity of striatal regions in response to pleasurable stimuli (Kaye et al., 2009). This resulted in the notion that AN patients suffer from general anhedonia unable to experience pleasure. However this view was challenged by Fladung et al. (2010) showing increased activity in the reward system in response to visual stimuli depicting underweight women. In response to food stimuli, previous studies have also frequently reported heightened salience processing in AN resulting in an increased response in the insula and orbitofrontal cortex (OFC) (Frank et al., 2012; Uher et al., 2004) and dorsolateral prefrontal cortex (Brooks et al., 2011; Brooks et al., 2012a).
When challenging cognitive control, reduced prefrontal cortex (PFC) activity has mostly been revealed in AN patients compared to healthy controls (Lock et al., 2011; Oberndorfer et al., 2011). But again when using stimuli with inherent rewarding properties for AN patients, as physical activity stimuli, we were able to show enhanced attentional engagement towards these stimuli (Giel et al., 2013)and increased PFC activity when challenging inhibitory control (Kullmann et al., 2013a; Zastrow et al., 2009).
These studies indicate that mesolimbic reward activations in conjunction with PFC activations are highly dependent on the task and stimuli. A unique advantage provided by resting-state fMRI is that it allows examining task-independent activations. The importance of studying intrinsic brain networks has been illustrated by altered functional connectivity in several different medical conditions such as schizophrenia (Meda et al., 2009), depression (Greicius et al., 2007) and Alzheimer's disease (Greicius et al., 2004). Recently, it has also been discovered that obesity is related to prominent alterations in resting-state and task-based functional networks mainly in prefrontal regions (Garcia-Garcia et al., 2012; Kullmann et al., 2012; Kullmann et al., 2013b). Furthermore, latest studies have evaluated resting-state functional connectivity in AN patients and those recovered from the disease. Favaro et al. (2012) evaluated exclusively the organization of visuospatial and somatosensory brain areas, revealing hypoconnectivity within these networks. In recovered AN patients, on the other hand, increased resting-state functional connectivity was identified in the default mode network, important for self-referential processing and cognitive control (Cowdrey et al., 2012). Interestingly, McFadden et al. (2013) observed reduced resting-state functional connectivity in the default mode network of currently ill AN patients suggesting state-dependent abnormalities. However, they observed reduced functional connectivity in both AN patients and recovered women within the anterior cingulate cortex of the salience network (McFadden et al., 2013). These studies used independent component analyses to investigate functional connectivity in AN, by identifying separable sets of brain regions or networks (Cole et al., 2010). Graph theory based network measures, on the other hand, characterize functional connectivity within the whole-brain network, taking into account a given region's relationship to the whole brain (Bullmore and Sporns, 2009). Degree centrality (DC) is a graph theory based network analysis to assess the centrality or functional importance, such that the complexity of the whole-brain network can be captured as a whole (Zuo et al., 2012). Since the neural mechanisms underlying the disorder in AN are poorly understood and multiple brain systems are affected, we used DC to analyze functional connectivity within the whole-brain network. However, to investigate the directional aspect of possible alterations, we used the Granger causality analysis (GCA), which is a statistical method originally used in the field of economics to assess directional influences between simultaneously recorded time series (Granger, 1969; Zhou et al., 2009). GCA has meanwhile been widely applied to reveal causal effects amongst brain regions by using time-prediction between BOLD fMRI series (Ding et al., 2006; Jiao et al., 2011; Qi et al., 2013; Stephan and Roebroeck, 2012; Uddin et al., 2009; Zhou et al., 2011).
To further delineate the neurobiological profile of AN, we sought to identify in this study brain regions that show altered functional connectivity within the whole-brain network in currently ill AN patients using degree centrality (DC) and then use GCA to analyze effective connectivity to understand the directional aspect of these alterations.
We evaluated resting-state connectivity in AN patients compared to two healthy control groups, displaying different levels of physical activity: healthy non-athletes (HC) and healthy athletes (HCA). Based on the extensive exercise in both AN and HCA groups, we predicted a similar connectivity pattern in sensorimotor brain regions between AN and athletes, while we hypothesized that the connectivity pattern of the prefrontal and striatal regions should be quite distinctive between athletes and AN patients.
2. Materials and methods
2.1. Participants
Twelve female individuals with AN (mean BMI 15.5 ± 1.5 kg/m2; mean age 23.3 ± 4.7 years) and twenty-six age-matched healthy female participants of normal weight were recruited for this study. Participants' characteristics have been described in detail in a recent publication (Kullmann et al., 2013a) (Table 1). AN patients were recruited in the Department of Psychosomatic Medicine and Psychotherapy at the University Hospital of Tübingen. We used the Eating Disorder Examination (EDE) to diagnose eating disorder and the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I (Fairburn and Cooper, 1993; Wittchen et al., 1997) to diagnose comorbid Axis I disorders in patients. Patients were excluded from the study for the following reasons: body mass index (BMI) < 12 kg/m2, intake of neuroleptics or benzodiazepines, a primary obsessive–compulsive or affective disorder, psychosis, bipolar disorder and substance abuse or addiction according to DSM-IV.
Table 1.
