Abstract
To advance our understanding of how the brain makes food decisions, it is essential to combine knowledge from two fields that have not yet been well integrated: the neuro-computational basis of decision-making and the homeostatic regulators of feeding. This Review integrates these two literatures from a neuro-computational perspective, with an emphasis in describing the variables computed by different neural systems and how they affect dietary choice. We highlight what is unique about feeding decisions, the mechanisms through which metabolic and endocrine factors affect the decision-making circuitry, why making healthy food choices is difficult for many people, and key processes at work in the obesity epidemic.
How do we choose what to eat? How is this decision different from choosing a pair of shoes? Why is consistent dieting rare and difficult? These are basic questions in behavioral neuroscience, and important ones, as our ability to address the obesity epidemic depends on our ability to answer them.
Solving these questions requires bringing together two areas of study that have been separated for too long: the neuro-computational basis of decision-making1,2, and the homeostatic regulation of feeding3. Decision-making research has focused on characterizing the computational and neurobiological substrates of choice that are common to many domains, from feeding to financial decisions to social exchange. In contrast, research on homeostatic regulation has focused on characterizing systems that are specific to feeding, and has paid limited attention to how they interact with the rest of the decision-making circuitry.
Here we examine how advances in both fields have made possible the beginnings of a synthesis with the potential to generate new insights, questions and applications. We review extensive evidence showing that a common set of processes is at work across virtually all of the types of decisions that have been studied, including food choices1,2. At the same time, it is well known that metabolic and endocrine factors have powerful effects on feeding3–5. This strongly suggests that these factors exert their influence by modulating the operations of the decision-making circuitry.
Our Review takes a neuro-computational perspective, which requires characterizing the variables computed by different neural systems and how they affect different types of decisions. We integrate up-to-date knowledge from decision neuroscience with what is known about the homeostatic regulation of feeding. We use this knowledge to propose answers to the following questions: what is unique about feeding decisions and why is making healthy food choices difficult to many people. Finally, we apply these concepts to the problem of obesity.
Framework
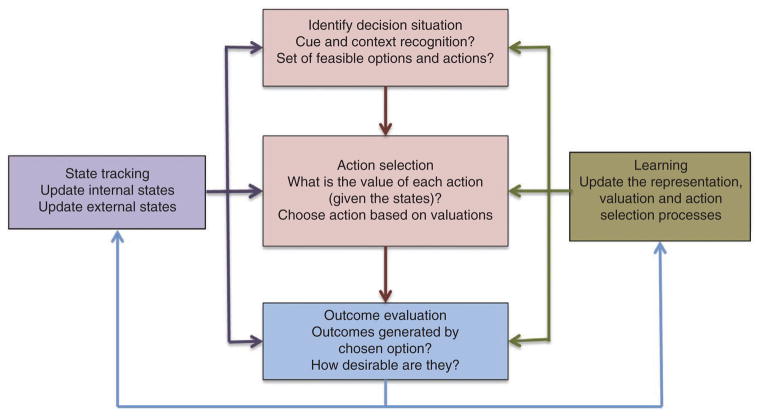
Figure 1 provides a description of the different types of computations that take place before, during and after making a feeding decision. The rest of the Review is organized around this framework. First, the organism needs to identify that it is in a decision situation and represent the options and actions available. This step can be triggered by internal states (for example, feeling of hunger) or by external ones (for example, sight of food). The fact that most animals engage in feeding bouts suggests that they make food decisions at selected situations, rather than at every instant. Second, the organism needs to choose among the available options (for example, steak or salad?). As we will see, this often involves assigning value to the different options and comparing those values to select one of them. Third, once a choice is made and food is consumed, the organism evaluates the outcome. This involves tracking the outcomes and states induced by previous food choices (for example, taste or gastrointestinal discomfort), and assigning outcome values to the experience. Fourth, the outcome information is used to learn how to make better decisions in the future by updating the representation, choice and state tracking systems. In particular, the organism uses the outcome values to update the value assigned to foods in future decisions. Learning can also affect the representation stage by changing how attention is deployed to different options. Finally, food consumption changes internal states (for example, energy stores and hunger levels), which affect how future choices are made through their effects on a variety of homeostatic mechanisms.
Figure 1.
Summary of computations that take place before, during and after decision-making.
Choice is guided by competing behavioral controllers
A sizable body of evidence has shown that decisions are controlled by three different systems6: a Pavlovian controller, a habitual controller and a goal-directed controller. This applies both to feeding and non-feeding decisions, but the distinction is especially central for dietary choice.
