Abstract
In the recent issue of Cell, Bonham et al. reveal that the sorting adapter TIRAP regulates the assembly of the myddosome upon Toll-like receptor activation from both the cell surface and endosomes through its promiscuous binding to lipids.
Toll-like receptors (TLRs) play critical roles in the innate immune system by recognizing pathogen-associated molecular patterns from microbes. TLR signaling results in a variety of cellular responses including the production of interferons (IFNs), pro-inflammatory cytokines and effector cytokines that direct the adaptive immune response. TLRs may be classified into two types based on their subcellular localizations: plasma membrane TLRs including TLR1, 2 and 4-6, and endosomal TLRs including TLR3, 7-9 and 11-13. All TLRs contain a cytoplasmic domain known as the Toll/interleukin-1 receptor (TIR) domain, which recruits TIR-containing adapter proteins upon activation. Among these adapter proteins, the signaling adapter MyD88 is needed by all TLRs except TLR3. MyD88 in turn assembles the Myddosome with kinases in the IRAK family through death domain interactions for TRAF6 activation and NF-κB nuclear translocation (Ferrao et al., 2012; Lin et al., 2010). TIRAP (also known as Mal) was defined as a sorting adapter because of its localization to the site of signal transduction before ligand engagement and has previously been shown to be critical for signal transduction of plasma membrane TLRs (Fitzgerald et al., 2001; Kagan and Medzhitov, 2006). In a recent issue of Cell, Bonham et al., led by senior author Kagan, provided direct evidence that TIRAP also regulates endosomal TLRs as a sorting adapter (Bonham et al., 2014).
Previous work has shown that TIRAP contains an N-terminal lipid binding domain of an unknown fold that binds to acidic phosphoinositides (PIs) and phosphatidylserine (PS), among which the interaction with phosphatidylinositol-4,5 bisphosphate (PI(4,5)P2), a plasma membrane-enriched lipid, was responsible for the sorting adapter role of TIRAP for TLR4 signaling (Kagan and Medzhitov, 2006). The plasma membrane placement of TIRAP may allow direct sensing of activated TLRs and assists in the recruitment of the important signaling adaptor MyD88. The latter is especially important because MyD88 TIR domain has limited ability to interact with receptor TIR domains directly (Brown et al., 2006; Ulrichts et al., 2007). The importance of TIRAP in plasma membrane TLRs was shown by genetic knockout studies (Horng et al., 2002; Yamamoto et al., 2002). However, the involvement of TIRAP in endosomal TLRs has previously been excluded because synthetic ligands robustly activated TLR7 and TLR9 in TIRAP-deficient cells (Horng et al., 2002; Yamamoto et al., 2002). As such, it is unclear how MyD88-dependent innate immune responses are activated by endosomal TLRs.
Given that high ligand concentrations could bypass the requirement of TIRAP for plasma membrane TLRs (Horng et al., 2002; Yamamoto et al., 2002), the authors reasoned that the resistance to endolysosomal nuclease digestion of some synthetic ligands and the highly endocytic nature of primary macrophages may lead to ligand accumulation, which removes the necessity for TIRAP. To test this hypothesis, instead of using synthetic CpG ligands, the authors used natural ligands from different strains of the DNA virus herpes simplex virus (HSV) to stimulate TLR9, a well studied endosomal TLR. Some of the HSV strains used engage TLR9 only. They found that IL-1β and IL-6 production were impaired in TIRAP knockout bone marrow-derived macrophages. Plasmacytoid dendritic cells (pDCs) exclusively utilize endosomal TLRs to detect infections and induce interferon production from endosomes rich in 3’ PIs. The authors further showed that TIRAP-deficient pDCs showed dimished IFN-α expression upon stimulation by HSV and influenza viruses, which are natural activators of TLR9 and TLR7 respectively. To provide additional mechanistic insights, the authors showed that the requirement for TIRAP in TLR9 signaling could also be bypassed by high concentrations of viral activators. Therefore, TIRAP is required for endosomal TLR signaling upon stimulation by viral ligands.
Because some of the TIRAP-interacting lipids such as PI(3)P and PI(3,5)P2 are enriched on endosomes, the authors proposed that some TIRAP would be located on endosomes to assist TLR signaling. Using GFP-tagging and confocal microscopy, they showed that in addition to the plasma membrane, the lipid-binding domain of TIRAP also localized to intracellular vesicles. In contrast, the pleckstrin homology (PH) domain of PLCδ1, which binds only to PI(4,5)P2, only resided on the plasma membrane. To address the functions of specific membrane targeting in TLR signaling, the authors replaced the lipid-binding domain of TIRAP with PLCδ1 PH domain, p40-phox endosomal localization domain that binds exclusively to PI(3)P, and the SLP2a PS-binding domain that localizes to both cell surface and endosomal membranes, respectively. They found that the PLCδ1 chimera specifically restored responsiveness to the TLR4 ligand LPS and the p40-phox chimera specifically rescued TLR9 signaling in immortalized TIRAP-deficient macrophages. Interestingly, the SLP2a chimera restored TLR9 signaling but not TLR4 signaling, suggesting that localization to a particular membrane species is not the only factor for assisting TLR proximal signal transduction. The requirement for PI interaction in TIRAP function may reflect the importance of PIs as docking sites for protein effectors. The levels of PIs in distinct cellular compartments are controlled by a group of PI-modifying enzymes including PI kinases, phosphatases and phospholipase. TLR signaling may be modulated by PI metabolism.
