Abstract
Ants are powerful model systems for the study of cooperation and sociality. In this review, we discuss how recent advances in ant genomics have contributed to our understanding of the evolution and organization of insect societies at the molecular level.
Keywords: Ant, Social insect, Caste determination, Communication, Division of labor, Epigenetics, Genome, Genomics, Immunity, Model, Mutualism
Introduction
Ants are among the most ecologically diverse and successful animals on our planet. They have colonized most terrestrial environments, where they occupy keystone positions and have strong ecological impacts owing to their crucial roles as scavengers, predators, granivores, herbivores and mutualists [1]. Understanding the ecological dominance of ants requires investigation into the complex organization of their societies.
The past three years have seen the publication of seven ant genomes [2-8] (Table 1; Figure 1), an achievement that has given the community studying social insects an unprecedented opportunity to investigate ant societies at the molecular level. The ant genomes provide insights into social insect biology that are complementary to those provided by the honeybee genome published in 2006 [9] because ants have a wider variety of social structures, morphotypes, behaviors, colony sizes and diets.
Table 1.
Summary of key parameters of seven sequenced ant genomes
| Harpegnathos saltator | Linepithema humile | Camponotus floridanus | Pogonomyrmex barbatus | Solenopsis invicta | Atta cephalotes | Acromyrmex echinatior | Average | Range | |
|---|---|---|---|---|---|---|---|---|---|
| Depth of coverage | 104x | 23x | 102x | 12x | 70x | 19x | 123x | 64.7x | (12x-123x) |
| Assembly size (Mb) | 297 | 215.6 | 240 | 235 | 353 | 317 | 300 | 279.7 | (215.6-353) |
| Total genome size (Mb) | 330 | 250.8 | 313 | 267 | 608.15 | 303 | 335 | 343.9 | (250.8-608.15) |
| Contig N50 (bp) | 38,027 | 35,858 | 24,134 | 11,606 | 14,674 | 14,240 | 62,705 | 28,749.1 | (11,606-62,705) |
| Scaffold N50 (bp) | 59,8192 | 138,6360 | 602,923 | 793,749 | 720,578 | 5,154,504 | 1,094,267 | 1,478,653.3 | (598192-5154504) |
| G+C composition (%) | 45 | 38 | 34 | 37 | 36 | 33 | 34 | 36.7 | (33-45) |
| Protein-coding genes | 18,564 | 16,123 | 17,064 | 17,177 | 16,569 | 18,093 | 17,278 | 17,266.9 | (16,123-18,564) |
| Orthologs + co-orthologs | 11,695 | 12,860 | 11,433 | 12,857 | 12,590 | 12,617 | 12,121 | - | - |
| Species-specific genes | 6,869 | 3,263 | 5,631 | 4,320 | 3,979 | 5,476 | 5,157 | 4,956.4 | (3,263-6,869) |
| Manually curated genes | 400 | 1,000 | 400 | 1,200 | 0 | 522 | 200 | 531.7 | (0-1,200) |
| Genes with EST support (%) | 84 | 51 | 81 | 43 | 56 | 40 | 84 | 62.7 | (40-84) |
| microRNA | 159 | 71 | 96 | 100 | NA | 68 | 93 | 97.8 | (68-159) |
| Total repeat content (%) | 26.9 | 23.5 | 15.1 | 11.5 | NA | 25.1 | 28 | 21.7 | (11.5-28) |
EST, expressed sequence tag; NA, not available.
Figure 1.
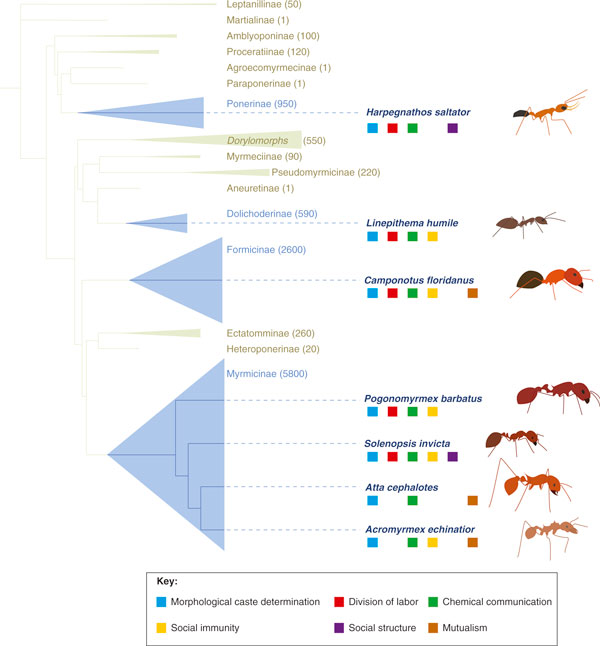
Phylogeny of the extant Formicidae. The figure highlights the subfamilies containing species with sequenced genomes (in blue). The heights of triangles are proportional to the number of described species in each subfamily (also indicated in parentheses). Branch lengths are proportional to the estimated divergence. The colored squares below the names of the sequenced species (on the right) indicate whether their genomes have been used to investigate the six topics discussed in this review (only the genome papers and all non-review publications citing the genome papers were considered). (Figure adapted from [81].)
In this review, we focus on six core aspects of ant biology: the production of alternative morphological castes, division of labor, chemical communication, alternative social organization, social immunity and mutualisms (Figure 2). While these issues have been extensively studied from a behavioral and physiological perspective, only now are we beginning to understand them at the molecular level [10-13]. For each issue, we discuss the advances provided by the ant genomes and their use in subsequent studies (Figure 3). Finally, we propose some avenues of research for future molecular studies.