Pavlovian control
Organisms automatically deploy many types of pre-programmed responses when exposed to specific stimuli. A famous example is the salivation response of Pavlov’s dogs when exposed to food. These behaviors are hardwired because they are effective and computationally simple responses to specific circumstances. With training, animals can also learn to deploy the Pavlovian behaviors in response to novel stimuli if they are predictive of the conditions that trigger the hardwired response. Thus, Pavlov’s dogs salivated not only to the sight of food, but also to the sound of a bell that predicted food.
The exact number of Pavlovian controllers is unknown and it is likely that different types of responses involve different neural subsystems6. For example, odorants trigger highly specific and stereotyped fear responses in rodents7, and each is expressed through different pathways, from amygdala and hypothalamus to periaqueductal gray8. In contrast, lesions to amygdala, orbitofrontal cortex (OFC) and ventral striatum (vStr) interfere with the expression of appetitive Pavlovian responses such as conditioned approach to cues associated with palatable foods9.
Two examples illustrate the role of Pavlovian control in feeding. Consummatory responses, such as pecking at physically proximate food, likely affect the initiation, rate and termination of eating. Preparatory behaviors, such as approaching cues that predict the delivery of food (for example, a restaurant sign), also influence when and what we eat.
Habitual control
More flexible behavioral responses can be generated using stimulus-action associations. More concretely, let rO(a | s) denote the reward generated by taking action a in state s. With repeated practice, and as long as the environment is stable, the habit system learns to assign a value to each state-action pair, denoted by V(a | s), which is proportional to the present discounted value of the rewards that follow10. The system then selects the actions with the highest value when exposed to cues associated with a given state.
Notably, the habit system is a model-free controller: it assigns values to action-state pairs by averaging the previous history of rewards without forming a model of the outcomes generated by each action. Given that its values are determined by previously experienced rewards, it has difficulty learning the future consequences of actions (for example, delayed health problems) that have not yet been experienced. This limits its ability to select optimal actions in environments in which actions have substantial long-term consequences or in settings with rapidly changing reward contingencies.
The dorsolateral striatum is critical for habitual control in both rodents11 and humans12,13. This area is connected in loops with motor cortex, which provides a mechanism through which cues can influence action selection6.
Two examples illustrate the role of habitual control in feeding. With sufficient training, rats tend to forage in cue-dependent locations associated with the receipt of previous rewards. Habits are likely to be in control in behaviors such as having a coffee at specific locations and following certain events (for example, lunch).
Goal-directed control
This controller allows for even more flexible behaviors by engaging in model-based control6,14. Let p(o | a, s) denote the probability of obtaining outcome o when taking action a in state s. This controller assigns a value to each action-state pair given by
As shown in this expression, the model of the choice situation has two components: the action-outcome associations represented by the probability function and the outcome-reward associations represented in the reward function. The superscript denotes the fact that this is the reward function used to evaluate potential outcomes at the time of decision.
In contrast with the habit system, values are now computed using the model of the action-outcome-reward contingencies, which is a forward-looking process. In particular, information about outcomes can be used to update values without having to experience them first. Given this, values can reflect delayed consequences well before they are experienced (for example, by assigning a low value to future health problems).
Evidence for goal-directed control has been established in rats15 and humans16–18. This requires showing that individuals prospectively modify their choices in response to changes to the action (that is, p(o | a, s)) or the reward contingencies (that is, rD(o | s)). Consistent with this, rats exhibit conditioned taste aversion, in which a previously favored action is no longer taken after the food reward associated with it is devaluated by pairing it with illness in a different context or by feeding to satiation15.
Several regions with distinct computational roles are thought to be involved in goal-directed control. The dorsomedial striatum is involved in the representation of action-outcome associations19. The hippocampus might have a similar function, although its role is not as well understood20. A large number of human functional magnetic resonance imaging (fMRI)21, monkey physiology22,23 and lesion studies24 have shown that areas of medial and central OFC, extending into ventromedial prefrontal cortex (vmPFC), compute the value of potential outcomes at the time of choice (that is, rD(o | s)). Notably, the same areas have been shown to assign value to a wide class of outcomes, from monetary payments to social rewards, and even appetitive and aversive foods25. Consistent with the properties of the goal-directed system, activity in the vmPFC decreases after outcomes are experimentally devalued16,17. These value signals can be computed in a few hundred milliseconds, which implies that the controller is capable of rapid decision-making26. A network of areas that includes dorsolateral prefrontral cortex (dlPFC), pre-supplementary motor area and bilateral inferior parietal sulcus take the vmPFC values as inputs, compares them to select a course of action and modulates activity in motor cortex to implement it27,28. Finally, experiments with insulalesioned rats suggest that it is involved in updating the value of food after changes in physiological states29, likely through its connectivity with vmPFC.
