Highlights
-
•
The role of B-type lamins in cellular organisation and function.
-
•
The role of B-type lamins in chromatin organisation.
-
•
The role of lamin B1 in cellular senescence.
-
•
The role of B-type lamins in organogenesis and tissue building.
Keywords: Lamin B1, Cellular senescence, Brain development, Autosomal dominant leukodystrophy
Abstract
For over two decades, B-type lamins were thought to have roles in fundamental processes including correct assembly of nuclear envelopes, DNA replication, transcription and cell survival. Recent studies have questioned these roles and have instead emphasised the role of these proteins in tissue building and tissue integrity, particularly in tissues devoid of A-type lamins. Other studies have suggested that the expression of B-type lamins in somatic cells influences the rate of entry into states of cellular senescence. In humans duplication of the LMNB1 gene (encoding lamin B1) causes an adult onset neurodegenerative disorder, termed autosomal dominant leukodystrophy, whilst very recently, LMNB1 has been implicated as a susceptibility gene in neural tube defects. This is consistent with studies in mice that reveal a critical role for B-type lamins in neuronal migration and brain development. In this review, I will consider how different model systems have contributed to our understanding of the functions of B-type lamins and which of those functions are critical for human health and disease.
1. Introduction
The nuclear lamina was originally defined as a fibrous structure lining the inner nuclear membrane (INM), which is resistant to detergent and salt extraction [1]. This structure was subsequently shown to be composed of intermediate filaments (IF) which in amphibian oocytes formed into a basket weave network that interlinked nuclear pore complexes (NPCs) [2–4]. Now classified as type V IF proteins, the lamins are the evolutionary progenitors of the IF supergene family [5]. Lamins are divided into two sub-types, the A-type lamins and the B-type lamins. A-type lamins (lamins A, C, AΔ10 and C2) are alternatively spliced products of the LMNA gene [6]. During development they are generally expressed at the time of organogenesis [7], form relatively thick filaments [8] and are soluble during mitosis [9]. The B-type lamins (lamins B1, B2 and B3) are the products of two distinct genes. Lamin B1 (in humans) is encoded by the gene LMNB1, located on chromosome 5q23.3-31.1, whilst lamins B2 and B3 are alternatively spliced products of LMNB2 located on chromosome 19p13.3 [10,11]. B-type lamins are anchored to the inner nuclear membrane via a prenylated cysteine residue at the C-terminus (see below) and are therefore associated with membranes during mitosis [9]. In addition, whilst lamin B3 is germ line specific, lamins B1 and B2 are expressed in most cells in embryos and adult animals [12–15], giving rise to the notion that they are essential for cell survival.
Like all IF proteins, lamins have a domain structure consisting of a (small) globular head domain, a central rod domain consisting of four coiled coil dimers and a (long) C-terminal globular domain. Lamins form obligate dimers (most likely homodimers), which then assemble into homopolymers. Lamin dimers are strongly predisposed towards proto-filament assembly via head-to-tail associations, which in turn form anti-parallel out of register lateral associations, eventually giving rise to 10–13 nm filaments [2,16,17].
The B-type lamins and prelamin A possess a CaaX motif that is a site for posttranslational modification. The CaaX motif is sequentially modified following translation by the addition of a 15-carbon farnesyl isoprenoid to the cysteine residue, proteolytic cleavage of the aaX residues and methylation of the now C-terminal cysteine. This renders the C-terminal domain hydrophobic and anchors permanently farnesylated lamins to the INM. Lamin A, however, undergoes further post-translational modification at the INM where the final 15 C-terminal amino acids are cleaved by the ZMPSTE24 endoproteinase, allowing the protein to migrate between the nucleoplasm and the lamina (see Levy and colleagues this edition, reviewed by Broers et al. [18]).
2. Lamina function in nuclear assembly, DNA replication, transcription and mitotic spindle assembly
The very first investigations indicating a functional role for B-type lamins arose from studies using cell-free extracts of Xenopus eggs that support the assembly of replication competent nuclei in vitro (Table 1). Physical or functional depletion of endogenous B-type lamins from these extracts led to the assembly of small fragile nuclei having functional nuclear pore complexes but which lacked a lamina and were unable to replicate DNA [19–21]. The mechanism by which lamins promote DNA replication in this system remains contentious and using similar approaches, some studies suggested that lamins assist in the elongation phase of DNA replication [22,23], whilst others have suggested that they contribute to the initiation phase of DNA replication [24,25]. Dominant negative lamin mutants can be used to disrupt pre-existing lamina filaments. When B-type lamina filaments are disrupted in this way in Xenopus oocytes, this leads to the sequestration of RNA polymerase II and inhibition of pol II dependent transcription [26]. B-type lamins have also been reported to interact with the NPC protein Nup153. Nup153 is a component of the nuclear pore basket which resides at the inner nuclear membrane. Depletion of either B-type lamins or Nup153 does not lead to gross abnormalities in NPC assembly or function but does lead to a phenotype in which NPCs float within the NE and cluster together [27]. Taken together these studies suggest that whilst B-type lamins are not essential for the assembly of transport competent NEs, they do have essential roles in NE integrity, maintaining the positioning of NPCs and in DNA replication and transcription. These essential functions of B-type lamins were supported by early siRNA depletion studies in somatic cells. These studies identified B-type lamins as essential for cell survival and their depletion led to apoptotic cell death [28].