Figure 2.
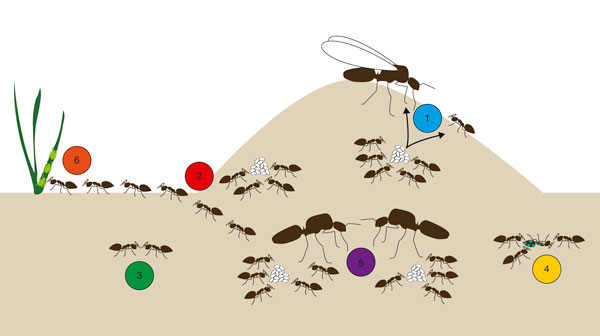
Diagram of a typical ant colony representing the six topics discussed in this review. (1) Morphological caste determination. In most ant species, a switch during larval development triggers alternative trajectories toward different female castes. Only the queen and one-worker caste are represented here, but some species also have different morphological worker castes. (2) Division of labor. Different groups of workers perform different tasks. Here, some workers nurse the brood inside the nest, whereas others forage for food outside. (3) Chemical communication. Ants rely on chemical communication for many aspects of their social organization. (4) Social immunity. Ants use both behavioral and physiological defenses to limit the transmission of pathogens and diseases in their societies. (5) Social structure. Ant species differ in the number of queens found in one nest, as well as the number of males that the queens mate with (not shown here). In some cases, these numbers vary between colonies of the same species. (6) Mutualism. Many ant species engage in mutualistic interactions with other organisms. Here, some ants tend and protect aphids in exchange for the sugary honeydew they produce.
Figure 3.
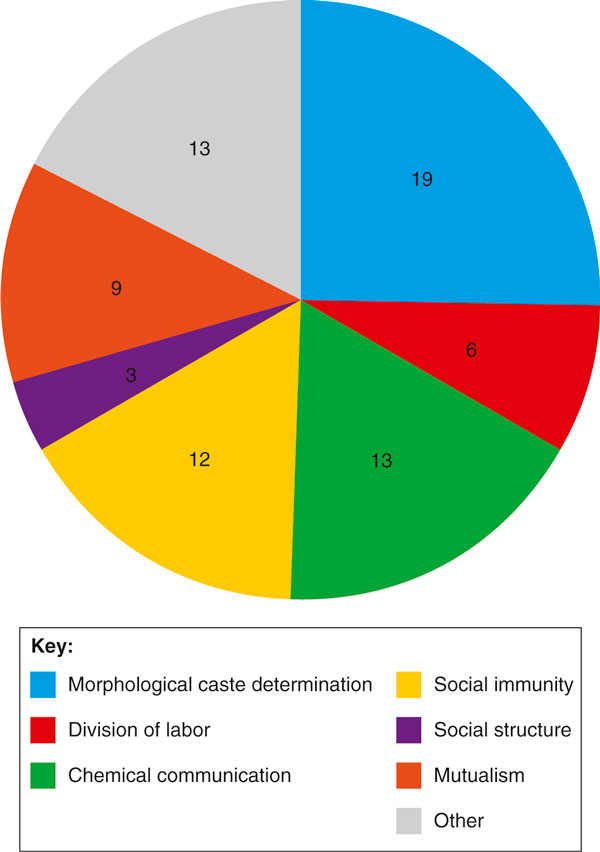
Pie chart representing the publication volume associated with each topic discussed in this review since the publication of the seven ant genomes. The numbers correspond to the number of studies investigating each topic, including the original genome papers and all publications on social insects citing the genome papers (reviews excluded). The category 'other' incorporates studies of topics that were not discussed in this review (such as sex determination, aging, circadian rhythms, invasiveness, metabolism and neurobiology), as well as studies focusing on social insects but not in a sociobiological context.
Caste determination and the division of labor
One of the most striking features of ants is the presence of queens and workers that can differ greatly in morphology, physiology and behavior. In some species, workers (which are all females) can also differ in size and morphology (such as minors, majors and soldiers). In some species, caste determination stems mostly from a developmental switch controlled by environmental factors, whereas, in others, strong genetic effects can also influence the process of caste determination. These genetic influences range from plastic genotypes that are biased toward queen or worker development to a strictly genetic determination [14]. Thus, ants provide an ideal system to investigate how differences in the social environment trigger developmental switches, changes in gene expression and the production of alternative phenotypes.
Recent studies implicate epigenetic processes in caste determination during larval development. Contrary to Drosophila fruit flies and Tribolium flour beetles, all sequenced ant and bee species have a complete set of DNA methylation enzymes [15]. Genome methylation in mammals and plants is widespread and primarily occurs in repetitive, transposable and regulatory elements, whereas it is found mostly in gene bodies in ants and bees, suggesting a fundamentally different function of DNA methylation [16-18]. In the carpenter ant Camponotus floridanus and the jumping ant Harpegnathos saltator, DNA methylation is correlated with caste-specific gene expression and alternative splicing, and patterns of DNA methylation change during development [16]. The genomes of the harvester ant Pogonomyrmex barbatus and the Argentine ant Linepithema humile revealed that annotated genes in the networks that underlie reproductive development, apoptosis and wing polyphenism have fewer CpG sites, the main target sites of DNA methylation, than the genome average [4,5]. This was interpreted as evidence for elevated rates of methylation in these genes because DNA methylation leads to increased mutation rates and thereby a depletion of CpG sites [19,20]. By contrast, the opposite pattern was observed in the leaf-cutting ant Atta cephalotes [6], suggesting that there might be important interspecific variation in how DNA methylation affects the production of alternative phenotypes. This is consistent with the recent finding that ant genomes exhibit distinct genome-wide depletion of observed relative to expected CpG sites [20]. Changes in gene transcription are also associated with histone modifications and changes in chromatin structure between castes and developmental stages in H. saltator and C. floridanus [2,21]. Both genome methylation and histone modification might therefore influence caste determination through transcriptional control and alternative splicing.
