Abstract
Background
To our knowledge, no prospective study has examined the association between vitamin D and cognitive decline or dementia.
Methods
We determined whether low levels of serum 25-hydroxyvitamin D (25[OH]D) were associated with an increased risk of substantial cognitive decline in the InCHIANTI population–based study conducted in Italy between 1998 and 2006 with follow-up assessments every 3 years. A total of 858 adults 65 years or older completed interviews, cognitive assessments, and medical examinations and provided blood samples. Cognitive decline was assessed using the Mini-Mental State Examination (MMSE), and substantial decline was defined as 3 or more points. The Trail-Making Tests A and B were also used, and substantial decline was defined as the worst 10% of the distribution of decline or as discontinued testing.
Results
The multivariate adjusted relative risk (95% confidence interval [CI]) of substantial cognitive decline on the MMSE in participants who were severely serum 25 (OH)D deficient (levels <25 nmol/L) in comparison with those with sufficient levels of 25(OH)D (≥75 nmol/L) was 1.60 (95% CI, 1.19-2.00). Multivariate adjusted random-effects models demonstrated that the scores of participants who were severely 25(OH)D deficient declined by an additional 0.3 MMSE points per year more than those with sufficient levels of 25(OH)D. The relative risk for substantial decline on Trail-Making Test B was 1.31 (95% CI, 1.03-1.51) among those who were severely 25(OH)D deficient compared with those with sufficient levels of 25(OH)D. No significant association was observed for Trail-Making Test A.
Conclusion
Low levels of vitamin D were associated with substantial cognitive decline in the elderly population studied over a 6-year period, which raises important new possibilities for treatment and prevention.
It is estimated that between 40% and 100% of older, community-living adults in the United States and Europe are vitamin D deficient.1 Vitamin D deficiency is associated with fractures, various chronic conditions, and mortality.2-5 Cognitive decline and dementia are also common in older adults, although their causes remain unclear.6-8 Vitamin D may help to prevent neurodegeneration because it plays an important role in the expression of neurotrophic factors, neurogenesis, calcium homeostasis, detoxification, and β-amyloid clearance.9-11
Animal and in vitro experiments suggest that vitamin D is neuroprotective.9,12,13 However, several small clinical studies provide equivocal evidence linking low serum 25-hydroxyvitamin D (25[OH]D) levels to cognitive dysfunction.14-20 No cross-sectional association between serum 25(OH)D levels and verbal memory was observed in older adults from the Third National Health and Nutrition Examination Survey.21 However, cross-sectional associations between 25(OH)D levels and cognitive dysfunction in older adults were observed using data from the Health Survey for England,22 the European Male Aging Study,23 and the Nutrition and Memory in Elders Study.24 Vitamin D supplementation studies have reported improved attention and reaction times after 6 months in 139 ambulatory participants with a history of falls,25 and a small improvement in clock drawing performance, though not verbal fluency, over 4 weeks in 25 nursing home residents who were 25(OH)D deficient at baseline.26
To our knowledge no previous prospective population-based studies have examined the relationship between vitamin D status and cognitive decline or incident dementia. We examined the association between vitamin D and subsequent cognitive decline using data from a large sample of older people enrolled in the InCHIANTI study,27 a prospective cohort study conducted in Tuscany, Italy.
METHODS
STUDY POPULATION
The InCHIANTI study was designed to identify risk factors for late-life disability and has been described extensively elsewhere.27 Briefly, the InCHIANTI study is a prospective population-based study that used a multistage sampling method to evaluate randomly selected older adults living in Greve, Chianti, and Bagno a Ripoli, Tuscany, Italy. A total of 1270 adults 65 years or older were randomly selected from the population using city registries, and 1154 participants agreed to take part in the study. A total of 858 participants completed at least 1 follow-up cognitive assessment between 1998 and 2006 with follow-up assessments every 3 years and were included in the present analysis (mean [SD] follow-up, 5.2 [1.3] years; median, 6 years). Compared with the analyzed group, those lost to follow-up were generally older (mean [SD] age, 79.7 [8.4] vs 74.0 [6.8] years), less educated (44.3% vs 28.0% with no educational qualifications), had lower baseline cognitive function (mean [SD] Mini-Mental State Examination [MMSE] score,28 20.9 [7.5] vs 25.2 [3.8]) and lower levels of serum 25(OH)D (mean [SD], 35.2 [20.2] vs 51.6 [37.1] nmol/L) (P<.001 for all comparisons), but they were no more likely to be female (56.6% vs 56.8%) (P>.99). The Instituto Nazionale Riposo e Cura Anziani institutional review board provided ethical approval for the study. Participants gave informed consent to participate, or if they were unable to do so, a close relative provided surrogate consent.