Participants' characteristics.
| Female anorexia nervosa patients (AN) (n = 12) |
Female non-athletes (HC) (n = 14) |
Female athletes (HCA) (n = 12) |
Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | M | SD | M | SD | M | SD | F | df | p | Post-hoc difference |
| Age (years) | 23.3 | 4.7 | 24.6 | 2.9 | 24.1 | 3.2 | .379 | 35 | .687 | – |
| Current BMI (kg/m2) | 15.5 | 1.5 | 21.4 | 1.5 | 22.0 | 1.9 | 57.87 | 35 | <.001 | AN < HCA, HC |
| Leptin (ng/dl) | 0.7 | 0.4 | 5.89 | 3.2 | 4.46 | 3.6 | 10.691 | 34 | <.001 | AN < HCA, HC |
| Hunger rating (cm) | 0.5 | 0.7 | 0.7 | 0.7 | 1.0 | 1.3 | 1.07 | 35 | .352 | – |
| CES | 6.5 | 2.6 | 4.12 | 1.9 | 5.55 | 1.6 | 4.329 | 35 | .021 | AN > HC |
| BAS | 3.1 | 0.4 | 3.18 | 0.4 | 3.24 | 0.2 | .455 | 35 | .638 | – |
| BIS | 3.5 | 0.5 | 2.98 | 0.4 | 2.83 | 0.5 | 7.247 | 35 | .002 | AN > HCA, HC |
| Depression score | 11.3 | 4.5 | 1.8 | 1.5 | 1.8 | 1.9 | 44.512 | 35 | <.001 | AN > HCA, HC |
| State anxiety score | 61.0 | 10.4 | 31.9 | 6.7 | 32.7 | 5.6 | 56.198 | 35 | <.001 | AN > HCA, HC |
| EDI-2 | 309.8 | 54.68 | 186.57 | 36.92 | 194.08 | 54.68 | 32.127 | 35 | <.001 | AN > HCA, HC |
| EDEQ | 3.43 | 1.46 | ||||||||
| Vigorous activity (h/week) | 10.51 | 13.21 | 6.87 | 2.21 | 3.08 | 3.1 | 2.98 | 35 | 0.06 | AN > HC |
Data are presented as mean ± SD. = p-Values for comparison of unadjusted data by ANOVA. AN: Anorexia nervosa patient; HC: healthy non-athlete control group; HCA: healthy athlete control group; BIS: behavioral inhibition system; BAS: behavioral activation system; CES: Commitment to Exercise Scale; EDI-2: Eating Disorder Inventory; EDEQ: Eating Disorder Examination Questionnaire.
Twenty-six age-matched healthy female participants of normal weight were recruited through local advertisement for two healthy control groups. One control group consisted of healthy athletes (HCA, 12 participants; mean BMI 22 ± 1.9 kg/m2; mean age 24.1 ± 3.2 years), required to perform competitively exercise in an endurance sport of at least 5 h a week for at least 1 year. The other control group consisted of healthy non-athletes, only included when performing casual physical exercise (HC, 14 participants; mean BMI 21.4 ± 1.5 kg/m2; mean age 24.6 ± 2.9 years). As assessed by the SCID-I, the healthy female participants had no history of an eating disorder or any other psychiatric, serious medical or neurological diseases and were not on any psychoactive medication. The local medical faculty's ethics committee approved the study. Written informed consent was obtained from all participants after complete description of the study to the participants.
Participants completed several self-report assessments as recently reported (Kullmann et al., 2013a). Of special importance to this study are questionnaires related to eating disorder symptoms (Eating Disorder Inventory-2 [EDI-2]) (Garner, 1991; Paul and Thiel, 2005), reward sensitivity and behavioral inhibition (behavioral activation/inhibition system [BAS/BIS]) (Gray, 1970; Strobel et al., 2001) and excessive exercise (Commitment to Exercise Scale [CES]) (Davis et al., 1993). Participants had a standardized breakfast (staff supervised) 1 h before the fMRI measurement, consisting of a bread roll with butter, jam or honey and herbal tea. In addition hunger was assessed by a 10 cm visual analogue scale ranging from 0 cm [not hungry at all] to 10 cm [strongest feeling of hunger] just before the fMRI measurement. All fMRI measurements were performed between 9 and 11 am.
2.2. Data acquisition
Whole-brain functional magnetic resonance imaging (fMRI) data was obtained by using a 3.0 T scanner (Siemens Tim Trio, Erlangen, Germany). Functional data were collected by using echo-planar imaging sequence: TR = 3 s, TE = 30 ms, FOV = 192 mm2, matrix 64 × 64, flip angle 90°, voxel size 3 × 3 × 3 mm3, slice thickness 3 mm, and the images were acquired in an interleaved order. Each brain volume comprised 47 axial slices and each functional run contained 200 image volumes, resulting in a total scan time of 10.06 min. In addition, high-resolution T1 weighted anatomical images (MPRage: 176 slices, matrix: 256 × 240 × 192, 1 × 1 × 1 mm3) of the brain were obtained. All participants were instructed not to focus their thoughts on anything in particular and to keep their eyes closed during the resting state MR acquisition.
2.3. Data preprocessing
Preprocessing was carried out by using Data Processing Assistant for Resting-State fMRI (DPARSF) (Chao-Gan and Yu-Feng, 2010) (http://www.restfmri.net) which is based on Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm) and Resting-State fMRI Data Analysis Toolkit (Song et al., 2011) (REST, http://www.restfmri.net). Functional images were realigned and co-registered to the T1 structural image. The anatomical image was normalized to the Montreal Neurological Institute (MNI) template using DARTEL, and the resulting parameter file was used to normalize the functional images (voxel size: 3 × 3 × 3 mm3). Finally the normalized images were smoothed with a three-dimensional isotropic Gaussian kernel (FWHM: 6 mm). A temporal filter (0.01–0.08 Hz) was applied to reduce low frequency drifts and high frequency physiological noise. Nuisance regression was performed using white matter, CSF, and the six head motion parameters as covariates. No participant had head motion with more than 2.0 mm maximum displacement or 2.0° of any angular motion.