Table 1.
The effects of lamin depletion or knock-down, on sub-cellular organisation or cellular function.
| Experimental system | Lamin sub-type | Cellular defects | Reference |
|---|---|---|---|
| Xenopus egg extracts | Lamin Liii | Fragile nuclei, DNA replication failure, NPC positioning, Spindle assembly defects | [19,20,27,29] |
| Xenopus oocytes | Lamin Liii | Pol II transcription failure | [26] |
| HeLa cells | Lamin B1 and Lamin B2 | Apoptosis | [28] |
More recently, B-type lamins have been implicated in the correct assembly and maintenance of mitotic spindles. Xenopus lamin Liii was detected as a matrix-like component of mitotic spindles in Xenopus egg extracts. Moreover, depletion of lamin B1 and lamin B2 from somatic cells led to a number of spindle defects including unfocused spindle poles, poor spindle morphology and lack of chromosome congression [29]. B-type lamin association with mitotic spindles is dependent upon RanGTP, Nudel and dynein and appears to occur at prometaphase [30,31]. B-type lamins appear to antagonise kinesin Eg5 to restrain spindle pole separation prior to anaphase and thus ensure correct spindle orientation and function [32] (Table 1).
3. Lamin B1 and cellular senescence
Recent reports have implied that changes in expression levels of lamin B1 are a hallmark of cellular senescence. Cellular senescence is a state of permanent cell cycle arrest triggered by a number of stresses including extended cell proliferation, persistent DNA damage, oxidative stress and oncogene activation. This in turn leads to activation of tumour suppressor genes, including p53 and Rb via p16 or p21 [33]. Recently it has been reported that lamin B1 accompanies replicative and oncogene induced senescence and that silencing of lamin B1 expression slowed proliferation rates and promoted cellular senescence via p53 and Rb dependent mechanisms [34]. In a complementary study Freund and co-workers also reported that loss of lamin B1 expression was a hallmark of senescence in vitro and in vivo but were equivocal as to whether this loss of expression was a cause or a consequence of senescence [35]. In an apparently contradictory study, entry into a senescent state as a result of MAPK activation and oxidative stress was accompanied by up-regulated expression of lamin B1 suggesting a novel mechanism of ATM-independent stress sensing [36]. The apparent discrepancies between the two studies was seemingly resolved by Dreesen and co-workers who showed that indeed up-regulation or down-regulation of lamin B1 expression could accompany cellular senescence but that lamin B1 silencing whilst causing cell cycle arrest did not in itself give rise to senescence. However, lamin B1 silencing in the presence of partial silencing of A-type lamins did [37]. This observation is apparently crucial, since replicative senescence is also accompanied by progressive oxidation of the lamin A tail leading to its functional inactivation [38]. Thus it appears that the ratio of lamin B1 to lamin A/C expression is critical and might be a key component of cellular ageing [39] (Fig. 1).
Fig. 1.
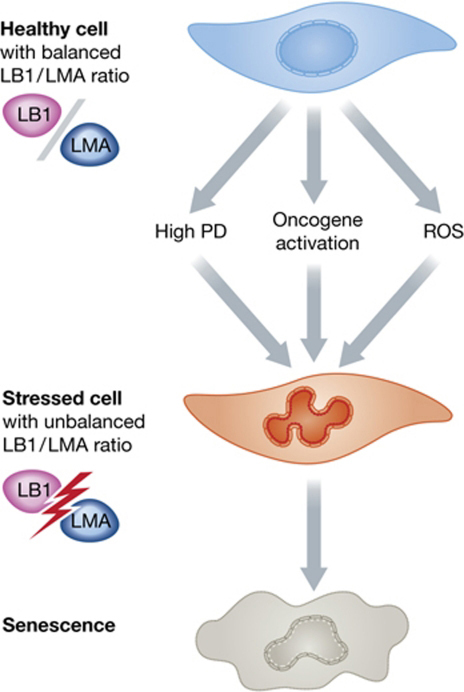
The effects of different cellular stresses that lead to senescence on lamin A to lamin B1 ratios. Healthy early passage cells have a balanced LMA to LB1 ratio. In response to senescence-inducing events, LB1 expression is altered and/or LMA is oxidized. This figure was reproduced with kind permission from EMBO Journal.