SERUM 25(OH)D CONCENTRATION
Blood samples obtained after the patient had fasted for 12 hours and rested for at least 15 minutes were centrifuged and stored at −80°C until analyzed.29 Serum levels of 25(OH)D were measured by radioimmunoassay (RIA kit; DiaSorin, Stillwater, Minnesota): intraassay and interassay coefficients of variation were 8.1% and 10.2%, respectively.
COGNITIVE TESTS
Tests of cognitive function were administered at baseline, after 3 years, and after 6 years. The 30-item MMSE28 is the most widely used neuropsychological measure of cognitive function and is an effective screening instrument for dementia in the general population. Scores for the MMSE range from 0 to 30, with lower scores reflecting worse cognitive function. Trail-Making Tests A and B (hereinafter referred to as Trails A and B) were administered to measure visuospatial scanning, sequential processing, motor speed, attention, and executive function. Follow-up data for Trails A was available for 680 participants, and 487 participants also completed at least 1 follow-up assessment on Trails B. Trails A involves connecting a series of consecutively numbered circles and focuses particularly on attention, whereas Trails B incorporates an alternating sequence of numbered and lettered circles and places greater emphasis on executive function. Worse performance is indicated by longer times to complete the Trails A and B.
STATISTICAL ANALYSIS
Multivariate logistic regression models were used to determine the relationship of serum 25(OH)D levels to substantial cognitive decline, which we defined as (1) a decline in MMSE score of 3 or more points at any stage of follow-up (n=290); and (2) as scoring in the worst 10% of cognitive decline or having the testing discontinued owing to multiple mistakes in Trails A (n=165) and Trails B (n=275). We divided levels of serum 25(OH)D into clinical groups to aid interpretation: severely 25(OH)D deficient (<25 nmol/L); 25(OH)D deficient (≥25 to <50 nmol/L); 25 (OH)D insufficient (≥50 to <75 nmol/L); and 25(OH)D sufficient (≥75 nmol/L).1 In unadjusted models, we controlled for baseline cognitive score only. In fully adjusted models, we adjusted for variables that have been identified as potential confounders in studies of cognition or 25(OH)D levels: age in years, sex, education (whether they completed elementary school), season during which blood samples were obtained, current smoking status, depressive symptoms (score ≥16 on the Italian Center for Epidemiologic Studies Depression Scale30), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), alcohol consumption (g/d), total energy intake (kcal/d) estimated by food frequency questionnaire,31 serum vitamin E level (alpha-tocopherol [μmol/L]),32 impaired mobility (mean gait speed, ≤0.4 m/s during 2 timed 4-m walks at normal pace33 or self-reported difficulty walking 100 m without stopping), and length of follow-up in years. There was no evidence of overfitting or colinearity. Linear trends across clinical 25(OH)D groups were tested by introducing 25(OH)D groups into separate logistic regression models as a continuous variable (rather than as a categorical variable). We corrected for possible overestimation of odds ratios using the Zhang and Yu34 method of deriving risk ratios.
We used random-effects models to examine the association between serum 25(OH)D level and MMSE scores modeled as a continuous variable at baseline and the 3- and 6-year follow-up waves. Random-effects models were valuable in this context because they allowed us to take into account both variation between subjects and autocorrelation between repeated measurements of the same participants over time.35 Random-effects terms included both the intercept and slope of cognitive scores over time. Candidate fixed-effects terms included all baseline covariates and their interaction with time (years of follow-up). The Bayesian Information Criterion was used to decide which fixed effects to include.36
Possible 2-way interactions between 25(OH)D level and baseline cognitive function were tested by including product terms in fully adjusted logistic regression models. In a series of preplanned secondary analyses, we excluded those participants with dementia at baseline (n=29) as diagnosed by geriatricians and a psychologist with expertise in cognitive impairment according to criteria set out in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).37 We investigated whether any observed association was mediated by medical conditions thought to be associated with both vitamin D status and cognitive function: stroke (neurologic signs indicating stroke or medical history); diabetes (using an antidiabetic agent, medical history, or having a fasting plasma glucose level ≥126 mg/dL); and hypertension (using antihypertensive agents, medical history, or having a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg). (To convert glucose to millimoles per liter, multiply by 0.0555.)
Levels of 25(OH)D were also analyzed as a log-transformed continuous variable in a series of further random-effects models. Because differential loss to follow-up has the potential to bias results, we performed weighted logistic regression analyses. We derived weights for each cognitive test using the inverse probability of having completed at least 1 repeated cognitive assessment using a logistic regression model with key variables as predictors (log-transformed 25[OH]D level, baseline cognitive performance, age in years, sex, education, and season of serum collection). Since no difference in the pattern of results was observed whether weighting was used or not, we report only the results of the unweighted analyses.