In humans, all aspects of feeding can be controlled by the goal-directed system. In particular, we are able to control what, when and how we eat based on cognitive goals (for example, lose weight), albeit not always successfully. Also, new information can influence food choices in the absence of previous experience (for example, a friend’s recommendation). This controller must also be closely coupled with homeostatic regulators of feeding, as the values that it computes are sensitive to changes in the physiological states that they encode (for example, hunger and ghrelin levels).
Competition and interactions among controllers
A basic problem with having multiple controllers is that they might favor different actions30. For example, in the presence of a plate full of tasty food, the Pavlovian system might activate a consumatory eating response, whereas the goal-directed system might favor stopping.
Evolving control
The strength with which the Pavlovian and habitual controllers are activated in response to stimuli evolves over time, as the underlying associations have to be learned and updated. Habitual control is deployed only after repeated training in both rodents15 and humans13. It is also highly context dependent, so that actions selected in one context or state might not be chosen in another.
Computation of goal-directed values
To make good choices, the controller in charge needs to select the action that has the highest value to the individual, taking into account both short and long-term consequences. For the reasons described above, the Pavlovian and habitual systems may select actions inconsistent with this long-term view. For example, they might favor eating dessert, as it generates a strong and immediate hedonic response, while ignoring future health consequences, even if this entails a decision-making mistake.
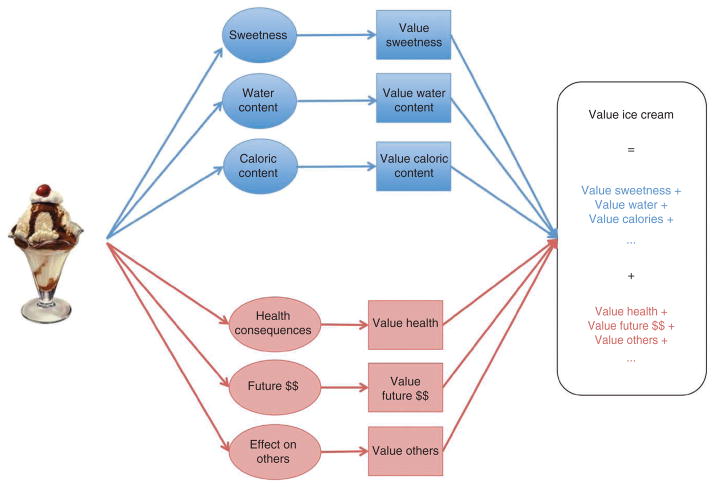
Given the limitations of Pavlovian and habitual control, it is critical to understand how goal-directed values are computed and the extent to which they correctly weight long-term consequences. Figure 2 summarizes our current understanding of how these values are computed: outcomes are mapped into a space of attributes, value is assigned to each of those attributes on the basis of their individual contribution to reward and the attribute values are summed to get an overall outcome value. This algorithm is able to compute the value of any potential outcome, even unfamiliar ones, as long as it can be mapped to a space of basic attributes to which it can assign value. Although Figure 2 lists some specific attributes as an example, we emphasize that the actual dimensions used by this system remain unknown.
Figure 2.
Description of how the goal-directed system can compute the value of a stimulus; for example, an ice-cream sundae. First, the item is mapped into the set of attributes that describe it. Second, a value is assigned to each attribute based on the current physiological state and the pleasure or pain associated with consumption of that attribute in the past. Third, the attribute values are integrated to compute an overall value for the item. There are two classes of attributes: basic attributes (such as sweetness or taste quality, depicted in blue) that are taken into account by all decision-makers and more ‘abstract’ attributes (such as health, depicted in red) that are only taken into account by those who make healthy choices. We emphasize that the actual attributes used by the goal-directed system have not been identified, and the ones highlighted here were chosen solely for the purpose of providing an example.
Notably, there seem to be two distinct types of attributes: those associated with basic and immediate outcomes (for example, taste), and those associated with abstract and delayed ones (for example, health). A good decision requires computing value signals in vmPFC that weight both types of attributes properly, including discounting future outcomes at an appropriate rate. However, a human fMRI study comparing how healthy and unhealthy eaters compute values suggests that this is not always done31. In particular, the vmPFC value signals of healthy eaters reflected both the health and taste of foods, whereas in unhealthy eaters they only reflected taste. In addition, in healthy eaters, but not in unhealthy ones, the left dlPFC modulated activity in vmPFC in a way consistent with helping it take into account the values of health attributes. Follow-up work has shown that giving non-dieters a reminder to pay attention to health information improves the healthiness of their food choices, and that the extent to which this happens is correlated with the degree to which the dlPFC-vmPFC network is activated32.
The mechanisms described here provide insight into why successful dieting is hard and rare. The Pavlovian and habit systems ignore long-term consequences. They can be inhibited, but only when the goal-directed system computes the correct overall value, detects a conflict and inhibits the competing responses. In addition, the goal-directed system assigns the correct value to food choices only when sufficient attention is paid to attributes such as health. Dietary self-control is hard because all of these processes have to be deployed correctly for it to occur and because we are constantly exposed to stimuli that trigger competing urges through the Pavlovian and habitual systems.
