Abstract
Laboratory studies suggest that evening light before bedtime can suppress melatonin. Here we measured the range of evening light intensity people can generate with their household lights, and for the first time determined if varying home light before usual bedtime can shift circadian phase. This was a 3-week study with two counterbalanced conditions separated by a 5-day break. In a dim week, 8 healthy subjects minimized their home light exposure from 4 hours before habitual bedtime until a self-selected bedtime. In a bright week, the subjects maximized their home lighting for the same time. The dim light melatonin onset (DLMO) was assessed after each week. On average subjects maximized their lights to ~65 lux and minimized their lights to ~3 lux. Wrist actigraphy indicated that subjects went to bed slightly later when lights were maximized (average 14 minutes later, p=0.05), but wake time did not change. Every subject had a later DLMO after the week of maximum versus minimum light exposure (average 1:03 h later, p<0.001). These results demonstrate that the light intensity people can generate at home in the few hours before habitual bedtime can alter circadian timing. People should reduce their evening light exposure to lessen circadian misalignment.
INTRODUCTION
Adults often delay their bedtime and wake time by an average of ~1 hour on days that they do not work (1). These later sleep times lead to more evening light exposure and less morning light exposure, which delays the timing of the internal central circadian clock (2–4). On subsequent mornings when people must wake earlier to get to work or school, they wake at an earlier circadian phase, and this contributes to the reduced alertness (4, 5) and performance (6, 7) experienced by many. Importantly, when this occurs chronically, such “social jet lag” is also associated with greater use of alcohol, nicotine and caffeine, and increased risk for depression and obesity (8–10).
What is not known is whether common household lighting can impact circadian timing when sleep is held relatively stable. In other words, can the light intensity people generate at home, in the hours before their usual bedtime, impact circadian timing? Two recent laboratory based studies have shown that evening room light of ~100–200 lux before usual bedtime can suppress melatonin (11, 12), suggesting such light has potential to phase delay the circadian clock. However the room light in these laboratory based studies was brighter than the evening light observed in field studies, where people typically receive ~40 lux or less in their homes in the few hours before bedtime (2, 13, 11). Therefore we conducted a field study to measure the range in light intensity that people can generate at home using their household lighting and to determine for the first time if manipulating home lighting before usual bedtime can alter circadian timing.
MATERIALS AND METHODS
Subjects
Eight healthy subjects participated (3 men, 5 women; mean age ± SD 25.6 ± 5.5 years; BMI 24.7 ± 2.1 kg/m2). Subjects were nonsmokers, medication free, consumed moderate caffeine (<300 mg/day) and alcohol doses (<2 drinks/day), reported no medical, psychiatric or sleep disorders, and passed a urine drug screen. No subject had worked night shifts or travelled across more than 1 time zone in the month preceding the study. The self-reported mean (± SD) sleep schedule in the week before the study was 00:04 ± 1.0 to 07:59 ± 1.0 hours. All subjects gave written informed consent prior to their participation. The study was approved by the Rush University Medical Center Institutional Review Board.
Protocol
The 3 week study consisted of two conditions, run in counterbalanced order and separated by a 5 day break. In each condition subjects were instructed to alter their lighting at home for 7 consecutive days beginning at a set clock time which was 4 hours before their habitual bedtime (determined from sleep diaries prior to study start) until a self-selected bedtime each night, at which time they turned off all lights. In the “dim” condition subjects were instructed to minimize their home lighting as much as possible, providing that they could still safely navigate their home environment. Subjects were provided “blue blockers” (visible light transmission 45%, light <540 nm transmission 2%, SCT-Orange Lens in Skyper frame, Uvex, Fürth, Germany) and were asked to put them on at the time they were instructed to dim their lights. They were permitted to remove the blue blockers at any time after this, providing that they noted the time and reason for removal on an event log. The blue blockers were included in the protocol as it was unclear what the intensity of light would be in the dim light condition, and in general how likely people are to wear blue blockers at home. In the “bright” condition subjects were instructed to maximize all of their home lighting, but were not to move their existing lighting or bring new lights into their home. After the 7 days in each condition, subjects came to the laboratory for a dim light phase assessment. There was a 5 day break between the two conditions where subjects were instructed to follow their usual sleep schedule. Four subjects completed the dim condition first and 4 subjects completed the bright condition first. Subjects were run in groups of two or three and the order of condition was counterbalanced within each group to control for the external photoperiod.