A recent comparative analysis suggested that the abundance and diversity of transcription factor binding sites (TFBSs) might also play a role in the evolution of caste-specific patterns of gene expression [20]. TFBSs were found to be more divergent within ants than between social and solitary insects, and genes exhibiting important changes in the abundance of TFBSs between social and solitary insects showed higher levels of gene expression plasticity between castes in C. floridanus (high polymorphism and reproductive division of labor) compared with H. saltator (low polymorphism and reproductive division of labor). Furthermore, the ant genomes revealed that the networks commonly known to exhibit phenotypic plasticity between castes (such as the neuroendocrine system) were preferentially targeted for regulatory changes during the evolution of sociality [20].
The genome sequences also facilitate the identification and study of candidate genes in the process of caste differentiation [5-7,20]. For example, analysis of insulin signaling, juvenile hormone and vitellogenin expression during artificial hibernation and hormone manipulation revealed how the environmental cues experienced by Pogonomyrmex rugosus queens are translated into the production of new queens and workers [22]. Similarly, the fire ant (Solenopsis invicta) was found to harbor two insulin receptors, which might play a role in the process of caste determination [23]. Finally, a study on molecular evolution in S. invicta revealed a positive association between caste-biased gene expression and the rate of gene evolution, the latter being driven largely by variation in the strength of purifying selection [24]. This study also showed that high rates of gene evolution actually preceded gene expression bias associated with the evolution of castes, suggesting that fast-evolving genes are more likely to be recruited to the processes underlying phenotypic plasticity [24].
Genetic effects on morphological caste determination have been found in L. humile [25] and the leaf-cutting ant Acromyrmex echinatior [26,27]. An extreme case of genetic caste determination occurs in the genus Pogonomyrmex, where some populations contain differentiated genetic lineages, most or all of which derive from historical hybridization between the harvester ants P. rugosus and P. barbatus [28-32]. These lineages always occur in pairs [33], and queens in each lineage-pair mate multiple times with males of their own as well as with males of the alternative lineage. Inter-lineage offspring develop into workers, whereas intra-lineage offspring develop into queens. Thus, the only way that a queen can produce a colony with both workers and queens is by mating with males of both lineages. Crossing experiments revealed that intra-lineage individuals are developmentally constrained to become queens [32]. Inter-lineage individuals have partly retained plasticity and can develop into queens under some conditions, but the association of genotype and caste is very strong, with almost no adult females presenting a mismatch between the genotype and expected phenotype [34]. This provides an interesting system to compare the epigenetic and gene expression changes across developmental stages between individuals for which caste fate is already known [17].
In addition to the reproductive division of labor between queens and workers, there is also usually a strong division of labor between workers, which specialize in different tasks. Worker behavior is influenced by several factors, including size and morphology [35-37], age [38,39], individual experience [40,41] and genetic background [42,43]. Worker behavior and task specialization in ants are often regulated by genes that also affect behavior in solitary insects (the foraging gene and circadian clock genes, for example) [44-48].
Reproduction and behavior are often interconnected in animals. In solitary wasps, for instance, females with developed ovaries lay eggs, whereas females with undeveloped ovaries forage for food. Accordingly, studies in the honeybee Apis mellifera suggest that physiological pathways regulating reproduction and behavior in solitary insects have been co-opted for the regulation of worker behavior in social insect species [49,50]. Studies in the ant Pogonomyrmex californicus revealed that nurses and foragers differ in ovary activity [51] and juvenile hormone levels [52], which are known to affect the production of vitellogenin (typically involved in reproduction) [22]. Interestingly, phylogenetic analyses revealed the existence of multiple genes encoding vitellogenin in most of the ant genomes sequenced [53], suggesting that an initial duplication of the ancestral vitellogenin gene occurred after ants diverged from bees and wasps. The ant vitellogenin genes cluster in two paralogous gene families that show caste- and behavior-specific expression in S. invicta [7] and P. rugosus [53], suggesting that vitellogenin in ants not only regulates reproduction but also the behavior of sterile workers [53]. The finding that vitellogenin plays similar roles in ants and bees, which evolved sociality independently, supports the hypothesis that the co-option of reproductive pathways plays a major role in social evolution.
Chemical communication
Effective communication is an important ingredient for any society to function efficiently. Ants rely heavily on chemical communication for social organization and to discriminate nestmates from non-nestmates [1]. The importance of chemical communication in ants is reflected by large expansions of chemosensory gene families and metabolic pathways for cuticular hydrocarbons in all ant genomes that have been studied in this respect compared with other sequenced hymenopterans [2,4,5,7,54]. A comparative analysis of antennal transcriptomes between C. floridanus and H. saltator identified many chemoreceptors differentially expressed between males and females, as well as between species, suggesting that sex- and species-specific biology is likely to have shaped the expression patterns of genes involved in communication [54].