Outcome evaluation
After an action is taken, the brain keeps track of two types of information: the outcomes generated by the different actions and their desirability. The first class of variables is used to learn the action-outcome associations underlying goal-directed choice (that is, p(o | a, s)). The hippocampus is thought to be involved in tracking these associations20.
The second class is used to learn the model-free action values used by the habit system and the reward function rD(o | s) used by the goal-directed controller. To do this, an experienced reward function rO(o | s) is computed at the time of outcome. Note that these are two distinct computations: rD(o | s) is used at the time of decision to compute goal-directed values, whereas rO(o | s) is computed at the time of outcome to evaluate the outcome of previous decisions. This distinction parallels the well-known concepts of wanting and liking proposed by Berridge33.
To do this, the brain encodes a continuous stream of hedonic states, which provide a measure of experienced reward at any given instant34. These hedonic signals measure the short-term effect of actions, but do not reflect their delayed consequences. As a result, any learning heavily influenced by these hedonic signals, as is the case in habitual control, will not incorporate the value of future outcomes.
An influential series of studies have found a network of ‘hedonic spots’ in nucleus accumbens (NAcc), ventral pallidum35 and brainstem. Notably, a pleasurable state is registered only when all of the areas respond in concert36. In human fMRI studies, medial and central OFC reliably correlate with subjective reports of hedonic experience37,38, including to taste rewards. Medial OFC receives inputs from all of the five senses, as well as at the insula, which can aid in the construction of these representations. OFC’s hedonic responses to basic stimuli such as liquids and odors depend not only on their chemistry and the physiological state of the individual, but also on cognitive beliefs about the experience, such as the price of a wine39. This suggests that OFC integrates multiple levels of information to compute hedonic value.
The hedonic effect of food is mediated by μ-opioid transmission in at least NAcc, ventral pallidum40 and basolateral amygdala41. However, it is not mediated by phasic dopamine responses, which instead are involved in value learning.
Learning
The brain utilizes the outcome tracking and hedonic signals to update how the three controllers operate.
Habit learning
The habitual system updates its action values through the computation of a reward prediction error (RPE) signal at the time of outcome given by
where the subscripts denote time, a is the action taken, s is the active state and o is the current outcome generated by the action10. The RPE can then be used to update the action values by
where λ is a learning rate controlling the speed of learning.
The phasic responses of midbrain dopamine neurons have been shown to encode the computation of RPE signals in a wide class of paradigms42–44, involving rewards as distinct as food45,46, money and social outcomes47. According to the model, stronger hedonic responses at outcome should lead to stronger dopaminergic responses, thereby increasing the likelihood that the action just taken will be repeated in the future. Consistent with this, blood oxygen level–dependent (BOLD) responses in dorsal striatum after ingestion of palatable food are proportional to subjects’ hedonic reports48. On the basis of rodent lesion49 and human fMRI studies13,17, habit learning is thought to depend on the release of dopamine RPE signals into dorsal striatum.
We emphasize again that the hedonic and phasic dopaminergic responses involve different computations and systems. Consistent with this, hyper-dopaminergic mice consume more sweet rewards, but do not seem to experience more pleasure from them50. In addition, dopaminergic responses to food rewards can arise independently of taste signaling, which suggests that post-ingestive mechanisms (for example, measures of caloric intake) can trigger RPE activity directly51. Notably, a sizable body of evidence suggests that, in contrast with the phasic responses, tonic dopamine levels influence activities that entail energy expenditure, such as exploration of the choice environment52.
Pavlovian learning
One important class of Pavlovian responses are those triggered by general appetitive (for example, approach) and aversive (for example, withdrawal) stimuli. The central nucleus of the amygdala and vStr have been shown to be involved in this type of learning. Anatomically, the amygdala projects directly to the lateral hypothalamus and brainstem nuclei associated with initiating conditioned autonomic reflexes53, and vStr sends indirect projections to motor nuclei in brainstem that have an analogous role9,54. In addition, dopamine projections to these areas are necessary for Pavlovian learning to occur55.
Goal-directed learning
The goal-directed system needs to learn p(o | a, s) and rD(o | s). It is well-known that the hippocampus and surrounding medial temporal lobe are important for learning stimulus-stimulus associations, for generalizing knowledge among them and for establishing ‘episodic’ memories even after single episodes20. The hippocampus is involved in learning p(o | a, s), but not at test time20. Notably, dopamine neurons project to hippocampus and modulate its plasticity56, which could help to prioritize which associations are learned on the basis of their reward implications.