Light, sleep, mood and performance at home
Subjects wore two actigraphy monitors with photosensors (30 second epochs, Actiwatch-L, Respironics, Bend, OR): one monitor around their neck to measure light exposure, and one monitor on their nondominant wrist to measure activity (2, 3). The photosensor worn around the neck was removed and placed upwards on a bedside table during sleep times, which also permitted recording of bedroom light intensity. Activity on the photosensors around the neck was checked to ensure subjects wore them as instructed. The light data was edited to ensure zero lux when the wrist actigraphy (see below) indicated subjects were asleep. Daytime light exposure was examined by binning each day of light data from wake time to lights set time into 24 bins, each approximately 30 mins long, depending on the variation in wake duration each day. Light immediately surrounding the lights set time and wake time were examined with 30 mins bins averaged relative to bed and wake time as per previous studies (2, 3). Sunglasses were not permitted throughout the study. The subjects noted their bedtime and wake times on sleep diaries which guided the analysis of the wrist activity with the Actiware 5.70.1 program (Respironics, Bend, OR). Actigraphic estimates of sleep onset and offset times, total sleep time and wake after sleep onset were extracted. In the 15 minutes before their self-selected bed and wake times, subjects rated their mood on visual analogue scales (“sleepy”, “hungry”, “happy” on a 10 cm line anchored by “not very” to “very”) and completed a 5 minute psychomotor vigilance task (PVT) on a portable handheld device. The PVT variables mean reciprocal reaction time (RT), fastest 10% RT and slowest 10% reciprocal RT were extracted (14).
Circadian phase assessments
After each week of dim or bright home light, each subject participated in a dim light phase assessment in the laboratory to determine their dim light melatonin onset (DLMO) - a reliable marker of the circadian clock (15, 16). Methodological details of the phase assessments have been previously described (2, 3). Briefly, subjects remained awake and seated in dim light (<5 lux, at level of the eyes, in direction of gaze) and gave a saliva sample every 30 minutes from 7 hours before to 1 hour after their average bedtime. Subjects were not permitted to consume any alcohol or caffeine at least 24 hours before each phase assessment and were breathalyzed on arrival at the laboratory. Non-steroidal anti-inflammatory drugs were not permitted throughout the study. Saliva samples were radioimmunoassayed for melatonin by Solidphase Inc (Portland, ME) using commercially available ALPCO kits (sensitivity 0.5 pg/ml). A DLMO was calculated for each phase assessment and defined as the time (with linear interpolation) when the melatonin concentration exceeded the mean of 3 low consecutive daytime values plus twice the standard deviation of these points (17).
RESULTS
All statistical analyses included an order effect that was consistently not significant (p>0.05). Light exposure during the day, between wake time and lights set time, did not differ between the bright and dim light conditions (Condition main effect p=0.67, range 11–1580 lux). As expected, the only significant difference in light exposure between the conditions occurred in the 4 hours before bedtime (bright evening light (mean ±SD) 63.9 ± 10.5 lux (range 46.0–82.2 lux) vs. average dim light 2.7 ± 1.2 lux, (range 1.6–5.3 lux) Figure 1A). When corrected for blue blocker use, the average dim light decreased to 2.0 ± 1.1 lux. The blue blockers were relatively well tolerated, as on average subjects wore the blue blockers 69.8% of the time, removing them because of personal grooming (44%), going to sleep (22%), difficulty seeing (19%) or discomfort from the glasses (15%).
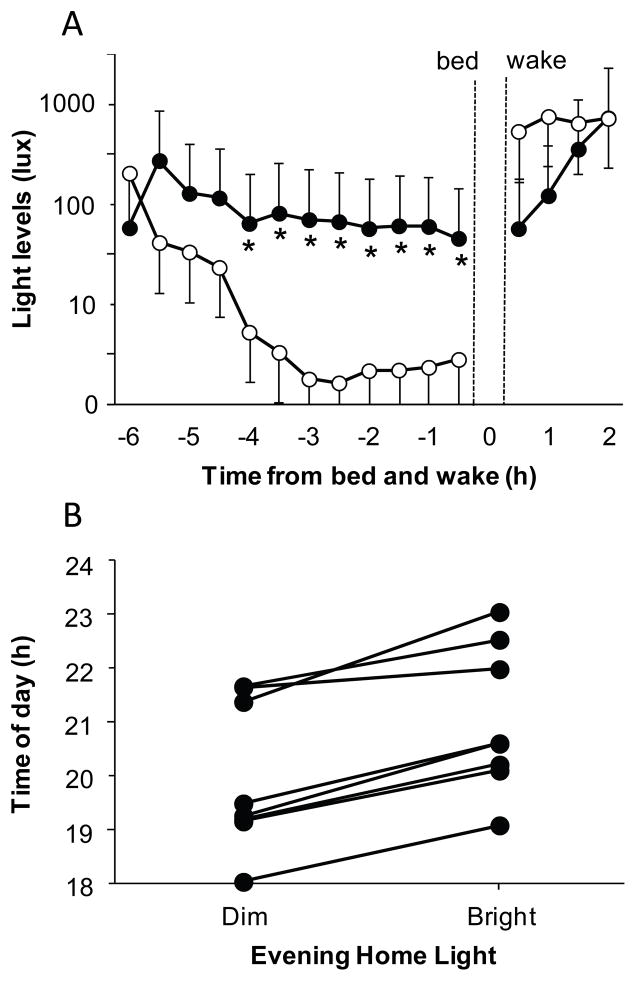
Figure 1.