4. Lamin B1 and gene silencing
Lamin B1 has been implicated with gene silencing via a number of studies. It has been known for decades that heterochromatin preferentially associates with the nuclear lamina. More recently, it has been shown that lamin B1 associates with large lamina-associated domains (LADs) of chromatin which are devoid of active histone marks but enriched for repressive marks [40,41]. LADs are in general gene-poor, consistent with the idea that chromosomes which are preferentially associated with the nuclear periphery are also gene poor [42]. Forced repositioning of genes to the nuclear lamina, leads to transcriptional silencing [43] implying that the lamina is a silencing environment. Finally, whilst in general LADs maintain their lamina association during mouse ESC differentiation, local gene repositioning to the nuclear interior does occur and this is correlated with gene activation [44]. Taken together these observations suggest that the lamina is a repressive environment and have given rise to the notion that lamin B1 is involved in gene silencing. However, when ESCs devoid of B-type lamins (see section on mouse models) are induced to differentiate into trophectoderm altered patterns of gene expression and repositioning of genes occurs as efficiently as in wild type ESCs implying that B-type lamins do not directly regulate gene expression [45].
Redistribution of heterochromatin from the nuclear periphery to senescence-associated heterochromatic foci (SAHFs) is also a hallmark of cellular senescence. Since down-regulation of lamin B1 accompanies cellular senescence [34–37] it is possible that this contributes to SAHF formation. Two separate studies have revealed quite subtle changes in gene associations with the nuclear lamina during senescence, which depend upon the type of histone marks present. Genome wide mapping demonstrated that during senescence, lamin B1 is preferentially depleted from LADS enriched for H2K9me3 and that LMNB1 silencing promotes the relocation of H2K9me3 from a predominantly perinuclear distribution to SAHFs [46]. However, despite a global reduction in lamin B1 levels in senescent cells lamin B1 binding to a sub-set of genes enriched for H3K27me3 increases and those genes become repressed [46,47]. Both studies suggest that lamin B1 depletion contributes to global chromatin reorganisation during senescence, although neither study demonstrates that lamin B1 contributes directly to gene silencing.
5. Studies in whole organisms
The notion that B-type lamins have essential functions and are therefore required for cell survival has been questioned by studies in whole organisms and their fundamental roles have now been studied in Caenorhabditis elegans, Drosophila and mouse models. Drosophila expresses two lamin sub-types, LamDm0, which is a ubiquitously expressed B-type lamin and LamC which is developmentally regulated [48]. Depletion of LamDm0 using RNAi leads to a range of severe developmental phenotypes, although some individuals survive to late pupal stages or early adulthood [49]. One problem with studies in Drosophila is that eggs maintain a pool of maternal LamDm0 protein which could contribute to early development. In addition, expression of LamC during larval stages could compensate for LamDm0 depletion. Nevertheless, it is clear that absence or depletion of LamDm0 did not cause gross NE defects or loss of cell proliferation in a majority of tissues suggesting that these lamins had more subtle roles in tissue building rather than basic functions in DNA replication and transcription.
Similar results were obtained using RNAi mediated depletion of the single (B-type) lamin Ce-lam in C. elegans. Ce-lam depletion did lead to clear cell proliferation and chromosome segregation defects resembling a cut-phenotype in many cells. None-the-less whilst a majority of individuals were embryonic lethal, organogenesis did occur and a small number survived as short lived sterile adults [50,51]. Like Drosophila, C. elegans embryos possess maternally derived Ce-lam protein and this might account for the survival of some cells. However, it appears unlikely that the maternal pool could account for survival to adulthood, again suggesting that this B-type lamin is not required for all basic cellular functions but instead has roles in ensuring tissue building and integrity. Never-the-less, the prevalence of mitotic defects in embryos does imply that spindle assembly and function could be an important if not essential role for B-type lamins.
Studies in mice have reinforced the view that B-type lamins have important roles in building some if not all tissues and that these roles could be mediated via the interaction of these lamins with mitotic spindles. The earliest mouse model for lamin B1 function was an insertional mutant containing the first five Lmnb1 exons joined in-frame to a βgeo reporter gene and thus lacked part of coil 2B and the entire C-terminal globular domain (termed Lmnb1Δ). Mice that were homozygous for this mutation were bred from heterozygotes and did not survive after birth. However, at embryonic day 18.5 they were present at an expected Mendelian ratio, indicating that mice could have been born live. Lmnb1Δ/Δ mice were smaller than wild-types. Most internal organs were grossly normal but the lungs either lacked or had fewer alveoli, suggesting that death occurred through respiratory failure. Bone structure was grossly abnormal including curvature of the spine, decreased length of long bones and abnormal cranial structures. Mouse embryo fibroblasts (MEFs) derived from Lmnb1Δ/Δ mice were able to proliferate and differentiate into adipocytes but became polyploidy and senesced prematurely. Taken together these results indicate that lamin B1 deficiency does not affect cell survival and differentiation per se but does affect the development of certain tissues and has important functions in maintaining genome stability in somatic cells [52].