Much less is known about how rD(o | s) is learned. Although it is natural to speculate that dopamine is also involved here, the evidence suggests that, at most, it provides a partial account6. For example, transgenic mice without dopamine are able to successfully learn the location of food rewards57. There are also aspects of the reward function that are hard to explain solely on the basis of the RPE account of dopamine. For example, it is easier to establish aversive conditioning between flavors and toxins that induce illness than between flavors and electric shocks58, which implies that rO(o | s) cannot be the sole driver of this type of learning.
Homeostatic regulation of feeding
A unique aspect of feeding decisions is the existence of dedicated homeostatic systems that regulate energy intake and stores. These systems include hormonal regulators of hunger, satiety and fat levels, such as leptin, ghrelin and insulin, among others3–5. A basic question is whether the homeostatic systems provide a parallel system for controlling feeding decisions or whether they operate by modulating the decision-making circuitry described above. The former view is common in the literature, which often has made a distinction between ‘homeostatic feeding’ and ‘hedonic feeding’. As others have previously4,5,59,60, here we argue for the latter view.
Given the richness of the homeostatic regulators and the detailed pathways that have been identified4,5,59,60, we do not attempt to be comprehensive in our discussion of how these mechanisms interact with the decision-making circuitry. Instead, we showcase three pathways that have been the subject of extensive investigation: leptin, ghrelin and the lateral hypothalamus.
Leptin is a circulating hormone secreted by adipocytes that signals the size of peripheral energy stores. It provides a negative homeostatic feedback loop to decrease feeding as energy stores increase. Consistent with this, meal size is reduced by exogenous administration of leptin61, and leptin-deficient mice become obese62. Leptin receptors have been found in many areas, including the hypothalamus63, ventral tegmental area (VTA)64 and hippocampus65. Rodent studies have found that leptin administration decreases the firing of VTA dopamine neurons after food consumption64. Human fMRI studies have found that leptin replacement down-modulates responses in the vStr when subjects are exposed to appetitive foods66. Notably, although leptin receptors are found in the taste buds67, leptin can modulate dopamine responses even when food is administered intragrastrically, which suggests that leptin’s effects on dopamine do not operate solely by decreasing the reward experienced from food consumption68. Leptin reduces food intake by enhancing response to satiety signals, similar to the release of cholecystokinin by the gut in response to gastric distension69. The hippocampus is densely populated with leptin and insulin receptors70, and administration of leptin into this region enhances long-term potentiation71. Together, these findings suggest that leptin can reduce feeding by modulating at least three different channels. First, it reduces phasic responses by dopamine neurons associated with the computation of RPEs, which decrease future feeding by lowering the habit values of food consumption. Second, it reduces the hedonic response to palatable food, which reduces the value assigned to these foods by the habit and goal-directed systems. Third, it enhances the learning of action-outcome associations by the goal-directed system, and, through this, its ability to control decisions.
Ghrelin is a peptide hormone secreted by the gut. It is thought to influence food initiation and termination, as its concentration increases in parallel with hunger before meals and falls with satiation afterwards72. Exogenous administration of ghrelin increases food intake in animals73 and humans74. Ghrelin receptors are found in the hypothalamus73, VTA75, NAcc, amygdala76 and hippocampus77. However, it does not seem to increase the hedonic responses to food (as measured by licking responses at the time of consumption)78. Instead, local ghrelin injections to rodent VTA increase dopamine release into the striatum, as well as subsequent feeding75. Ghrelin administration also increases BOLD responses to food pictures in areas such as OFC, striatum and amygdala, which control the computation of value in goal-directed choice and influence the responses of appetitive Pavlovian controllers79. Ghrelin also modulates hippocampal activity and memory performance80. Together, these findings suggest that ghrelin modulates feeding through (at least) the following channels. First, it increases the goal-directed values assigned to food at meal initiation and decreases them after satiation. Second, it modulates the computation of RPEs by the dopamine system. Third, it modulates the activation of Pavlovian appetitive responses, such as approaching food cues. Notably, ghrelin responses to food consumption are modulated not only by the food’s nutritional content, but also by cognitive beliefs about how much was consumed81, which suggests that these metabolic signals might be more complex than commonly thought.
Neurons expressing orexin and melanin hormones in the lateral hypothalamus act as the metabolic detectors and are critical for the regulation of feeding. Projections from lateral hypothalamus to caudolateral OFC (the secondary taste area) seem to carry satiety information that can be used in the computation of hedonic and goal-directed values82. This region also seems to be important for the control of feeding Pavlovian responses. For example, electrical stimulation of lateral hypothalamus induces intense eating83, and this effect can be blocked through leptin administration or feeding to satiation. These neurons are also involved in goal-directed control through their projections to OFC, insula and amygdala5.