A. Mean light levels in the hours before self-selected bedtime and after self-selected wake time. Asterisks indicate when the light intensity in the bright condition (closed circles) was significantly different to the light intensity in the dim condition (open circles; paired t-tests, all p≤0.024). Data is in 30 minute bins and error bars represent SEs. The brighter morning light in the dim condition was not significant, and was due to one subject going outside shortly after wake time on two mornings. B. The dim light melatonin onsets (DLMOs) observed in each individual subject after a week of dim evening or bright evening light at home. The DLMOs for each subject are connected by a line. All subjects had later DLMOs after the week of bright home light (p<0.001).
Every subject had a later DLMO after the week of bright home light than after the week of dim home light (average dim DLMO 19:59 ± 1.4 h vs. average bright DLMO 21:02 ±1.4 h, paired t-test p<0.001, Figure 1B). Subjects had a slightly later sleep onset time in the bright week, but there was no change in sleep offset, total sleep time or wake after sleep onset (see Table 1). Therefore the DLMO to sleep onset phase angle was longer after the dim light week than bright light week (average dim DLMO-bedtime interval 4.2 ± 1.1 h vs. average bright DLMO-bedtime interval 3.4 ± 1.1 h, paired t-test p<0.002). There was a trend towards subjects feeling less sleepy before bedtime in the bright than dim condition (5.1 ± 2.3 cm vs. 6.4 ± 2.4 cm, Time of Day x Condition interaction, p=0.079), and less hungry on the mornings after the evening bright home light (1.9 ± 1.7 cm vs. 3.4 ± 2.3 cm, Time of Day x Condition interaction, p=0.078), but no change in happiness (p=0.27). The mean reciprocal RT and fastest 10% RT on the PVT showed no change (p>0.12). However, the slowest 10% reciprocal RT results indicated subjects performed faster before bedtime (0.0025 ± 0.001 vs. 0.0024 ± 0.001 msec) but slower after wake time (0.0023 ± 0.0009 vs. 0.0025 ± 0.0009 msec) in the bright versus dim light condition (Condition x Time interaction, p=0.044).
Table 1.
Sleep parameters derived from wrist actigraphy in the dim versus bright condition.
| Dim Light | Bright Light | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Sleep onset (time)† | 00:13 | 1.0 h | 00:27 | 0.9 h |
| Sleep offset (time) | 07:47 | 1.1 h | 07:52 | 1.0 h |
| Total sleep time (mins) | 453.8 | 78.0 | 445.0 | 67.0 |
| Wake after sleep onset (mins) | 48.8 | 25.1 | 50.2 | 27.4 |
p≤0.05 Condition main effect
DISCUSSION
In this 3 week field study we measured the range of light intensities people can generate in their homes with their household lighting. We demonstrated for the first time that manipulating light intensity in the home environment with little change in sleep times can significantly impact circadian timing. During the evenings of dim light at home subjects successfully reduced their light exposure to very low levels (~3 lux), usually by restricting their light source to a single remote lamp and/or TV. The intermittent use of blue blockers would have further reduced exposure to short wavelength light, but regardless of individual use (ranging from 10–98% of the dim light time), all subjects showed similar changes in the timing of their DLMO. Given the very dim light levels subjects were able to create in their homes, the use of the blue blockers is unlikely to have had much additional effect. When subjects maximized their lights at home on average they were exposed to ~65 lux. As expected this is higher than the ~40 lux previously reported in field studies (2, 13), but lower than the ~100–200 lux levels used in laboratory based studies (11, 12). Thus laboratory simulations of home lighting should consider using room light of less than 100 lux. In the bright condition, subjects performed faster before bedtime, likely in part due to an alerting effect of the light. However, the following morning, they performed more slowly perhaps due to the relative delay in circadian timing. Notably, apart from a slightly later sleep onset, there were no other differences in sleep between conditions, although wrist actigraphy may not have reflected the subtle changes in sleep that can occur following bright light exposure before bedtime (11).