2.3.1. Degree centrality analysis
In the present study, we used network degree centrality to identify regions of high connectivity by mapping the degree of functional connectivity across the brain (Buckner et al., 2009; Zuo et al., 2012). Degree centrality is defined by the number of edges connecting to a node. For a weighted graph, as used in this study, it is the weighted sum of positive correlations by requiring each connection's statistical significance to exceed a threshold of p < 0.001. Subject-level Z-score maps were created by subtracting the mean degree centrality value for the entire brain from each voxel and by dividing the corresponding standard deviation. Degree centrality has been shown to represent the most local and directly quantifiable centrality measure and has been widely used to examine node characteristics of intrinsic network connectivity (for review see Zuo et al., 2011). The degree centrality maps were transferred to z values for group comparisons.
2.3.1.1. Statistical analysis: degree centrality
The degree centrality maps were entered into SPM8 for group comparison. One-way ANOVA with group as the between subject factor was used to examine differences between AN patients, HCA and HC. All group tests were thresholded at p < 0.05, family-wise error (FWE) corrected for multiple comparisons.
2.3.2. Effective connectivity analysis
Effective connectivity was analyzed by Granger causality as described previously (Qi et al., 2013) using REST-GCA in the REST toolbox (Zang et al., 2012). Based on the results of the degree centrality analysis we selected the seed regions, which showed significant differences between AN patients and the control groups (left and right inferior frontal gyrus; MNI coordinates (x, y, z) ±48, 9, 24). Effective connectivity was calculated between the reference time series of the seed region (right and left inferior frontal gyrus, respectively), defined as x, and the time series of each voxel within the whole brain, defined as y. The direct influence of x on y (Fx?y) and y on x (Fy?x) was calculated voxel by voxel across the whole brain. The residual-based F was normalized (F') and standardized to Z scores for each voxel (Zx?y and Zy?x, subtracting the global mean F' values, divided by the standard deviation) (Zang et al., 2011).
2.3.2.1. Statistical analysis: effective connectivity from and to the inferior frontal gyrus
Granger causality maps were obtained for each seed region for each direction (Zx?y and Zy?x) for both AN patients and healthy controls (HCA and HC). These Granger causality maps were entered into SPM8 for group comparison. Separate one-way ANOVAs with group as the between subject factor were used to examine differences between AN patients, HCA and HC for each direction and seed (left and right IFG) separately. All group tests were thresholded at p < 0.05, family-wise error (FWE) corrected for multiple comparisons.
Pearson correlations (two-sided) were calculated between behavioral measurements and degree centrality and effective connectivity measures (6 brain regions; 8 behavioral measurements) for each group separately and over the whole group (p < 0.001, Bonferroni corrected) using SPSS (version 20; IBM Corporation, Armonk, NY).
3. Results
3.1. Resting-state functional connectivity in anorexia nervosa and healthy controls
Degree centrality was used to measure voxel-wise network centrality. Specifically, we found reduced degree centrality within the bilateral inferior frontal gyrus (IFG) (MNI coordinates (x, y, z) ±48, 9, 24; tmax = 4.74; p < 0.05 FWE-corrected for multiple comparisons) (Fig. 1) in AN patients compared to healthy controls. No significant differences were observed between HC and HCA (p > 0.05, FWE-corrected).
Fig. 1.
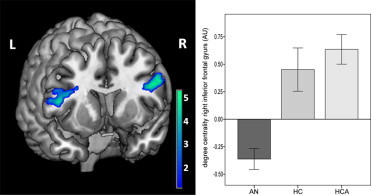
Decreased functional connectivity in the inferior frontal gyrus measured by degree centrality in anorexia nervosa patients compared to healthy controls (p < 0.05, FWE corrected) (AN: anorexia nervosa; HC: healthy non-athlete controls; HCA healthy athlete controls).
3.2. Effective connectivity from and to the inferior frontal gyrus
Compared with healthy controls, patients with AN showed decreased effective connectivity from the right IFG to the midcingulum (Table 2; Fig. 2A; p < 0.05, FWE corrected).
Table 2.
Altered effective connectivity in anorexia nervosa patients compared to healthy controls from and to the inferior frontal gyrus (IFG).
| Regions | Hem | BA | MNI (mm) (x, y, z) | Size |
T value* |
|---|---|---|---|---|---|
| Increased effective connectivity to the right IFG | |||||
| Medial frontal orbital gyrus | Bilateral | 47 | 0, 36, -18 | 48 | 5.6 |
| Decreased effective connectivity from the right IFG | |||||
| Midcingulum | Bilateral | 24 | ±3, 12, 33 | 52 | 4.22 |
| Increased effective connectivity to the left IFG | |||||
| Insula | Left | 13 | -39, -15, 12 | 49 | 4.53 |
| Insula | Right | 13 | 39, 0, -3 | 19 | 4.15 |
p < 0.05, family wise error corrected for multiple comparisons.
Fig. 2.