Uniqueness of dietary choice
Although the core decision-making circuitry is used to make both feeding and non-feeding decisions, several properties of feeding make it a unique choice problem. First, feeding can be regulated by any of the three controllers, and Pavlovian and habitual controllers are likely to be important for feeding. In contrast, decisions such as which shoes to buy are mostly under goal-directed control. Second, feeding decisions have to be made with high frequency. This is important because it gives rise to the possibility of frequent and rapid learning, which can help explain why animals exposed to highly palatable diets become rapidly habitized. Third, feeding is modulated by dedicated homeostatic mechanisms. In contrast, analogous homeostatic mechanisms have not been identified for any other behaviors, except those that involve basic physiological drives such as breathing or hydration. Fourth, there are feeding-specific Pavlovian mechanisms, which likely control consummatory behaviors such as approaching food in hunger states. In contrast, dedicated Pavlovian controllers have not been identified for other consumption decisions. Fifth, feeding-related learning is constrained in the type of associations that can be learned. This is exemplified by the relative ease with which organisms learn associations between flavors and illness, even after a single exposure58. In contrast, associations between flavors and other types of outcomes are harder to learn. Sixth, consumption of sweet and fatty foods activates the hedonic circuitry with unusual power. This is important because it can lead to a rapid transfer of control to the Pavlovian and habit systems. Consistent with this, rats exhibit a strong preference for a calorie-free saccharine solution over intravenous self-administration of cocaine84, and blocking opioid receptors with naloxone reduces hyperphagia of highly palatable foods, but has no effect on the consumption of regular food85. Also, conditioned preferences for flavors associated with fat content administered intragastrically are hard to extinguish and long-lived86.
Other behavioral decisions share some of these properties, but only feeding combines all of them (Table 1). This is important because it is the interaction of these features that makes dietary choice difficult and unique. Understanding this uniqueness is essential to understand why there is an obesity epidemic, but not an increase in decision mistakes in other domains.
Table 1.
Properties of different decision problems
| Feeding | Breathing | Sex | Consumption goods (for example, clothing) | Drugs of abuse | Kin altruism | |
|---|---|---|---|---|---|---|
| Necessary and frequent consumption | ✓ | ✓ | × | × | × | × |
| Regulated by multiple controllers | ✓ | Possible, but limited | ✓ | ✓ | ✓ | ✓ |
| Dedicated homeostatic mechanisms | ✓ | ✓ | ? | × | × | × |
| Dedicated Pavlovian controllers | ✓ | ✓ | ? | × | × | ? |
| Dedicated learning systems | ✓ | ? | ? | × | × | ? |
| Powerful activation of hedonic circuitry at consumption | ✓ | × | ✓ | × | ✓ | × |
Obesity
Obesity results from a sustained excess in calories consumed minus calories used. Thus, it is affected by feeding decisions, energy consumption and metabolic factors that regulate how excess calories are stored and used. Here we focus on the role of feeding decisions, which some view as the critical and most challenging component of the equation.
It is important to highlight that, from a neuroeconomic perspective, obesity is almost always associated with mistakes in decision-making. A mistake occurs when an individual’s choice does not maximize the net present value of rewards associated with the decision, appropriately discounted. The fact that obesity is accompanied by frequent and (often) unsuccessful attempts to lose weight demonstrates that individuals know that healthier eating is the optimal option, but that they are unable to systematically make the choices necessary to accomplish this goal.
Potential mechanisms
In principle, excess food consumption can result from faulty processing in any of the decision processes described above. For example, an individual who experiences unusually large hedonic responses (rO(o | s)) or reward prediction errors (δt) after consuming fats or sugars is likely to increase their consumption substantially, through all of the controllers, which would result in obesity. Some traits seem to be associated with an increased incidence of obesity, although cause and effect are often difficult to sort out in these studies. For example, there is a positive correlation between body mass index and the discounting of future monetary rewards87, and individuals with stronger sensitivity for rewards (of any kind) exhibit stronger BOLD responses in OFC and NAcc when exposed to food images88.
Likely mechanisms
Although a wide range of mechanisms can be responsible for individual cases of obesity, two key facts suggest that the current obesity epidemic is likely attributable to a sub-set of the potential mechanisms. First, healthy rats and humans only develop hyperphagia when repeatedly exposed to cafeteria style diets89, which suggests that environmental variables are critical. Second, obesity rates have increased over a short time span, which makes it highly unlikely that a purely biological cause is at work.
Given these critical facts, and the findings reviewed above, we hypothesize that increases in obesity result from a change in environmental factors and its interactions with the properties of the feeding circuitry. First, there has been a substantial increase in food cues and availability of unhealthy foods. For example, portion sizes have increased substantially90 and food prices have decreased91. Second, this has resulted in increased activation of Pavlovian and habitual controllers, with all of the short-comings that this entails. Third, this has increased the demands placed on the goal-directed controller, making it more likely to fail. This problem might be further exacerbated by lifestyle and work-place changes that have led to increased cognitive demands and stress in many individuals. Consistent with this, experiments have shown that cue-triggered Pavlovian and habitual control is more likely to take over under cognitive load92, and an fMRI study of dieters32 showed that attention is important to compute correct goal-directed values.