A recent study of chemosensory protein genes revealed contrasting modes of evolution between genes occurring only in ants and those also found in the honeybee [55]. Clades with ant-specific expansions showed evidence of faster evolution and elevated levels of positive selection compared with clades of one-to-one orthologs across all ants and the honeybee. A possible explanation is that the more conserved chemosensory protein genes that occur in both ants and bees are associated with more general features of social insect biology, whereas genes in ant-specific expansions might be related to more idiosyncratic environmental and social conditions [55]. At the same time, ant-specific desaturase gene families, which are involved in the production of chemical signals, show an elevated number of genes and high variability in both sequence and expression, possibly reflecting an increased demand for diversity in the chemical signals used in ant communication [20].
Social structure, social immunity and mutualisms
There is tremendous variation across ant species in social organization and the number of queens per nest. Such variation in the breeding system can also occur within species, as in S. invicta, where colonies can have one queen (monogyne) or many queens (polygyne). As is the case in other ants, the two social forms not only differ in the number of queens but also in many other traits (such as the reproductive potential of queens, odor and size of both queens and workers, aggressiveness of workers and number of sperms produced by males) [7,56-58]. The social phenotype of S. invicta is completely associated with two allelic variants at a single locus (Gp-9) encoding an odorant binding protein [56,59,60]. Gp-9 was recently found to be located on a pair of heteromorphic social chromosomes (SB and Sb) comprising a large (12.7 Mb) non-recombining genomic region [57]. It is likely that several of the 600-plus genes in the non-recombining region are involved in the many phenotypic differences characterizing individuals harboring the alternative social chromosomes. Comparative studies revealed that these social chromosomes have many properties typical of sex chromosomes. First, the lack of recombination is also associated with several inversions. Second, one of the two variants (the Sb chromosome) occurs only in one type of social organization (the polygyne form), just as the Y chromosome occurs only in males. Third, the Sb chromosome cannot recombine with itself because individuals having two copies of this chromosome die within weeks after reaching the adult stage. Finally, the inability of the Sb chromosome to recombine with itself or with the SB chromosome has been associated with the accumulation of many repetitive elements, in a manner similar to that of the Y chromosome [57]. While this is the first description of a social chromosome, it is likely that such supergenes also exist in other social insects. Polymorphism in social organization has evolved independently numerous times in ants, where many species have both monogyne and polygyne colonies. The occurrence of the polygyne social form is associated almost invariably with a polygyny syndrome whereby, as in S. invicta, polygyne queens are smaller, accumulate reduced amounts of fat during sexual maturation, have lower fecundity and initiate new colonies with the help of workers, rather than independently [61]. Interestingly, it appears that variation in queen number is also associated with a single non-recombining region in the ant Formica selysi (J Purcell, A Brelsford and M Chapuisat, unpublished data).
Another important aspect of social life is that it facilitates the transmission of pathogens and diseases owing to the associated high population densities and frequent social contacts [62]. Thus, one could expect social insects to have more efficient immune systems and more genes involved in immunity compared with solitary species. Surprisingly, early comparative analyses reported that both the honeybee and the ants have fewer immune genes than do Drosophila melanogaster and Tribolium castaneum [4-6,9,63-66]. Social insects have multiple collective behavioral defenses against pathogens, such as grooming other colony members [67] or the intake of tree resin with anti-pathogenic properties [68]. It has thus been proposed that such prophylactic behaviors might reduce the selective pressure for increasing the number of immune genes in the genome [4-6]. However, a recent comparative-genomic analysis shows that only 3 of 16 immune-gene families differed significantly between social and solitary insect species [20]. This finding, combined with the fact that the non-social parasitoid wasp Nasonia vitripennis also contains fewer immune genes than do flies and beetles [69], suggests that the depletion of immune genes in social insects is not as dramatic as initially proposed and might not be directly associated with sociality.
Specific features of the ant immune system could also account for the lower number of immune genes in their genomes. The genome of C. floridanus revealed the antimicrobial peptide hymenoptaecin to be a large precursor protein with multiple bioactive domains [65,66], suggesting a diverse array of possible immune functions. Hymenoptaecin is present in all other ants, the honeybee, as well as Nasonia vitripennis [5,65,69]. Finally, behavioral analyses combined with RNA-seq of genes implicated in physiological immune defenses in A. echinatior confirmed the existence of efficient prophylactic behaviors and showed that, in most conditions, pathogenic challenges triggered an increase in immune gene expression. However, ants challenged with a fungus-garden pathogen showed a decrease in immune gene expression while displaying more prophylactic behaviors, suggesting that trade-offs might occur between physiological and behavioral immune responses [70].
Another key type of interspecific interaction in ants lies in the evolution of diverse and ecologically important forms of mutualisms with plants (Acacia trees, for example) [71] and other insects (such as aphids) [72]. Approximately 50 million years ago, one ant clade also evolved a complex system of fungus farming [73]. The current repertoire of sequenced ant genomes includes two species of fungus farming leaf-cutting ants (A. cephalotes and A. echinatior), which cultivate their food fungus on leaf fragments harvested from the vegetation surrounding their nest [1]. The analysis of their genomes revealed that the genes necessary for the biosynthesis of the amino acid arginine are lacking (in contrast to C. floridanus, S. invicta and H. saltator), but that they are present in their symbiotic fungus [3,6]. This suggests that leaf-cutting ants have become completely dependent on their food fungus over evolutionary time. These findings highlight the benefit of genomic resources for identifying the potential physiological consequences of evolution in complex societies and pave the way for further studies to better understand the genomic impact of obligate mutualisms in ants and other organisms.