Altered effective connectivity from and to the IFG in anorexia nervosa compared to healthy controls. (A) Increased effective connectivity from the bilateral OFC (red) to the right IFG and decreased effective connectivity from the right IFG to the midcingulum (blue) (p < 0.05, FWE corrected). (B) Increased effective connectivity from the bilateral insula (red) to the left IFG (p < 0.05, FWE corrected). (C) Schematic overview of changes in effective connectivity measured by Granger causality in anorexia nervosa patients compared to healthy controls. The red arrows indicate increased effective connectivity from the insula and OFC to the IFG; the blue arrow indicates decreased effective connectivity from the IFG to the MC in AN patients compared to healthy controls (IFG: inferior frontal gyrus, MC: midcingulum; OFC: orbitofrontal cortex).
From the bilateral OFC to the right IFG, AN patients showed increased effective connectivity compared to healthy controls (Table 2; Fig. 2A); and from the bilateral insula cortex to the left IFG AN patients showed increased effective connectivity compared to healthy controls (Table 2; Fig. 2B).
No significant differences were observed between HC and HCA (p > 0.05, FWE-corrected).
3.3. Correlation results
After correcting for multiple comparisons, we only found, in AN patients, a significant negative correlation between the degree centrality of the right IFG with vigorous physical activity (r = -0.764, p = 0.001).
For the whole group, we found a significant negative correlation between the degree centrality of the left IFG and state anxiety score (r = -0.519, p = 0.001) and for the right IFG with the depression sum score (r = -0.528, p = 0.001) and state anxiety score (r = -0.521, p = 0.001). For the differential effective connectivity patterns, our analyses revealed positive correlations between the increased effective connectivity from the orbital frontal cortex to the right IFG and the depression sum score (r = 0.657, p < 0.001), state anxiety score (r = 0.593, p < 0.001), and the Eating Disorder Inventory (r = 0.509, p = 0.001). For the decreased effective connectivity from the right IFG to the midcingulum, we found negative correlations with the depression sum score (r = -0.516, p = 0.001) and state anxiety score (r = -0.628, p < 0.001). And for the increased effective connectivity from the insula cortex to the left IFG, we found a positive correlation with the depression sum score (r = -0.569, p < 0.001), state anxiety score (r = -0.521, p = 0.001) and the Eating Disorder Inventory (r = 0.549, p < 0.001).
Additionally, significant correlations, uncorrected for multiple comparisons, are reported in Supplementary Table 1.
4. Discussion
This is the first study, to our knowledge, investigating whole-brain resting-state functional and effective connectivity alterations in currently ill AN patients compared to healthy controls. Using degree centrality, we were able to show that the bilateral IFG is a region of special functional importance in AN patients revealing reduced functional connectivity. To further investigate the influence of directionality, we applied GCA to evaluate changes in effective connectivity. AN patients showed reduced effective connectivity from and increased effective connectivity to the IFG. Specifically, we were able to show decreased effective connectivity from the right IFG to the midcingulum and increased effective connectivity from the bilateral OFC to the right IFG in AN compared to healthy controls. For the left IFG, we only observed increased connectivity from the bilateral insula to the left IFG in AN compared to healthy controls. Of note, scores reflecting vigorous physical activity in AN patients correlated with connectivity patterns of the right IFG.
The major region affected by AN is the inferior frontal cortex, which is a key area for executive functions, encompassing multiple high level processes to control and organize other cognitive operations. Inhibition of inappropriate behaviors, for instance, is one critical component of cognitive control and especially the right IFG, as found in our study, is of special importance for inhibitory control of motor responses (Asahi et al., 2004; Duann et al., 2009). Imaging studies in AN have found, when challenging cognitive control, reduced prefrontal activity in AN patients compared to healthy controls (Lock et al., 2011; Oberndorfer et al., 2011). While these studies have shown that AN patients need less inhibitory resources to maintain behavioral performance, we were able to show in a recent study that in response to stimuli depicting physical activity AN patients need more inhibitory resources to maintain behavioral performance, resulting in hyperactivation of the prefrontal cortex. These results suggest that physical activity stimuli might place an increased demand on the inhibitory control system in AN patients (Kullmann et al., 2013a). Similarly, processing of food in AN goes along with increased prefrontal activations, which has been speculated to impinge on cognitive control over food consumption (Brooks et al., 2011; Brooks et al., 2012a).
In the current study, we were able to observe a significant relationship between vigorous physical activity and altered network integrity of the right IFG. Particularly AN patients displayed hyperactivity, ranging from lengthy walks to vigorous exercise. Our results indicate that the more vigorous AN patients exercise the stronger the reduction of the functional connectivity in the IFG. Since the IFG is pivotal for inhibitory behavior, including motor response, altered integrity within the whole-brain network could contribute to hyperactivity symptoms in AN. However, we cannot disentangle, in this cross sectional study, whether hyperactivity is a cause or effect of the altered connectivity pattern. Yet, there are multiple hypotheses trying to explain the role of hyperactivity in AN. Some approaches conceptualize it as a secondary order symptom to deliberately lose weight (Bruch, 1962). However, in many cases hyperactivity has been shown to precede food restriction (Davis et al., 2005). Evolutionary approaches, on the other hand, consider hyperactivity as a primary reinforcer by increasing foraging during food restriction (Fessler, 2002; Scheurink et al., 2010). Furthermore, exercise can also be considered as a coping strategy to compensate anxiety and depression (Brewerton et al., 1995; Penas-Lledo et al., 2002), which is elevated in AN patients, or as a thermoregulatory behavior (Carrera et al., 2012).