The results of this study highlight that the light intensity people can generate at home with their household lighting in the few hours before usual bedtime has potential to affect their circadian timing. In this field study there was no baseline condition, and it is possible that the DLMO advanced relative to baseline in the dim condition, or delayed relative to baseline in the bright condition, or both. However, as the DLMO to sleep onset phase angle lengthened to 4.2 h in the dim condition and there was only a small change in sleep onset, it appears likely that there was a phase advance in the DLMO in the dim condition. This result is consistent with a recent study that found significant phase advances in the DLMO when people went camping and were no longer exposed to artificial light (18). We also observed that the shift in the DLMO was remarkably consistent as every subject had later circadian timing (by ~ 1 hour) after maximizing versus minimizing their light exposure at home. Phase delays of ~1 hour have been shown to negatively impact alertness (4, 5), performance (6, 7), and subjective wellbeing (5), even in double blind conditions (5). While modest in size, when such phase delays occur chronically, they contribute to social jet lag which can have significant physical and mental health consequences (8–10). Thus these results corroborate clinical advice to patients with delayed sleep phase disorder to dim their household lights in the hours before bedtime to minimize further phase delays (19). More generally, these findings also add to the increasing recognition of the impact of evening light exposure on human circadian timing (12, 11, 20–22) and suggest that reducing household light exposure before bedtime is a simple and effective step towards reducing circadian misalignment.
Acknowledgments
We thank Dr Stephanie Crowley, Marissa Dziepak, Andrew Eiden, Amy Feehan, Brock Peiffer, Muneer Rizvydeen, Christina Suh and Asantewaa Ture for their assistance with data collection and analysis, Dr Louis Fogg for his statistical advice, and our Medical Director, Margaret Park, M.D. We also thank Professor Leon Lack for his comments on the manuscript. This project was supported by an R01 grant (HL083971) from the National Heart Lung and Blood Institute to HJB. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Heart Lung and Blood Institute or the National Institutes of Health. The National Heart Lung and Blood Institute and the National Institutes of Health had no involvement in designing the study, data collection, data analysis and interpretation, writing of the manuscript, nor in the decision to submit the manuscript for publication.
References
- 1.National Sleep Foundation. [Accessed on 3 December 2013];Sleep in America Poll Summary of Findings. 2011 Available at: http://www.sleepfoundation.org.
- 2.Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–118. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395:191–195. doi: 10.1016/j.neulet.2005.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep Biol Rhythms. 2008;6:172–179. [Google Scholar]
- 5.Yang CM, Spielman AJ, D’Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]
- 6.Yang CM, Spielman AJ. The effect of a delayed weekend sleep pattern on sleep and morning functioning. Psychol Health. 2001;16:715–725. [Google Scholar]
- 7.Burgess HJ, Legasto CS, Fogg LF, Smith MR. Can small shifts in circadian phase affect performance? Appl Ergon. 2012;44:109–111. doi: 10.1016/j.apergo.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 10.Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Hidalgo MP, Allebrandt KV. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28:771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- 11.Santhi N, Thorne HC, van der Veen DR, Johnsen S, Mills SL, Hommes V, Schlangen LJ, Archer SN, Dijk DJ. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 12.Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheuermaier K, Laffan AM, Duffy JF. Light exposure patterns in healthy older and young adults. J Biol Rhythms. 2010;25:113–122. doi: 10.1177/0748730410361916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–591. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 16.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 17.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 18.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, Johnston SH, Allen R, Kelly KA, Souetre E, Schultz PM, Starz KE. Phase-shifting effects of bright morning light as treatment for delayed sleep phase syndrome. Sleep. 1990;13:354–361. [PubMed] [Google Scholar]
- 20.Burgess HJ. Evening ambient light exposure can reduce circadian phase advances to morning light independent of sleep deprivation. J Sleep Res. 2013;22:83–88. doi: 10.1111/j.1365-2869.2012.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czeisler CA. Perspective: casting light on sleep deficiency. Nature. 2013;497:S13. doi: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- 22.Wahnschaffe A, Haedel S, Rodenbeck A, Stoll C, Rudolph H, Kozakov R, Schoepp H, Kunz D. Out of the lab and into the bathroom: evening short-term exposure to conventional light suppresses melatonin and increases alertness perception. Int J Mol Sci. 2013;14:2573–2589. doi: 10.3390/ijms14022573. [DOI] [PMC free article] [PubMed] [Google Scholar]