Concluding remarks
Analysis of the seven sequenced ant genomes has already led to significant advances in our understanding of important aspects of ant biology. Below, we highlight three avenues of research that we believe will prove fruitful as this endeavor continues.
First, large comparative analyses of the genomes of ants, social bees and social wasps (which evolved sociality independently) with the genomes of solitary bees, wasps and other insects will be needed to investigate the key evolutionary changes associated with sociality in the Hymenoptera. The only study that has conducted such a comparative analysis so far has provided valuable information on the evolution of several aspects of social organization (caste determination, chemical communication and social immunity), as discussed above [20].
A second important step will be to perform functional studies to validate experimentally the numerous hypotheses generated by comparative analyses. So far, only a handful of studies have manipulated gene expression in ants using RNA interference [74,75], hormonal treatments [22,76] or pharmacological manipulations [47], and none of them investigated the consequences at the genome scale. Forward genetics, using, for example, random mutagenesis, has been prohibitive in ants and other social insects because these approaches require substantial subsequent crossing. This is not feasible in ants mainly owing to their long generation times and the difficulty to breed most species in the laboratory. However, reverse-genetic approaches for social insects using new and highly effective tools for targeted genome editing have now come within reach. These approaches use engineered nucleases, such as zinc-finger nucleases (ZFNs) [77], transcription activator-like effector nucleases (TALENs) [78] or CRISPR RNA-guided Cas9 nucleases [79,80]. Although these techniques will not be able to overcome the fundamental experimental limitations posed by many ant species, they are now opening up the possibility of creating transgenics and genetic knockouts for a subset of carefully chosen model species that can be propagated in the laboratory. Studies using combinations of genetic, pharmacological, social, hormonal and pheromonal manipulations will be necessary to provide a better understanding of the actual roles of different genes and physiological pathways in regulating ant social life.
Finally, understanding the molecular organization of ant societies will require precise behavioral data at the individual level to investigate the links between communication, gene expression, physiology and behavior. Collecting such data has long been a difficult task, but a new automated tracking system now allows the automatic quantification of all social interactions between all individuals in an ant colony over the course of several weeks. Using this system has already demonstrated the importance of spatial distribution in the regulation of age-related division of labor in insect societies [39]. The use of such sophisticated behavioral tracking and quantification, combined with next-generation genomic data, could well provide the next answers to the big questions in the biology of social insects.
In conclusion, social insects have played a central role in our understanding of the organization and behaviors of complex animal societies, principally from ethological and ecological perspectives. The advent of next-generation sequencing techniques now provides opportunities to use social insects to study how genetic and environmental contributions interact to control societal organization.
Contributor Information
Romain Libbrecht, Email: romain.libbrecht@gmail.com.
Peter R Oxley, Email: poxley@mail.rockefeller.edu.
Daniel JC Kronauer, Email: dkronauer@mail.rockefeller.edu.
Laurent Keller, Email: laurent.keller@unil.ch.
Acknowledgements
We thank Sean McKenzie for comments on the manuscript. This work was supported by a Marie Curie postdoctoral fellowship to RL, a Leon-Levy postdoctoral fellowship to PRO, grant 1DP2GM105454-01 from the NIH to DJCK and several grants from the Swiss National Science Foundation and an ERC advanced grant to LK.
References
- Holldobler B, Wilson E. The Ants. Belknap Press; 1990. [Google Scholar]
- Bonasio R, Zhang G, Ye C, Mutti NS, Fang X, Qin N, Donahue G, Yang P, Li Q, Li C, Zhang P, Huang Z, Wang J, Leibig J. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science. 2010;14:1068–1071. doi: 10.1126/science.1192428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard S, Zhang G, Schiott M, Li C, Wurm Y, Hu H, Zhou J, Ji L, Qiu F, Rasmussen M, Pan H, Hauser F, Krogh A, Grimmelikhuizen CJP, Wang J, Boomsma J. The genome of the leaf-cutting ant Acromyrmex echinatior suggests key adaptations to advanced social life and fungus farming. Genome Res. 2011;14:1339–1348. doi: 10.1101/gr.121392.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Zimin A, Holt C, Abouheif E, Benton R, Cash E, Croset V, Currie CR, Elhaik E, Elsik CG, Fave MJ, Fernandes V, Gadau J, Gibson JD, Graur D, Grubbs KJ, Hagen DE, Helmkampf M, Holley J-A, Hu H, Viniegra ASI, Johnson BR, Johnson RM, Khila A, Kim JW, Laird J, Mathis KA, Moellert JA, Muñoz-Torres MC, Murphy MC. et al. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc Natl Acad Sci USA. 2011;14:5673–5678. doi: 10.1073/pnas.1008617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR, Smith CD, Robertson HM, Helmkampf M, Zimin A, Yandell M, Holt C, Hu H, Abouheif E, Benton R, Cash E, Croset V, Currie CR, Elhaik E, Elsik CG, Fave M-J, Fernandes V, Gibson JD, Graur D, Gronenberg W, Grubbs KJ, Hagen DE, Viniegra ASI, Johnson BR, Johnson RM, Khila A, Kim JW, Mathis KA, Murphy MC. et al. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc Natl Acad Sci USA. 2011;14:5667–5672. doi: 10.1073/pnas.1007901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen G, Teiling C, Li L, Holt C, Abouheif E, Bornberg-Bauer E, Bouffard P, Caldera EJ, Cash E, Cavanaugh A, Denas O, Elhaik E, Fave M-J, Gadau J, Gibson JD, Graur D, Grubbs KJ, Hagen DE, Harkins TT, Helmkampf M, Hu H, Johnson BR, Kim J, Marsh SE, Moeller JA, Murphy MC, Naughton MC, Nigam S, Overson R. et al. The genome sequence of the leaf-cutter ant Atta cephalotes reveals insights into its obligate symbiotic lifestyle. PLoS Genet. 2011;14:e1002007. doi: 10.1371/journal.pgen.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm Y, Wang J, Riba-Grognuz O, Corona M, Nygaard S, Hunt BG, Ingram KK, Falquet L, Nipitwattanaphon M, Gotzek D, Dijkstra MB, Oettler J, Comtesse F, Shih C-J, Wu W-J, Yang C-C, Thomas J, Beaudoing E, Pradervand S, Flegel V, Cook ED, Fabbretti R, Stockinger H, Li L, Farmerie WG, Oakey J, Boomsma JJ, Pamilo P, Yi SV, Heinze J. et al. The genome of the fire ant Solenopsis invicta. Proc Natl Acad Sci USA. 2011;14:5679–5684. doi: 10.1073/pnas.1009690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadau J, Helmkampf M, Nygaard S, Roux J, Simola DF, Smith CR, Suen G, Wurm Y, Smith CD. The genomic impact of 100 million years of social evolution in seven ant species. Trends Genet. 2012;14:14–21. doi: 10.1016/j.tig.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock GM, Robinson GE, Gibbs RA, Worley KC, Evans JD, Maleszka R, Robertson HM, Weaver DB, Beye M, Bork P. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;14:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman BJ, Woodard SH, Robinson GE. Molecular evolutionary analyses of insect societies. Proc Natl Acad Sci USA. 2011;14:10847–10854. doi: 10.1073/pnas.1100301108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf AC, Benton R, Keller L. The molecular basis of social behavior: models, methods and advances. Curr Opin Neurobiol. 2013;14:3–10. doi: 10.1016/j.conb.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Smith C, Toth A, Suarez A, Robinson G. Genetic and genomic analyses of the division of labour in insect societies. Nat Rev Genet. 2008;14:735–748. doi: 10.1038/nrg2429. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: social life in molecular terms. Nat Rev Genet. 2005;14:257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- Schwander T, Lo N, Beekman M, Oldroyd B, Keller L. Nature versus nurture in social insect caste differentiation. Trends Ecol Evol. 2010;14:275–282. doi: 10.1016/j.tree.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Drewell RA, Lo N, Oxley PR, Oldroyd BP. Kin conflict in insect societies: a new epigenetic perspective. Trends Ecol Evol. 2012;14:367–373. doi: 10.1016/j.tree.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Bonasio R, Li Q, Lian J, Mutti NS, Jin L, Zhao H, Zhang P, Wen P, Xiang H, Ding Y, Jin Z, Shen SS, Wang Z, Wang W, Wang J, Berger SL, Liebig J, Zhang G, Reinberg D. Genome-wide and caste-specific DNA methylomes of the ants Camponotus floridanus and Harpegnathos saltator. Curr Biol. 2012;14:1755–1764. doi: 10.1016/j.cub.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR, Mutti NS, Jasper WC, Naidu A, Smith CD, Gadau J. Patterns of DNA methylation in development, division of labor and hybridization in an ant with genetic caste determination. PLoS One. 2012;14:e42433. doi: 10.1371/journal.pone.0042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glastad K, Hunt B, Yi S, Goodisman M. DNA methylation in insects: on the brink of the epigenomic era. Insect Mol Biol. 2011;14:553–565. doi: 10.1111/j.1365-2583.2011.01092.x. [DOI] [PubMed] [Google Scholar]
- Elango N, Hunt BG, Goodisman MAD, Yi SV. DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci USA. 2009;14:11206–11211. doi: 10.1073/pnas.0900301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola DF, Wissler L, Donahue G, Waterhouse RM, Helmkampf M, Roux J, Nygaard S, Glastad K, Hagen DE, Viljakainen L, Reese JT, Hunt BG, Graur D, Elhaik E, Kriventseva E, Wen J, Parker BJ, Cash E, Privman E, Childers CP, Munos-Torres MC, Boomsma JJ, Bornberg-Bauer E, Currie C, Elsik CG, Suen G, Goodisman MAD, Keller L, Liebig J, Rawls A, Social insect genomes exhibit dramatic evolution in gene composition and regulation while preserving regulatory features linked to sociality. Genome Res. 2013. doi: 10.1101/gr.155408.113. [DOI] [PMC free article] [PubMed]
- Simola DF, Ye C, Mutti NS, Dolezal K, Bonasio R, Liebig J, Reinberg D, Berger SL. A chromatin link to caste identity in the carpenter ant Camponotus floridanus. Genome Res. 2013;14:486–496. doi: 10.1101/gr.148361.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht R, Corona M, Wende F, Azevedo DO, Serrao JE, Keller L. Interplay between insulin signaling, juvenile hormone and vitellogenin regulates maternal effects on polyphenism in ants. Proc Natl Acad Sci USA. 2013;14:11050–11055. doi: 10.1073/pnas.1221781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HL, Pietrantonio P. Insect insulin receptors: insights from sequence and caste expression analyses of two cloned hymenopteran insulin receptor cDNAs from the fire ant. Insect Mol Biol. 2011;14:637–649. doi: 10.1111/j.1365-2583.