The Granger causality analysis revealed altered effective connectivity patterns between the IFG and the midcingulum, OFC and insula. The former is part of the cingulate cortex, which is especially known for its functional heterogeneity subdivided in several distinct regions. The midcingulum is central to skeletomotor regulations (Vogt et al., 1992) on top of its role in cognitive tasks, especially related to attention (Torta and Cauda, 2011). In terms of connectivity, the midcingulum has been shown to be part of a network including the thalamus, midbrain, and anterior insular cortex (Torta and Cauda, 2011). Together these regions belong to the so called core network recruited by cognitive demanding tasks controlling goal-directed behavior (Dosenbach et al., 2006; Dosenbach et al., 2007; Seeley et al., 2007). There is growing evidence that the core network can be divided into two distinct subnetworks for salience processing and executive control (Seeley et al., 2007). The anterior cingulate cortex and anterior insula respond to personal salience including motivational, emotional or cognitive tasks by integrating sensory data (Bush et al., 2000; Critchley, 2005), as for example processing taste reward (Keating et al., 2012), while the prefrontal cortex is exceedingly involved in cognitive control operating on identified salience by directing attention towards relevant stimuli (Seeley et al., 2007). Of note, within the resting-state salience network, McFladden and colleagues identified the anterior cingulate cortex as a possible trait-related biomarker due to the reduced functional connectivity in AN patients and recovered women (McFadden et al., 2013). In our study, we postulate that the reduced IFG functional and effective connectivity to the midcingulum may lead to a dysfunctional inhibitory response directing attention away from food and lowering the drive to approach food. Whether this is associated with an increased or decreased PFC activation during cognitive demanding tasks probably depends on the input the IFG receives from the insula and OFC. The latter is crucially involved in reward processing and is part of the secondary gustatory cortex (Rolls et al., 1990). As a multisensory neural node, the insular cortex integrates perception, emotion, interoceptive awareness, cognition, and gustation (Frank et al., 2013; Nunn et al., 2008), the anterior part of the insular cortex is also recognized, amongst other functions, as the primary gustatory cortex (Rolls, 2005; Yaxley et al., 1988). Previous studies have frequently reported heightened salience processing in AN patients resulting in an increased response to food cues in the insula and OFC (Frank et al., 2012; Uher et al., 2004) and altered effective connectivity to food cues between the insula and IFG (Kim et al., 2012). This imbalanced convergence on the insula has been proposed to increase the experience of anxiety and fear, mostly on the monitoring of the bodily states (Brooks et al., 2012b). Concomitantly, we observed a heightened effective connectivity from the insula and OFC to the IFG, which could induce an overregulated inhibitory system in order to restrict the stronger interceptive response evoked by salient stimuli.
Even though both the AN and HCA groups displayed intensive exercise, we found no similarities in functional and effective connectivity. Both control groups revealed the same connectivity pattern. This is probably related to the fact that the main regions consistently involved in altered cerebral function in AN are the frontal and cingulate cortex as well as striatal regions (Kaye et al., 2009; Pietrini et al., 2011) and not sensorimotor areas. However when challenging inhibitor control with physical activity stimuli, we were able to show a similar sensorimotor response in athletes and AN patients (Kullmann et al., 2013a).
A limitation of our study is the small sample size making this study a preliminary study that needs further replication. Hence, differences in functional and effective connectivity can also be due to comorbidities associated with AN, as depression and anxiety. Also, we could not differentiate restricting versus purging subgroups due to the small sample size. Further studies are needed to evaluate differences between these subtypes with respect to hyperactivity and brain function.
Taken together, AN patients showed reduced connectivity within the cognitive control system of the brain and increased connectivity within regions important for salience/reward processing. These results are also consistent with clinic findings showing aberrant cognitive control and overregulation (Tchanturia et al., 2004; Vitousek and Manke, 1994).
Disclosures
None of the authors declares a conflict of interest.
Acknowledgments
We gratefully thank Karoline Pfeiffer and Eva Schäflein for their help with patient recruitment; and Maike Borutta and Katrin Stingel for excellent technical support. This work was supported by a grant from the Center of Nutritional Medicine Tübingen/Hohenheim (grant number: ZEM 24.A II-08).
Appendix A. Supplementary material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.nicl.2014.04.002.
Appendix A. Supplementary materials
Correlation analyses between altered functional and effective connectivity and behavioral characteristics for the whole group.
References
- Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR). Washington, DC. American Psychiatric Association.