Vicious circles
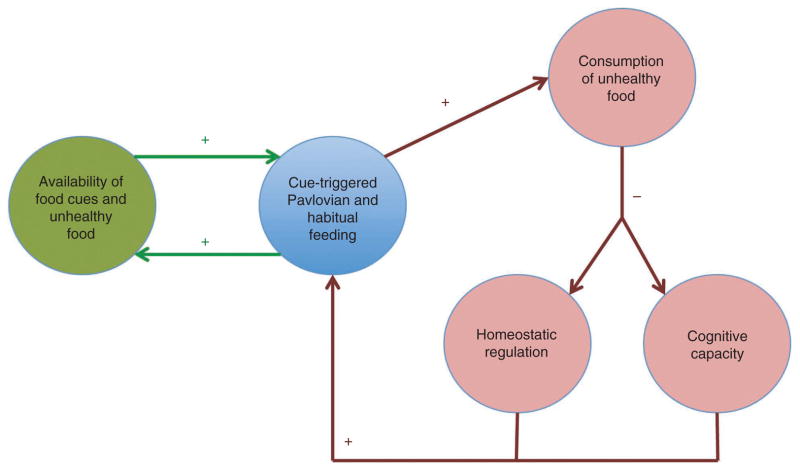
These mechanisms give rise to three vicious circles that further worsen the problem (Fig. 3). First, increased consumption of unhealthy foods impairs the ability of metabolic and endocrine factors to restore homeostatic balance. This impairment can operate through different channels. For example, elevated dietary fat (directly or indirectly) confers insensitivity to peptides that regulate body weight through their effect on the hypothalamus93,94. Second, high-fat diets have been associated with the development of cognitive impairments that can interfere with the performance of the goal-directed system, further increasing the extent to which feeding is governed by habitual and Pavlovian control95. Consistent with this, hippocampal damage produces hyperphagia in humans96,97 and rats98, and obese individuals have stronger BOLD responses to food cues in NAcc99. Third, as Pavlovian and habitual control take over, behavior becomes more responsive to the cues associated with these systems (for example, big portions) and less responsive to cognitive factors (for example, prices, information or health goals). This introduces economic incentives for food suppliers to further increase the supply of cues (for example, marketing) and unhealthy foods (for example, fat, salt and sugar content). This highlights the importance of policies that target the source of the problem directly, through regulation of the food environment100.
Figure 3.
External (green) and internal (dark red) vicious circles in obesity.
Final remarks
Substantial progress has been made toward understanding the neurocomputational basis of decision-making, as well as the nature of the various systems that serve as homeostatic regulators of feeding. The next challenge is to bring the two fields together. Here we have tried to showcase that this integrative agenda is feasible, has the potential to generate new insights and questions, and could substantially advance our understanding of diseases such as obesity and anorexia. Needless to say, the work required to integrate these two fields has just begun.
Acknowledgments
The support of the National Science Foundation (AR3.SELFCNTRL-1-NSF.ARR1), the Lipper Foundation, and the Betty and Gordon Moore Foundation is gratefully acknowledged.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daw N, O’Doherty JP. Multiple systems for value learning. In: Glimcher P, Fehr E, editors. Neuroeconomics: Decision Making and the Brain. 2. Academic Press; New York: 2013. [Google Scholar]
- 7.Stowers L, Cameron P, Keller JA. Ominous odors: olfactory control of instinctive fear and aggression in mice. Curr Opin Neurobiol. 2013;23:339–345. doi: 10.1016/j.conb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 9.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niv Y, Montague PR. Theoretical and empirical studies of learning. In: Glimcher PW, Fehr E, Camerer C, Poldrack R, editors. Neuroeconomics: Decision-Making and the Brain. Elsevier; 2008. [Google Scholar]
- 11.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 12.de Wit S, et al. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012;32:12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 16.Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27:4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci. 2009;29:11330–11338. doi: 10.1523/JNEUROSCI.1639-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 20.Wimmer EG, Shohamy D. The striatum and beyond: contributions of the hippocampus to decision making. In: Delgado M, Phelps EA, Robbins TW, editors. Attention and Performance XXII. Oxford University Press; Oxford: 2011. [Google Scholar]
- 21.Rangel A, Clithero J. The computation of stimulus values in simple choice. In: Glimcher P, Fehr E, editors. Neuroeconomics: Decision Making and the Brain. 2. Academic Press; 2013. [Google Scholar]
- 22.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 24.Camille N, Griffiths CA, Vo K, Fellows LK, Kable JW. Ventromedial frontal lobe damage disrupts value maximization in humans. J Neurosci. 2011;31:7527–7532. doi: 10.1523/JNEUROSCI.6527-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris A, Adolphs R, Camerer C, Rangel A. Dynamic construction of stimulus values in the ventromedial prefrontal cortex. PLoS ONE. 2011;6:e21074. doi: 10.1371/journal.pone.0021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare TA, Schultz W, Camerer CF, O’Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proc Natl Acad Sci USA. 2011;108:18120–18125. doi: 10.1073/pnas.1109322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basten U, Biele G, Heekeren HR, Fiebach CJ. How the brain integrates costs and benefits during decision making. Proc Natl Acad Sci USA. 2010;107:21767–21772. doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. J Neurosci. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 31.Hare TA, Camerer C, Rangel A. Self-control in decision-making involves modulation of the vMPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 32.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 34.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 35.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith KS, Berridge KC. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 38.Rolls ET, et al. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 39.Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 41.Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci USA. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- 47.Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci. 2012;7:274–281. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 49.Wang LP, et al. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher ‘wanting’ but not ‘liking’ for sweet rewards. Behav Pharmacol. 2004;15:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Araujo IE, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 52.Beeler JA, Fraixer CRM, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci. 2012;6:49. doi: 10.3389/fnint.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-Amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parkinson JA, et al. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- 56.Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- 57.Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychon Sci. 1966;4:123–124. [Google Scholar]
- 59.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 60.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 61.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 62.Szczypka MS, Rainey MA, Palmiter RD. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet. 2000;25:102–104. doi: 10.1038/75484. [DOI] [PubMed] [Google Scholar]
- 63.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 64.Hommel JD, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 65.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 66.Farooqi IS, et al. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shigemura N, Miura H, Kusakabe Y, Hino A, Ninomiya Y. Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Arch Histol Cytol. 2003;66:253–260. doi: 10.1679/aohc.66.253. [DOI] [PubMed] [Google Scholar]
- 68.Domingos AI, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276:R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 70.Lathe R. Hormones and the hippocampus. J Endocrinol. 2001;169:205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- 71.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 73.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 74.Druce MR, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 75.Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carlini VP, et al. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 77.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol. 2012;303:R259–R269. doi: 10.1152/ajpregu.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Diano S, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 81.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol. 2011;30:424–429. doi: 10.1037/a0023467. [DOI] [PubMed] [Google Scholar]
- 82.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 83.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 84.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense Sweetness Surpasses Cocaine Reward. PLoS ONE. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Apfelbaum M, Mandenoff A. Naltrexone suppresses hyperphagia induced in the rat by a highly palatable diet. Pharmacol Biochem Behav. 1981;15:89–91. doi: 10.1016/0091-3057(81)90344-0. [DOI] [PubMed] [Google Scholar]
- 86.Drucker DB, Ackroff K, Sclafani A. Nutrient-conditioned flavor preference and acceptance in rats: effects of deprivation state and nonreinforcement. Physiol Behav. 1994;56:701–707. doi: 10.1016/0031-9384(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 87.Ikeda S, Kang MI, Ohtake F. Hyperbolic discounting, the sign effect, and the body mass index. J Health Econ. 2010;29:268–284. doi: 10.1016/j.jhealeco.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Beaver JD, et al. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord. 2011;44:203–211. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wansink B. Mindless Eating: Why We Eat More Than We Think. Bantam; New York: 2010. [Google Scholar]
- 91.Cutler DM, Glaeser EL, Shapiro JM. Why have Americans become more obese? J Econ Perspect. 2003;17:93–118. [Google Scholar]
- 92.Mann T, Ward A. Attention, self-control and health behaviors. Curr Dir Psychol Sci. 2007;16:280–283. [Google Scholar]
- 93.Clegg DJ, et al. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–R986. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 94.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 95.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Higgs S. Memory and its role in appetite regulation. Physiol Behav. 2005;85:67–72. doi: 10.1016/j.physbeh.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychol Sci. 1998;9:392–396. [Google Scholar]
- 98.Clifton PG, Vickers SP, Somerville EM. Little and often: ingestive behavior patterns following hippocampal lesions in rats. Behav Neurosci. 1998;112:502–511. doi: 10.1037//0735-7044.112.3.502. [DOI] [PubMed] [Google Scholar]
- 99.Rothemund Y, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 100.Marteau TM, Hollands GJ, Fletcher PC. Changing human behavior to prevent disease: the importance of targeting automatic processes. Science. 2012;337:1492–1495. doi: 10.1126/science.1226918. [DOI] [PubMed] [Google Scholar]