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- Hunt BG, Ometto L, Wurm Y, Shoemaker D, Soojin VY, Keller L, Goodisman MA. Relaxed selection is a precursor to the evolution of phenotypic plasticity. Proc Natl Acad Sci USA. 2011;14:15936–15941. doi: 10.1073/pnas.1104825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht R, Schwander T, Keller L. Genetic components to caste allocation in a multiple-queen ant species. Evolution. 2011;14:2907–2915. doi: 10.1111/j.1558-5646.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- Hughes W, Boomsma J. Genetic royal cheats in leaf-cutting ant societies. Proc Natl Acad Sci USA. 2008;14:5150–5153. doi: 10.1073/pnas.0710262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WOH, Sumner S, Van Borm S, Boomsma JJ. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc Natl Acad Sci USA. 2003;14:9394–9397. doi: 10.1073/pnas.1633701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms Cahan S, Parker JD, Rissing SW, Johnson RA, Polony TS, Weiser MD, Smith DR. Extreme genetic differences between queens and workers in hybridizing Pogonomyrmex harvester ants. Proc R Soc Lond B Bio. 2002;14:1871–1877. doi: 10.1098/rspb.2002.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian GE, Fewell JH, Gadau J, Johnson RA, Larrabee D. Genetic determination of the queen caste in an ant hybrid zone. Proc Natl Acad Sci USA. 2002;14:8157–8160. doi: 10.1073/pnas.112222099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volny VP, Gordon DM. Genetic basis for queen-worker dimorphism in a social insect. Proc Natl Acad Sci USA. 2002;14:6108–6111. doi: 10.1073/pnas.092066699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms Cahan SH, Keller L. Complex hybrid origin of genetic caste determination in harvester ants. Nature. 2003;14:306–309. doi: 10.1038/nature01744. [DOI] [PubMed] [Google Scholar]
- Helms Cahan SH, Julian GE, Rissing SW, Schwander T, Parker JD, Keller L. Loss of phenotypic plasticity generates genotype-caste association in harvester ants. Curr Biol. 2004;14:2277–2282. doi: 10.1016/j.cub.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Schwander T, Helms Cahan SH, Keller L. Characterization and distribution of Pogonomyrmex harvester ant lineages with genetic caste determination. Mol Ecol. 2007;14:367–387. doi: 10.1111/j.1365-294X.2006.03124.x. [DOI] [PubMed] [Google Scholar]
- Schwander T, Keller L, Helms Cahan SH. Two alternate mechanisms contribute to the persistence of interdependent lineages in Pogonomyrmex harvester ants. Mol Ecol. 2007;14:3533–3543. doi: 10.1111/j.1365-294X.2007.03407.x. [DOI] [PubMed] [Google Scholar]
- Wilson EO. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behav Ecol Sociobiol. 1980;14:143–156. doi: 10.1007/BF00299520. [DOI] [Google Scholar]
- Detrain C, Pasteels J. Caste differences in behavioral thresholds as a basis for polyethism during food recruitment in the ant, Pheidole pallidula (Hymenoptera: Myrmicinae). J Insect Behav. 1991;14:157–176. doi: 10.1007/BF01054609. [DOI] [Google Scholar]
- Robinson E, Feinerman O, Franks N. Flexible task allocation and the organization of work in ants. Proc R Soc Lond B Bio. 2009;14:4373–4380. doi: 10.1098/rspb.2009.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EO. The Insect Societies. Cambridge, MA: Harvard University Press; 1971. [Google Scholar]
- Mersch DP, Crespi A, Keller L. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science. 2013;14:1090–1093. doi: 10.1126/science.1234316. [DOI] [PubMed] [Google Scholar]
- Theraulaz G, Bonabeau E, Deneubourg J. Threshold reinforcement and the regulation of division of labour in social insects. Proc R Soc Lond B Bio. 1998;14:327–333. doi: 10.1098/rspb.1998.0299. [DOI] [Google Scholar]
- Ravary F, Lecoutey E, Kaminski G, Chaline N, Jaisson P. Individual experience alone can generate lasting division of labor in ants. Curr Biol. 2007;14:1308–1312. doi: 10.1016/j.cub.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Oldroyd BP, Fewell JH. Genetic diversity promotes homeostasis in insect colonies. Trends Ecol Evol. 2007;14:408–413. doi: 10.1016/j.tree.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Libbrecht R, Keller L. Genetic compatibility affects division of labor in the argentine ant Linepithema humile. Evolution. 2012;14:517–524. doi: 10.1111/j.1558-5646.2012.01792.x. [DOI] [PubMed] [Google Scholar]
- Ingram KK, Kleeman L, Peteru S. Differential regulation of the foraging gene associated with task behaviors in harvester ants. BMC Ecol. 2011;14:19. doi: 10.1186/1472-6785-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram KK, Krummey S, LeRoux M. Expression patterns of a circadian clock gene are associated with age-related polyethism in harvester ants, Pogonomyrmex occidentalis. BMC Ecol. 2009;14:7. doi: 10.1186/1472-6785-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram KK, Kutowoi A, Wurm Y, Shoemaker D, Meier R, Bloch G. The molecular clockwork of the fire ant Solenopsis invicta. PLoS One. 2012;14:e45715. doi: 10.1371/journal.pone.0045715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C, Sokolowski MB. Molecular basis for changes in behavioral state in ant social behaviors. Proc Natl Acad Sci USA. 2009;14:6351–6356. doi: 10.1073/pnas.0809463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Ben-Shahar Y. Social behavior and comparative genomics: new genes or new gene regulation? Genes Brain Behav. 2002;14:197–203. doi: 10.1034/j.1601-183X.2002.10401.x. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc Natl Acad Sci USA. 2003;14:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE Jr. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Natl Acad Sci USA. 2004;14:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal AG, Johnson J, Hölldobler B, Amdam GV. Division of labor is associated with age-independent changes in ovarian activity in Pogonomyrmex californicus harvester ants. J Insect Physiol. 2013;14:519–524. doi: 10.1016/j.jinsphys.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Dolezal AG, Brent CS, Hölldobler B, Amdam GV. Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. J Exp Biol. 2012;14:454–460. doi: 10.1242/jeb.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona M, Libbrecht R, Wurm Y, Riba-Grognuz O, Studer RA, Keller L. Vitellogenin underwent subfunctionalization to acquire caste and behavioral specific expression in the harvester ant Pogonomyrmex barbatus. PLoS Genet. in press . [DOI] [PMC free article] [PubMed]