- Asahi S., Okamoto Y., Okada G., Yamawaki S., Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. 15309395 [DOI] [PubMed] [Google Scholar]
- Brewerton T.D., Stellefson E.J., Hibbs N., Hodges E.L., Cochrane C.E. Comparison of eating disorder patients with and without compulsive exercising. International Journal of Eating Disorders. 1995;17:413–416. doi: 10.1002/1098-108x(199505)17:4<413::aid-eat2260170414>3.0.co;2-0. 7620482 [DOI] [PubMed] [Google Scholar]
- Brooks S.J., O'Daly O., Uher R., Friederich H.C., Giampietro V., Brammer M., Williams S.C., Schioth H.B., Treasure J., Campbell I.C. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PloS One. 2012;7:e34000. doi: 10.1371/journal.pone.0034000. 22479499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., O'Daly O.G., Uher R., Friederich H.C., Giampietro V., Brammer M., Williams S.C., Schioth H.B., Treasure J., Campbell I.C. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PloS One. 2011;6:e22259. doi: 10.1371/journal.pone.0022259. 21799807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., Rask-Andersen M., Benedict C., Schioth H.B. A debate on current eating disorder diagnoses in light of neurobiological findings: is it time for a spectrum model? BMC Psychiatry. 2012;12:76. doi: 10.1186/1471-244X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosomatic Medicine. 1962;24:187–194. doi: 10.1097/00006842-196203000-00009. 13873828 [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. 19211893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. 19190637 [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. 10827444 [DOI] [PubMed] [Google Scholar]
- Carrera O., Adan R.A., Gutierrez E., Danner U.N., Hoek H.W., van Elburg A.A., Kas M.J. Hyperactivity in anorexia nervosa: warming up not just burning-off calories. PloS One. 2012;7:e41851. doi: 10.1371/journal.pone.0041851. 22848634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J.C., Blackmore E., Sutandar-Pinnock K., Woodside D.B. Relapse in anorexia nervosa: a survival analysis. Psychological Medicine. 2004;34:671–679. doi: 10.1017/S0033291703001168. 15099421 [DOI] [PubMed] [Google Scholar]
- Casper R.C., Jasbine L.N. An eight-year follow-up: outcome from adolescent compared to adult onset anorexia nervosa. Journal of Youth and Adolescence. 1996;25:499–517. [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. 20577591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. 20407579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdrey F.A., Filippini N., Park R.J., Smith S.M., McCabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Human Brain Mapping. 2014 doi: 10.1002/hbm.22202. 23033154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. 16254997 [DOI] [PubMed] [Google Scholar]
- Davis C., Blackmore E., Katzman D.K., Fox J. Female adolescents with anorexia nervosa and their parents: a case–control study of exercise attitudes and behaviours. Psychological Medicine. 2005;35:377–386. doi: 10.1017/s0033291704003447. 15841873 [DOI] [PubMed] [Google Scholar]
- Davis C., Brewer H., Ratusny D. Behavioral frequency and psychological commitment: necessary concepts in the study of excessive exercising. Journal of Behavioral Medicine. 1993;16:611–628. doi: 10.1007/BF00844722. 8126715 [DOI] [PubMed] [Google Scholar]
- Ding L., Worrell G.A., Lagerlund T.D., He B. Spatio-temporal source localization and Granger causality in ictal source analysis. Conference Proceedings: ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2006;1:3670–3671. doi: 10.1109/IEMBS.2006.259393. 17947049 [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. 17576922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Visscher K.M., Palmer E.D., Miezin F.M., Wenger K.K., Kang H.C., Burgund E.D., Grimes A.L., Schlaggar B.L., Petersen S.E. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. 16731517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J.R., Ide J.S., Luo X., Li C.S. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. 19675251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn C.G., Cooper Z. The Eating Disorder Examination. In: Fairburn C.G., Wilson G.T., editors. twelfth edition. Guilford Publications; New York: 1993. pp. 317–360. (Binge Eating: Nature Assessment and Treatment). [Google Scholar]
- Favaro A., Santonastaso P., Manara R., Bosello R., Bommarito G., Tenconi E., Di Salle F. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biological Psychiatry. 2012;72:864–870. doi: 10.1016/j.biopsych.2012.04.025. 22633945 [DOI] [PubMed] [Google Scholar]
- Fessler D.M. Pseudoparadoxical impulsivity in restrictive anorexia nervosa: a consequence of the logic of scarcity. International Journal of Eating Disorders. 2002;31:376–388. doi: 10.1002/eat.10035. 11948643 [DOI] [PubMed] [Google Scholar]
- Fladung A.K., Gron G., Grammer K., Herrnberger B., Schilly E., Grasteit S., Wolf R.C., Walter H., von Wietersheim J. A neural signature of anorexia nervosa in the ventral striatal reward system. American Journal of Psychiatry. 2010;167:206–212. doi: 10.1176/appi.ajp.2009.09010071. 19833790 [DOI] [PubMed] [Google Scholar]
- Frank G.K., Bailer U.F., Henry S.E., Drevets W., Meltzer C.C., Price J.C., Mathis C.A., Wagner A., Hoge J., Ziolko S., Barbarich-Marsteller N., Weissfeld L., Kaye W.H. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biological Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. 15992780 [DOI] [PubMed] [Google Scholar]