More recently a lamin B2 deficient mouse model has been produced by inserting a LacZ reporter into exon 1 of Lmnb2. This mutant specifically disrupts lamin B2, since the sperm specific lamin B3 uses an alternative exon1. Lmnb2−/− mice were bred from Lmnb2+/− mice and yielded homozygotes at expected Mendelian ratios. Lmnb2−/− mice were born but died within one hour. The mice had normal body weights and did not display any abnormalities in organ systems except the brain, which displayed grossly abnormal neuronal layering in the forebrain. More detailed investigations revealed that at E13.5 the neocortex appeared normal but subsequent neuronal migration into the cortex was impaired. MEFs derived from Lmnb2−/− mice displayed no growth defects, suggesting a highly specific role for lamin B2 in neuronal migration [53]. A more recent study investigated the role of farnesylation of B-type lamins in neuronal migration. Knock-in mice expressing non-farnesylated versions of lamin B1 or lamin B2 were created. In contrast, to Lmnb2−/− mice, mice expressing non-farnesylated lamin B2 developed normally and were apparently healthy. Mice expressing non-farnesylated lamin B1, however, died at birth and had striking neuronal defects. In particular, in migrating neurones the nuclear lamina was apparently torn away from chromatin, which was left ‘naked’ [54]. Thus, lamin B1 also appears to have a role in neuronal migration.
Whilst these mouse models suggest specific and overlapping functions for each B-type lamin, it has been argued that in the case of single knock-out mice the remaining B-type lamin could compensate for the lack of the other and therefore mask additional fundamental roles. To investigate this hypothesis, Embryonic Stem Cells (ESCs) that were null for lamin B1 and B2 were created from blastocysts obtained by mating Lmnb1+/− and Lmnb2+/− mice. Since ESCs do not express A-type lamins, these cells were effectively null for all lamin sub-types. Surprisingly, these ESCs formed colonies and displayed growth rates that were similar to wild type ESCs, expressed pluripotency markers and maintained ploidy, suggesting that lamins are not essential for ESC growth and survival. The Lmnb1−/−Lmnb2−/− ESCs were also able to differentiate into trophectoderm with similar gene expression changes to wild type ESCs. By crossing Lmnb1+/−Lmnb2+/− mice, the expected Mendelian distribution of Lmnb1−/−Lmnb2−/− embryos were observed from E12.5 to E18.5 but pups died immediately after birth through a failure to breath. The mice displayed multiple organ defects including poorly developed lungs, diaphragms and brains. However, other organs systems such as heart were normal. Similar to the Lmnb2−/− mice, brain abnormality in the double knock-out mice appeared to occur because of grossly impaired neuronal migration. The ability of some organ systems to develop normally in this mouse model might be explained because A-type lamins are more critical for the maintenance of some organs. It is intriguing to note from this perspective that in the lung and in neurones, A-type lamins are absent or expressed at very low levels [45].
The finding that B-type lamins are essential for the development and maintenance of some tissues but not others was reinforced by the finding that a conditional double knockout of Lmnb1 and Lmnb2 in keratinocytes has no deleterious effects. The mice developed normally and had normal lifespans with no gross abnormalities in skin morphology, hair production or production of nails throughout life. Moreover, unlike Lmnb1Δ/Δ MEFs, Lmnb1−/−Lmnb2−/− keratinocytes grew normally, had some abnormalities in nuclear morphology but did not display aneuploidy [55]. Similar results were obtained in a cre-recombinase knockout of Lmnb1 and Lmnb2 in hepatocytes. Hep-Lmnb1−/−Lmnb2−/− mice had normal liver function and morphology and in tissues had no nuclear defects. Isolated hepatocytes did display nuclear shape abnormalities but this did not reflect the in vivo situation [56]. Thus B-type lamins appear to be completely dispensable in some quite complex organ systems (Table 2).
Table 2.
The effects of depletion or knock-out of [74] lamin sub-types on cellular function and organogenesis in C. elegans, Drosophila melanogaster and mouse.
| Organism | Lamin sub-type | Cellular defects | Organ defects | Reference |
|---|---|---|---|---|
| C. elegans | Ce-lam | Chromosome segregation | Multiple embryonic defects, adult sterility | [74,51] |
| Drosophila | LamDm0 | None | Multiple | [49] |
| Mouse | Lamin B1 | Polyploidy, premature senescence | Small size, lung and bone, particularly spine and cranium | [52] |
| Mouse | Lamin B2 | Nuclear shearing in migrating neurones | Neuronal layering in forebrain | [53] |
| Mouse | Lamin B1 and B2 | None is ESCs | Small size, lung, bone and brain development | [45] |
6. The role of B-type lamins in human disease
It is now well established that mutations in LMNA give rise to a greater variety of genetic disorders than any other known gene (reviewed by Broers et al. [18], and other reviews of this edition: Azibani et al., Guénantin et al., Cau & Levy). In contrast, mutations in LMNB2 have yet to be directly associated with any human disease (although LMNB2 variants have been implicated in acquired partial lipodystrophies [57,58] see Guénantin et al., this edition), whilst diseases associated with LMNB1 are just emerging. Adult-onset autosomal dominant leukodystrophy (ADLD) is a very rare demyelinating neuropathy of the central nervous system that presents in the fourth or fifth decade of life. The disease is usually but not always associated with early autonomic symptoms and eventually ataxia [59–61]. Cardiovascular and skin defects have also been reported in one ADLD family leaving the possibility of additional hallmarks of the disease [62]. In ADLD patients, white matter defects are observed, particularly in the cerebellum, corticospinal tracts and corpus callosum, which leads to brain and spinal cord atrophy [63]. At a histological level, these lesions display astrogliosis but oligodendrocyte preservation [64].