- Zhou X, Slone JD, Rokas A, Berger SL, Liebig J, Ray A, Reinberg D, Zwiebel LJ. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 2012;14:e1002930. doi: 10.1371/journal.pgen.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulmuni J, Wurm Y, Pamilo P. Comparative genomics of chemosensory protein genes reveals rapid evolution and positive selection in ant-specific duplicates. Heredity. 2013;14:538–547. doi: 10.1038/hdy.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L, Ross K. Selfish genes: a green beard in the red fire ant. Nature. 1998;14:573–575. doi: 10.1038/29064. [DOI] [Google Scholar]
- Wang J, Wurm Y, Nipitwattanaphon M, Riba-Grognuz O, Huang Y-C, Shoemaker D, Keller L. A Y-like social chromosome causes alternative colony organization in fire ants. Nature. 2013;14:664–668. doi: 10.1038/nature11832. [DOI] [PubMed] [Google Scholar]
- Lawson LP, Vander Meer RK, Shoemaker D. Male reproductive fitness and queen polyandry are linked to variation in the supergene Gp-9 in the fire ant Solenopsis invicta. Proc R Soc B Bio. 2012;14:3217–3222. doi: 10.1098/rspb.2012.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger MJ, Ross KG. Identification of a major gene regulating complex social behavior. Science. 2002;14:328–332. doi: 10.1126/science.1065247. [DOI] [PubMed] [Google Scholar]
- Ross KG, Keller L. Genetic control of social organization in an ant. Proc Natl Acad Sci USA. 1998;14:14232–14237. doi: 10.1073/pnas.95.24.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke AFG, Franks NR. Social Evolution in Ants. Princeton University Press; 1995. [Google Scholar]
- Cremer S, Armitage SA, Schmid-Hempel P. Social immunity. Curr Biol. 2007;14:R693–R702. doi: 10.1016/j.cub.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Bucher G, Friedrich M, Grimmelikhuijzen CJ. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;14:949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF. The genome sequence of Drosophila melanogaster. Science. 2000;14:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Ratzka C, Förster F, Liang C, Kupper M, Dandekar T, Feldhaar H, Gross R. Molecular characterization of antimicrobial peptide genes of the carpenter ant Camponotus floridanus. PLoS One. 2012;14:e43036. doi: 10.1371/journal.pone.0043036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzka C, Liang C, Dandekar T, Gross R, Feldhaar H. Immune response of the ant Camponotus floridanus against pathogens and its obligate mutualistic endosymbiont. Insect Biochem Mol Biol. 2011;14:529–536. doi: 10.1016/j.ibmb.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Parasites in Social Insects. Princeton University Press; 1998. [Google Scholar]
- Christe P, Oppliger A, Bancala F, Castella G, Chapuisat M. Evidence for collective medication in ants. Ecol Lett. 2003;14:19–22. [Google Scholar]
- Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;14:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yek SH, Boomsma JJ, Schiøtt M. Differential gene expression in Acromyrmex leaf-cutting ants after challenges with two fungal pathogens. Mol Ecol. 2013;14:2173–2187. doi: 10.1111/mec.12255. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Coevolution of mutualism between ants and acacias in Central America. Evolution. 1966;14:249–275. doi: 10.2307/2406628. [DOI] [PubMed] [Google Scholar]
- Way MJ. Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol. 1963;14:307–344. doi: 10.1146/annurev.en.08.010163.001515. [DOI] [Google Scholar]
- Schultz TR, Brady SG. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA. 2008;14:5435–5440. doi: 10.1073/pnas.0711024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M-Y, Vander Meer RK, Coy M, Scharf ME. Phenotypic impacts of PBAN RNA interference in an ant, Solenopsis invicta, and a moth, Helicoverpa zea. J Insect Physiol. 2012;14:1159–1175. doi: 10.1016/j.jinsphys.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Lu HL, Vinson S, Pietrantonio PV. Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS. 2009;14:3110–3123. doi: 10.1111/j.1742-4658.2009.07029.x. [DOI] [PubMed] [Google Scholar]
- Rajakumar R, San Mauro D, Dijkstra MB, Huang MH, Wheeler DE, Hiou-Tim F, Khila A, Cournoyea M, Abouheif E. Ancestral developmental potential facilitates parallel evolution in ants. Science. 2012;14:79–82. doi: 10.1126/science.1211451. [DOI] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;14:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2012;14:49–55. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O'Connor-Giles KM. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013. in press . [DOI] [PMC free article] [PubMed]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JJ, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;14:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau CS. Inferring ant evolution in the age of molecular data (Hymenoptera: Formicidae). Myrmecol News. 2009;14:201–210. [Google Scholar]