- Frank G.K., Reynolds J.R., Shott M.E., Jappe L., Yang T.T., Tregellas J.R., O'Reilly R.C. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37:2031–2046. doi: 10.1038/npp.2012.51. 22549118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Kullmann S., Veit R. Food related processes in the insular cortex. Frontiers in Human Neuroscience. 2013;7:499. doi: 10.3389/fnhum.2013.00499. 23986683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia I., Jurado M.A., Garolera M., Segura B., Sala-Llonch R., Marques-Iturria I., Pueyo R., Sender-Palacios M.J., Vernet-Vernet M., Narberhaus A., Ariza M., Junque C. Alterations of the salience network in obesity: a resting-state fMRI study. Human Brain Mapping. 2012 doi: 10.1002/hbm.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner D.M. Eating Disorder Inventory—2: Professional Manual. Psychological Assessment Resources; 1991. [Google Scholar]
- Giel K.E., Kullmann S., Preissl H., Bischoff S.C., Thiel A., Schmidt U., Zipfel S., Teufel M. Understanding the reward system functioning in anorexia nervosa: crucial role of physical activity. Biological Psychology. 2013;94:575–581. doi: 10.1016/j.biopsycho.2013.10.004. 24161801 [DOI] [PubMed] [Google Scholar]
- Granger C. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- Gray J.A. The psychophysiological basis of introversion–extraversion. Behaviour Research and Therapy. 1970;8:249–266. doi: 10.1016/0005-7967(70)90069-0. 5470377 [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. 17210143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. 15070770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebebrand J., Bulik C.M. Critical appraisal of the provisional DSM-5 criteria for anorexia nervosa and an alternative proposal. International Journal of Eating Disorders. 2011;44:665–678. doi: 10.1002/eat.20875. 22072403 [DOI] [PubMed] [Google Scholar]
- Jiao Q., Lu G., Zhang Z., Zhong Y., Wang Z., Guo Y., Li K., Ding M., Liu Y. Granger causal influence predicts BOLD activity levels in the default mode network. Human Brain Mapping. 2011;32:154–161. doi: 10.1002/hbm.21065. 21157880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W.H., Fudge J.L., Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Wierenga C.E., Bailer U.F., Simmons A.N., Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends in Neurosciences. 2013;36:110–120. doi: 10.1016/j.tins.2013.01.003. 23333342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C., Tilbrook A.J., Rossell S.L., Enticott P.G., Fitzgerald P.B. Reward processing in anorexia nervosa. Neuropsychologia. 2012;50:567–575. doi: 10.1016/j.neuropsychologia.2012.01.036. 22349445 [DOI] [PubMed] [Google Scholar]
- Kim K.R., Ku J., Lee J.H., Lee H., Jung Y.C. Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neuroscience Letters. 2012;521:152–157. doi: 10.1016/j.neulet.2012.05.075. 22684096 [DOI] [PubMed] [Google Scholar]
- Kullmann S., Giel K.E., Hu X., Bischoff S.C., Teufel M., Thiel A., Zipfel S., Preissl H. Impaired inhibitory control in anorexia nervosa elicited by physical activity stimuli. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst070. 23677490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Veit R., Ketterer C., Schick F., Haring H.U., Fritsche A., Preissl H. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Human Brain Mapping. 2012;33:1052–1061. doi: 10.1002/hbm.21268. 21520345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S., Pape A.A., Heni M., Ketterer C., Schick F., Haring H.U., Fritsche A., Preissl H., Veit R. Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cerebral Cortex (New York, N.Y.: 1991) 2013;23:1247–1256. doi: 10.1093/cercor/bhs124. 22586138 [DOI] [PubMed] [Google Scholar]
- Lock J., Garrett A., Beenhakker J., Reiss A.L. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. American Journal of Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. 21123315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden K.L., Tregellas J.R., Shott M.E., Frank G.K. Reduced salience and default mode network activity in women with anorexia nervosa. Journal of Psychiatry and Neuroscience. 2013;38:130046. doi: 10.1503/jpn.130046. 24280181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S.A., Stevens M.C., Folley B.S., Calhoun V.D., Pearlson G.D. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PloS One. 2009;4:e7911. doi: 10.1371/journal.pone.0007911. 19936244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn K., Frampton I., Gordon I., Lask B. The fault is not in her parents but in her insula—a neurobiological hypothesis of anorexia nervosa. European Eating Disorders Review: the Journal of the Eating Disorders Association. 2008;16:355–360. doi: 10.1002/erv.890. 18711713 [DOI] [PubMed] [Google Scholar]
- Oberndorfer T.A., Kaye W.H., Simmons A.N., Strigo I.A., Matthews S.C. Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. International Journal of Eating Disorders. 2011;44:1–8. doi: 10.1002/eat.20750. 20127942 [DOI] [PubMed] [Google Scholar]
- Paul T., Thiel A. EDI-2. Eating Disorder Inventory—2. Deutsche Version. Hogrefe; Göttingen, Germany: 2005. [Google Scholar]
- Penas-Lledo E., Vaz Leal F.J., Waller G. Excessive exercise in anorexia nervosa and bulimia nervosa: relation to eating characteristics and general psychopathology. International Journal of Eating Disorders. 2002;31:370–375. doi: 10.1002/eat.10042. 11948642 [DOI] [PubMed] [Google Scholar]
- Pietrini F., Castellini G., Ricca V., Polito C., Pupi A., Faravelli C. Functional neuroimaging in anorexia nervosa: a clinical approach. European Psychiatry: the Journal of the Association of European Psychiatrists. 2011;26:176–182. doi: 10.1016/j.eurpsy.2010.07.011. 20934859 [DOI] [PubMed] [Google Scholar]