ADLD has been shown to be caused by duplications involving LMNB1 on chr. 5q32 [59]. Duplication boundaries have now been mapped in a large collection of ADLD families, confirming that the minimal duplicated regions necessary for the disease includes the whole of the LMNB1 gene, which can be inverted, and that probably arose through non-homologous end joining or replication-based defects [65]. Duplication of LMNB1 leads to increased lamin B1 mRNA and protein expression in brain tissue. It has been proposed that somehow, increased expression of lamin B1 causes suppressed transcription and lack of myelin basic protein and proteolipid protein in oligodendrocytes, inducing central myelin breakdown. LMNB1 is negatively regulated by miR-23, which is also implicated in ADLD variants and it has been proposed that an over representation of lamin B1 mRNA sequesters miR-23 leading to disturbances in myelin protein production [60,66].
More recently, LMNB1 has been implicated as a susceptibility gene in neural tube defects. In a mouse model of spina bifida and exencephaly (curly tail), a Lmnb1 polymorphic variant was implicated as a modifier of neural tube closure defects. The defect results in loss of a C-terminal glutamic acid residue that causes nuclear shape defects and premature senescence in MEFs. Crossing curly tail strains with wild-type Lmnb1 strains partially rescues spina bifida and exencephaly [67]. In a study involving 239 patients with ADLD, exon sequences revealed a number of polymorphisms in LMNB1, including five synonymous and three non-synonymous (missense) variants. Of these, p.A501V was detected in nine patients, whilst p.A436T and p.D448G were each only detected in one patient. Expression of p.A436T in HeLa cells promoted nuclear dysmorphism [68]. Whilst not definitive, these intriguing results indicate that LMNB1 might contribute to neural tube defects in humans.
More recently, LMNB1 has also been implicated in cancers. In combination with vimentin, circulating lamin B1 is diagnostic of early stage hepatocellular carcinomas [69,70]. In breast cancer, decreased expression of LMNB1 is associated with poor prognosis [71]. This study is consistent with a finding that over-expression of lamin B1 is associated with low grade differentiation in pancreatic cancer and that the drug betulinic acid down-regulates lamin B1 expression as part of its anti-cancer activity [72]. Finally, β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 over-expression [73]. Taken together, these studies suggest that lamin B1 might be an important new target for anti-cancer therapies in a range of epithelial tumours.
7. Conclusions
For years it was thought that B-type lamins had essential functions in processes associated with cell proliferation. The original studies supporting these views were carried out in highly specialised model systems with either extremely simplified nuclear envelopes (Xenopus eggs) or highly specialised chromosome organisation (Xenopus oocytes). These systems also express a single specialised germ line specific B-type lamin (lamin Liii – [19,20]). In contrast, complete elimination of B-type lamins from mouse ESCs has apparently few deleterious effects. Their elimination from developing embryos certainly causes lethality, but some tissues, particularly brain, lung and bones, appear more vulnerable to such loss than others (e.g. skin and liver) [33,71,56]. Altered lamin B1 expression is also implicated in cellular senescence where it might contribute to the formation of SAHFs [46]. However, in both mouse development and senescence it appears that any deleterious function resulting from reduced lamin B1 expression is also associated with reduced or absent expression of lamin A/C implying that serious problems only arise when A-type lamins cannot compensate for loss of B-type lamin expression [37,45]. This is most dramatically seen in the brain where loss of lamin B2 expression or altered lamin B1 farnesylation causes severe loss of brain organisation and neuronal migration in mice [53,54], whilst LMNB1 duplication leads to severe adult onset brain and CNS degeneration in humans [59]. The molecular mechanisms behind these effects remain unclear but appear to arise through direct effects on neuronal nuclear structure [54] or indirect effects on oligodendrocyte mediated neuronal myelination [60]. Future studies should focus on lamin B1 interactions (e.g. with miR-23) which will form the basis of understand the pathways that do depend on these lamins.