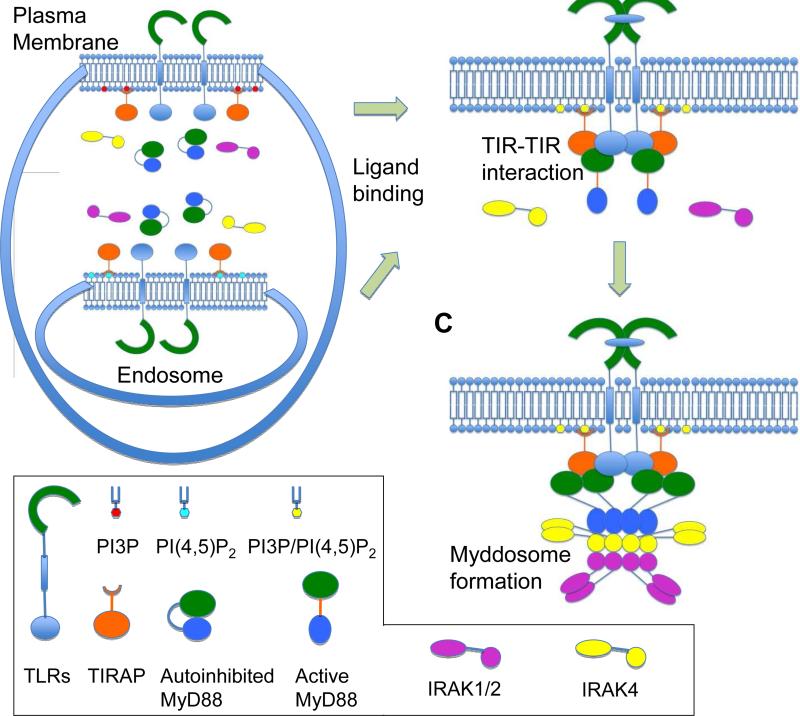
Bonham et al. showed that TIRAP is a new component of the Myddosome and promotes the assembly of Myddosomes at both the cell surface and the endosomes. The first step in this assembly requires the TIR-TIR interactions among receptor TIR domains and adapter TIR domains. Because of the low affinity between receptor TIR domains and MyD88 TIR domain, TIRAP most likely enhances the assembly by serving as a bridge in ternary TIR-TIR interactions (Figure 1). In this scenario, ligand binding induces TLR dimerization and possibly higher-order oligomerization to juxtapose the cytosolic TIR domains. Because of the proximity and because of the ease of two-dimensional diffusion (Wu et al., 2011), oligomerized receptor TIRs most likely first recruit the sorting adaptor TIRAP to activated receptors. The receptor-TIRAP complex may then further recruit MyD88, IRAK4 and IRAK2 from the cytosolic compartment to form the membrane-bound Myddosome. Such a scenario may also be consistent with the role of avidity in higher-order assembly of the Myddosome, in which multiple MyD88 molecules co-exist in a single Myddosome (Lin et al., 2010).
Figure 1. The sorting adaptor TIRAP is required for signaling by both plasma membrane and endosomal TLRs.
(A) Schematic image of the unstimulated states with constitutive localization of TIRAP to plasma and endosomal membranes.
(B) Upon ligand stimulation, TIRAP at either membranes interacts with activated TLRs and recruits MyD88 through TIR-TIR interaction.
(C) Further recruitment of IRAK family kinases leads to the assembly of the Myddosome. (The exact stoichiometry is not inferred in the drawing)
Collectively, these studies present an elegant model for TLR signaling in which the sorting adapter TIRAP, stationed at different membrane locations via somewhat promiscuous lipid binding, is able to detect even small quantities of ligand-bound TLRs to initiate Myddosome assembly. When large quantities of ligand-bound TLRs are present, receptor TIRs may directly engage MyD88 through increased local concentration and enhanced avidity (Horng et al., 2002; Yamamoto et al., 2002). However, not all TLRs have been tested. It will be interesting to see if TIRAP is a universal sorting adapter, or if other potential sorting adapters such as TRAM mediate signaling of some TLR pathways. It is also yet to be shown that endogenous TIRAP resides at the various membranes and colocalizes with TLRs upon ligand stimulation. Additionally, the interactions among TLRs, TIRAP and MyD88 should be reconsituted in vitro using purified proteins and suitable lipids. Specific recognition is usually very important in cell signaling. Here, Bonham et al. gave a contradictory example on the importance of ambiguous lipid specificity of TIRAP. Perhaps promiscuity is not always bad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, Kagan JC. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell. 2014;156:705–716. doi: 10.1016/j.cell.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Brown RA, Ozinsky A, Hesselberth JR, Fields S. Binding specificity of Toll-like receptor cytoplasmic domains. Eur J Immunol. 2006;36:742–753. doi: 10.1002/eji.200535158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrao R, Li J, Bergamin E, Wu H. Structural insights into the assembly of large oligomeric signalosomes in the toll-like receptor-interleukin-1 receptor superfamily. Sci Signal. 2012;5:re3. doi: 10.1126/scisignal.2003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichts P, Peelman F, Beyaert R, Tavernier J. MAPPIT analysis of TLR adaptor complexes. FEBS Lett. 2007;581:629–636. doi: 10.1016/j.febslet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475:510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]