- Qi R., Zhang L.J., Zhong J., Zhang Z., Ni L., Jiao Q., Liao W., Zheng G., Lu G. Altered effective connectivity network of the basal ganglia in low-grade hepatic encephalopathy: a resting-state fMRI study with Granger causality analysis. PloS One. 2013;8:e53677. doi: 10.1371/journal.pone.0053677. 23326484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiology & Behavior. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. 15924905 [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Yaxley S., Sienkiewicz Z.J. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Journal of Neurophysiology. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. 2258734 [DOI] [PubMed] [Google Scholar]
- Scheurink A.J., Boersma G.J., Nergardh R., Sodersten P. Neurobiology of hyperactivity and reward: agreeable restlessness in anorexia nervosa. Physiology & Behavior. 2010;100:490–495. doi: 10.1016/j.physbeh.2010.03.016. 20361989 [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. 17329432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. 21949842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Roebroeck A. A short history of causal modeling of fMRI data. Neuroimage. 2012;62:856–863. doi: 10.1016/j.neuroimage.2012.01.034. 22248576 [DOI] [PubMed] [Google Scholar]
- Strobel A., Beauducel A., Debener S., Brocke B. Eine deutschsprachige Version des BIS/BAS-Fragebogens von Carver und White. Zeitschrift Für Differentielle und Diagnostische Psychologie. 2001;22:216–227. [Google Scholar]
- Strober M., Freeman R., Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. International Journal of Eating Disorders. 1997;22:339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. 9356884 [DOI] [PubMed] [Google Scholar]
- Tchanturia K., Anderluh M.B., Morris R.G., Rabe-Hesketh S., Collier D.A., Sanchez P., Treasure J.L. Cognitive flexibility in anorexia nervosa and bulimia nervosa. Journal of the International Neuropsychological Society: JINS. 2004;10:513–520. doi: 10.1017/S1355617704104086. 15327730 [DOI] [PubMed] [Google Scholar]
- Torta D.M., Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. 21459151 [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human Brain Mapping. 2009;30:625–637. doi: 10.1002/hbm.20531. 18219617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R., Murphy T., Brammer M.J., Dalgleish T., Phillips M.L., Ng V.W., Andrew C.M., Williams S.C., Campbell I.C., Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. American Journal of Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. 15229057 [DOI] [PubMed] [Google Scholar]
- Vitousek K., Manke F. Personality variables and disorders in anorexia nervosa and bulimia nervosa. Journal of Abnormal Psychology. 1994;103:137–147. doi: 10.1037//0021-843x.103.1.137. 8040475 [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Finch D.M., Olson C.R. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex (New York, N.Y.: 1991) 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. 1477524 [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Wunderlich U., Gruschwitz S., Zaudig M. SKID-I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Hogrefe; Göttingen, Germany: 1997. [Google Scholar]
- Yaxley S., Rolls E.T., Sienkiewicz Z.J. The responsiveness of neurons in the insular gustatory cortex of the macaque monkey is independent of hunger. Physiology & Behavior. 1988;42:223–229. doi: 10.1016/0031-9384(88)90074-1. 3406148 [DOI] [PubMed] [Google Scholar]
- Zang Z.X., Yan C.G., Dong Z.Y., Huang J., Zang Y.F. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. Journal of Neuroscience Methods. 2012;203:418–426. doi: 10.1016/j.jneumeth.2011.10.006. 22020117 [DOI] [PubMed] [Google Scholar]
- Zastrow A., Kaiser S., Stippich C., Walther S., Herzog W., Tchanturia K., Belger A., Weisbrod M., Treasure J., Friederich H.C. Neural correlates of impaired cognitive–behavioral flexibility in anorexia nervosa. American Journal of Psychiatry. 2009;166:608–616. doi: 10.1176/appi.ajp.2008.08050775. 19223435 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Ding M., Chen Y., Wright P., Lu Z., Liu Y. Detecting directional influence in fMRI connectivity analysis using PCA based Granger causality. Brain Research. 2009;1289:22–29. doi: 10.1016/j.brainres.2009.06.096. 19595679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Wang X., Klahr N.J., Liu W., Arias D., Liu H., von Deneen K.M., Wen Y., Lu Z., Xu D., Liu Y. A conditional Granger causality model approach for group analysis in functional magnetic resonance imaging. Magnetic Resonance Imaging. 2011;29:418–433. doi: 10.1016/j.mri.2010.10.008. 21232892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel S., Lowe B., Reas D.L., Deter H.C., Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet. 2000;355:721–722. doi: 10.1016/S0140-6736(99)05363-5. 10703806 [DOI] [PubMed] [Google Scholar]
- Zipfel S., Mack I., Baur L.A., Hebebrand J., Touyz S., Herzog W., Abraham S., Davies P.S.W., Russell J. Impact of exercise on energy metabolism in anorexia nervosa. Journal of Eating Disorders. 2013;1:37. doi: 10.1186/2050-2974-1-37. 24499685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel S., Wild B., Gross G., Friederich H.C., Teufel M., Schellberg D., Giel K.E., de Zwaan M., Dinkel A., Herpertz S., Burgmer M., Lowe B., Tagay S., von Wietersheim J., Zeeck A., Schade-Brittinger C., Schauenburg H., Herzog W. Focal Psychodynamic Therapy, Cognitive Behaviour Therapy, and Optimised Treatment as Usual in Outpatients with Anorexia Nervosa (ANTOP Study): Randomised Controlled Trial. Lancet; 2013. [DOI] [PubMed] [Google Scholar]
- Zuo X.N., Ehmke R., Mennes M., Imperati D., Castellanos F.X., Sporns O., Milham M.P. Network centrality in the human functional connectome. Cerebral Cortex (New York, N.Y.: 1991) 2012;22:1862–1875. doi: 10.1093/cercor/bhr269. 21968567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation analyses between altered functional and effective connectivity and behavioral characteristics for the whole group.