Acknowledgements
I would like to thank the contributions of many students, post-docs and co-investigators over the years, particularly Jo Bridger, Juergen Meier and the late Keith Campbell who initiated work on lamins in my lab. I would also like to acknowledge the BBSRC, Wellcome Trust and AICR for recent financial support.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Dwyer N., Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol. 1976;70(3):581–591. doi: 10.1083/jcb.70.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;32:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 3.Fisher D.Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA. 1986;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeon F.D., Kirschner M.W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 5.Döring V., Stick R. Gene structure of nuclear lamin LIII of Xenopus laevis: a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J. 1990;9(12):4073–4081. doi: 10.1002/j.1460-2075.1990.tb07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin F., Worman H.J. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268(22):16321–16326. [PubMed] [Google Scholar]
- 7.Röber R.A., Weber K., Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1986;105(2):365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg M.W., Huttenlauch I., Hutchison C.J., Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci. 2008;121(Pt 2):215–225. doi: 10.1242/jcs.022020. [DOI] [PubMed] [Google Scholar]
- 9.Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- 10.Lin F., Worman H.J. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics. 1995;27(2):230–236. doi: 10.1006/geno.1995.1036. [DOI] [PubMed] [Google Scholar]
- 11.Biamonti G., Giacca M., Perini G., Contreas G., Zentilin L., Weighardt F. The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol Cell Biol. 1992;12(8):3499–3506. doi: 10.1128/mcb.12.8.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa K., Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993;12(1):97–106. doi: 10.1002/j.1460-2075.1993.tb05635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benavente R., Krohne G., Franke W.W. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell. 1985;41(1):177–190. doi: 10.1016/0092-8674(85)90072-8. [DOI] [PubMed] [Google Scholar]
- 14.Lehner C.F., Stick R., Eppenberger H.M., Nigg E.A. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol. 1987;105(1):577–587. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuurman N., Heins S., Aebi U. Nuclear lamins: their structure, assembly, and interactions. J Struct Biol. 1998;122(1–2):42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- 16.Karabinos A., Schünemann J., Meyer M., Aebi U., Weber K. The single nuclear lamin of Caenorhabditis elegans forms in vitro stable intermediate filaments and paracrystals with a reduced axial periodicity. J Mol Biol. 2003;325(2):241–247. doi: 10.1016/s0022-2836(02)01240-8. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Harush K., Wiesel N., Frenkiel-Krispin D., Moeller D., Soreq E., Aebi U. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol. 2009;386(5):1392–1402. doi: 10.1016/j.jmb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Broers J.L., Ramaekers F.C., Bonne G., Yaou R.B., Hutchison C.J. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86(3):967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 19.Meier J., Campbell K.H., Ford C.C., Stick R., Hutchison C.J. The role of lamin LIII in nuclear assembly and DNA replication, in cell-free extracts of Xenopus eggs. J Cell Sci. 1991;98(Pt 3):271–279. doi: 10.1242/jcs.98.3.271. [DOI] [PubMed] [Google Scholar]
- 20.Newport J.W., Wilson K.L., Dunphy W.G. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990;111(6 Pt 1):2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins H., Hölman T., Lyon C., Lane B., Stick R., Hutchison C. Nuclei that lack a lamina accumulate karyophilic proteins and assemble a nuclear matrix. J Cell Sci. 1993;106(Pt 1):275–285. doi: 10.1242/jcs.106.1.275. [DOI] [PubMed] [Google Scholar]
- 22.Spann T.P., Moir R.D., Goldman A.E., Stick R., Goldman R.D. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J Cell Biol. 1997;136(6):1201–1212. doi: 10.1083/jcb.136.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moir R.D., Yoon M., Khuon S., Goldman R.D. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J Cell Biol. 2000;149(6):1179–1192. doi: 10.1083/jcb.151.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis D.J., Jenkins H., Whitfield W.G., Hutchison C.J. GST-lamin fusion proteins act as dominant negative mutants in Xenopus egg extract and reveal the function of the lamina in DNA replication. J Cell Sci. 1997;110(Pt 20):2507–2518. doi: 10.1242/jcs.110.20.2507. [DOI] [PubMed] [Google Scholar]
- 25.Izumi M., Vaughan O.A., Hutchison C.J., Gilbert D.M. Head and/or CaaX domain deletions of lamin proteins disrupt preformed lamin A and C but not lamin B structure in mammalian cells. Mol Biol Cell. 2000;11(12):4323–4337. doi: 10.1091/mbc.11.12.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spann T.P., Goldman A.E., Wang C., Huang S., Goldman R.D. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol. 2002;156(4):603–608. doi: 10.1083/jcb.200112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smythe C., Jenkins H.E., Hutchison C.J. Incorporation of the nuclear pore basket protein nup153 into nuclear pore structures is dependent upon lamina assembly: evidence from cell-free extracts of Xenopus eggs. EMBO J. 2000;19(15):3918–3931. doi: 10.1093/emboj/19.15.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harborth J., Elbashir S.M., Bechert K., Tuschl T., Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114(Pt 24):4557–4765. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 29.Tsai M.Y., Wang S., Heidinger J.M., Shumaker D.K., Adam S.A., Goldman R.D. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311(5769):1887–1893. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- 30.Ma L., Tsai M.Y., Wang S., Lu B., Chen R., Iii J.R. Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat Cell Biol. 2009;11(3):247–256. doi: 10.1038/ncb1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Civelekoglu-Scholey G., Tao L., Brust-Mascher I., Wollman R., Scholey J.M. Prometaphase spindle maintenance by an antagonistic motor-dependent force balance made robust by a disassembling lamin-B envelope. J Cell Biol. 2010;188(1):49–68. doi: 10.1083/jcb.200908150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman B., Channels W., Qiu M., Iglesias P., Yang G., Zheng Y. Lamin B counteracts the kinesin Eg5 to restrain spindle pole separation during spindle assembly. J Biol Chem. 2010;285(45):35238–35244. doi: 10.1074/jbc.M110.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collado M., Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6(6):472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 34.Shimi T., Butin-Israeli V., Adam S.A., Hamanaka R.B., Goldman A.E., Lucas C.A. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25(24):2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freund A., Laberge R.M., Demaria M., Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23(11):2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barascu A., Le Chalony C., Pennarun G., Genet D., Imam N., Lopez B., Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31(5):1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreesen O., Chojnowski A., Ong P.F., Zhao T.Y., Common J.E., Lunny D. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200(5):605–617. doi: 10.1083/jcb.201206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pekovic V., Gibbs-Seymour I., Markiewicz E., Alzoghaibi F., Benham A.M., Edwards R. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell. 2011;10(6):1067–1079. doi: 10.1111/j.1474-9726.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- 39.Hutchison C.J. B-type lamins and their elusive roles in metazoan cell proliferation and senescence. EMBO J. 2012;31(5):1058–1059. doi: 10.1038/emboj.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickersgill H., Kalverda B., de Wit E., Talhout W., Fornerod M., van Steensel B. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet. 2006;38(9):1005–1014. doi: 10.1038/ng1852. [DOI] [PubMed] [Google Scholar]
- 41.Guelen L., Pagie L., Brasset E., Meuleman W., Faza M.B., Talhout W. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453(7197):948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 42.Elcock L.S., Bridger J.M. Exploring the relationship between interphase gene positioning, transcriptional regulation and the nuclear matrix. Biochem Soc Trans. 2010;38(Pt 1):263–267. doi: 10.1042/BST0380263. [DOI] [PubMed] [Google Scholar]
- 43.Reddy K.L., Zullo J.M., Bertolino E., Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452(7184):243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 44.Peric-Hupkes D., van Steensel B. Role of the nuclear lamina in genome organization and gene expression. Cold Spring Harb Symp Quant Biol. 2010;75:517–524. doi: 10.1101/sqb.2010.75.014. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y., Sharov A.A., McDole K., Cheng M., Hao H., Fan C.M. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334(6063):1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadaie M., Salama R., Carroll T., Tomimatsu K., Chandra T., Young A.R. Redistribution of the lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev. 2013;27(16):1800–1808. doi: 10.1101/gad.217281.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah P.P., Donahue G., Otte G.L., Capell B.C., Nelson D.M., Cao K. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27(16):1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riemer D., Stuurman N., Berrios M., Hunter C., Fisher P.A., Weber K. Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J Cell Sci. 1995;108(Pt 10):3189–3198. doi: 10.1242/jcs.108.10.3189. [DOI] [PubMed] [Google Scholar]
- 49.Osouda S., Nakamura Y., de Saint Phalle B., McConnell M., Horigome T., Sugiyama S. Null mutants of Drosophila B-type lamin Dm(0) show aberrant tissue differentiation rather than obvious nuclear shape distortion or specific defects during cell proliferation. Dev Biol. 2005;284(1):219–232. doi: 10.1016/j.ydbio.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., Rolef Ben-Shahar T., Riemer D., Treinin M., Spann P., Weber K. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11(11):3937–3947. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haithcock E., Dayani Y., Neufeld E., Zahand A.J., Feinstein N., Mattout A. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102(46):16690–16695. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vergnes L., Péterfy M., Bergo M.O., Young S.G., Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101(28):10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coffinier C., Chang S.Y., Nobumori C., Tu Y., Farber E.A., Toth J.I. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci USA. 2010;107(11):5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung H.J., Nobumori C., Goulbourne C.N., Tu Y., Lee J.M., Tatar A. Farnesylation of lamin B1 is important for retention of nuclear chromatin during neuronal migration. Proc Natl Acad Sci USA. 2013;110(21):E1923–E1932. doi: 10.1073/pnas.1303916110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S.H., Chang S.Y., Yin L., Tu Y., Hu Y., Yoshinaga Y. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011;20(18):3537–3544. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weisend C.M., Kundert J.A., Suvorova E.S., Prigge J.R., Schmidt E.E. Cre activity in fetal albCre mouse hepatocytes: Utility for developmental studies. Genesis. 2009;47(12):789–792. doi: 10.1002/dvg.20568. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hegele R.A., Cao H., Liu D.M., Costain G.A., Charlton-Menys V., Rodger N.W. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet. 2006;79:383–389. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao J., Li Y., Fu X., Luo X. A Chinese patient with acquired partial lipodystrophy caused by a novel mutation with LMNB2 gene. J Pediatr Endocrinol Metab. 2012;25:375–377. doi: 10.1515/jpem-2012-0007. [DOI] [PubMed] [Google Scholar]
- 59.Padiath Q.S., Saigoh K., Schiffmann R., Asahara H., Yamada T., Koeppen A. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38(10):1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 60.Lin S.T., Ptácek L.J., Fu Y.H. Adult-onset autosomal dominant leukodystrophy: linking nuclear envelope to myelin. J Neurosci. 2011;31(4):1163–1166. doi: 10.1523/JNEUROSCI.5994-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potic A., Pavlovic A.M., Uziel G., Kozic D., Ostojic J., Rovelli A. Adult-onset autosomal dominant leukodystrophy without early autonomic dysfunctions linked to lamin B1 duplication: a phenotypic variant. J Neurol. 2013;260(8):2124–2129. doi: 10.1007/s00415-013-6958-3. [DOI] [PubMed] [Google Scholar]
- 62.Guaraldi P., Donadio V., Capellari S., Contin M., Casadio M.C., Montagna P. Isolated noradrenergic failure in adult-onset autosomal dominant leukodystrophy. Auton Neurosci. 2011;159(1–2):123–126. doi: 10.1016/j.autneu.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Sundblom J., Melberg A., Kalimo H., Smits A., Raininko R. MR imaging characteristics and neuropathology of the spinal cord in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. AJNR Am J Neuroradiol. 2009;30(2):328–335. doi: 10.3174/ajnr.A1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melberg A., Hallberg L., Kalimo H., Raininko R. MR characteristics and neuropathology in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. AJNR Am J Neuroradiol. 2006;27(4):904–911. [PMC free article] [PubMed] [Google Scholar]
- 65.Giorgio E., Rolyan H., Kropp L., Chakka A.B., Yatsenko S., Gregorio E.D. Analysis of LMNB1 duplications in autosomal dominant leukodystrophy provides insights into duplication mechanisms and allele-specific expression. Hum Mutat. 2013;34(August (8)):1160–1171. doi: 10.1002/humu.22348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin S.T., Fu Y.H. miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis Model Mech. 2009;2(3–4):178–188. doi: 10.1242/dmm.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Castro S.C., Malhas A., Leung K.Y., Gustavsson P., Vaux D.J., Copp A.J. Lamin B1 polymorphism influences morphology of the nuclear envelope, cell cycle progression, and risk of neural tube defects in mice. PLoS Genet. 2012;8(11):e1003059. doi: 10.1371/journal.pgen.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson A., Partridge D., Malhas A., De Castro S.C., Gustavsson P., Thompson D.N. Is LMNB1 a susceptibility gene for neural tube defects in humans? Birth Defects Res A: Clin Mol Teratol. 2013;97(6):398–402. doi: 10.1002/bdra.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun S., Xu M.Z., Day P.J., Luk J.M. Circulating lamin B1 (LMNB1) biomarker detects early stage liver cancer in patients. J Proteome Res. 2010;9:70–78. doi: 10.1021/pr9002118. [DOI] [PubMed] [Google Scholar]
- 70.Wong K.F., Luk J.M. Discovery of lamin B1 and vimentin as circulating biomarkers of early stage hepatocellular carcinoma. Methods Mol Biol. 2012;909:295–310. doi: 10.1007/978-1-61779-959-4_19. [DOI] [PubMed] [Google Scholar]
- 71.Wazir U., Ahmed M.H., Bridger J.M., Harvey A., Jiang W.G., Sharma A.K. The clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor mRNA expression in human breast cancer. Cell Mol Biol Lett. 2013 doi: 10.2478/s11658-013-0109-9. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L., Kong X., Jia Z., Cui J., Gao J., Wang G. Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer. Clin Cancer Res. 2013;19:4651–4661. doi: 10.1158/1078-0432.CCR-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu L., Wang J., Lei S., Zhang W., Du X., Wang Z. β-Asarone induces senescence in colorectal cancer cells by inducing lamin B1 expression. Phytomedicine. 2013;20:512–520. doi: 10.1016/j.phymed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Liu B., Wang J., Chan K.M., Tjia W.M., Deng W., Guan X., Huang J.D., Li K.M., Chau P.Y., Chen D.J., Pei D., Pendas A.M., Cadiñanos J., López-Otín C., Tse H.F., Hutchison C., Chen J., Cao Y., Cheah K.S., Tryggvason K., Zhou Z. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11(7):780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]