P values were 2 sided throughout, and the type I error rate for statistical significance was preset at .05. Because this was a post hoc analysis of a previously assessed cohort, statistical power was not formally calculated prospectively. Analyses were performed using Stata SE, version 9.2 (StataCorp, College Station, Texas) with the exception of the random-effects models, which were performed using Mplus, version 5 (Muthen & Muthen, Los Angeles, California).
RESULTS
The characteristics of the study population are listed in Table 1. Unadjusted baseline MMSE, Trails A, and Trails B scores were significantly lower in those subjects who were 25(OH)D deficient or severely deficient than in those who were 25(OH)D sufficient, and just over half of participants with dementia were severely 25(OH)D deficient. Participants who were 25(OH)D deficient or severely deficient were also more likely than those who were 25(OH)D sufficient to be older and female; to have been tested between December and May; to have significant depressive symptoms, impaired mobility, and lower total energy intake; to have had a stroke; to have no educational qualifications; and to consume no alcohol.
Table 1. Baseline Characteristics of 858 InCHIANTI27 Participants by Serum 25(OH)D Concentrationa.
| Serum 25(OH)D Concentration, nmol/L |
|||||
|---|---|---|---|---|---|
| Characteristic | <25 (n=175) |
≥25 to <50 (n=360) |
≥50 to <75 (n=166) |
≥75 (n = 157) |
P Valueb |
| Age, mean (SD), y | 77.5 (7.9) | 74.1 (6.2) | 72.1 (6.0) | 71.6 (5.5) | <.001 |
| Women | 140 (80.0) | 220 (61.1) | 65 (39.2) | 62 (39.5) | <.001 |
| No educational qualificationsc | 109 (62.3) | 241 (66.9) | 31 (18.7) | 24 (15.3) | <.001 |
| Season tested | <.001 | ||||
| December-February | 81 (46.3) | 137 (38.1) | 43 (25.9) | 46 (29.3) | |
| March-May | 45 (25.7) | 93 (25.8) | 17 (10.2) | 8 (5.1) | |
| June-August | 8 (4.6) | 29 (8.1) | 22 (13.3) | 28 (17.8) | |
| September-November | 41 (23.4) | 101 (28.1) | 84 (50.6) | 75 (47.8) | |
| Alcohol consumption, g/d | <.001 | ||||
| 0 | 71 (40.6) | 101 (28.1) | 32 (19.3) | 28 (17.8) | |
| 0.1-29.9 | 93 (53.1) | 213 (59.2) | 92 (55.4) | 95 (60.5) | |
| ≥30.0 | 11 (6.3) | 46 (12.8) | 42 (25.3) | 34 (21.7) | |
| Current smoker | 19 (10.9) | 44 (12.2) | 30 (18.1) | 28 (17.8) | .09 |
| Depressive symptoms (CES-D score ≥16) | 85 (48.6) | 118 (32.8) | 43 (25.9) | 38 (24.2) | <.001 |
| BMI, mean (SD) | 27.2 (4.7) | 27.8 (4.2) | 27.3 (3.8) | 27.0 (3.3) | .19 |
| Total energy intake, mean (SD), kcal | 1756.2 (450.0) | 1903.7 (550.0) | 2020.2 (573.2) | 2100.6 (617.2) | <.001 |
| Serum vitamin E (alpha tocopherol), mean (SD), μmol/L | 30.0 (7.8) | 30.5 (8.8) | 30.4 (8.2) | 31.0 (8.2) | .71 |
| Impaired mobility | 64 (36.6) | 49 (13.6) | 13 (7.8) | 9 (5.7) | <.001 |
| Stroke | 14 (8.0) | 18 (5.0) | 2 (1.2) | 6 (3.8) | .03 |
| Diabetes | 25 (14.3) | 52 (14.4) | 16 (9.6) | 23 (14.7) | .45 |
| Hypertension | 155 (88.6) | 297 (82.5) | 135 (81.3) | 134 (85.4) | .22 |
| Dementia | 16 (9.1) | 10 (2.8) | 2 (1.2) | 1 (0.6) | <.001 |
| Cognitive function scores, mean (SD)d | |||||
| MMSE | 23.7 (5.3) | 25.2 (3.3) | 26.0 (3.0) | 26.3 (2.8) | <.001 |
| Trails A | 151.8 (94.7) | 114.9 (75.5) | 94.2 (69.7) | 87.2 (62.1) | <.001 |
| Trails B | 239.9 (78.4) | 219.6 (80.8) | 188.5 (32.4) | 180.5 (87.0) | <.001 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiologic Studies Depression Scale30; MMSE, Mini-Mental State Examination29 (range, 0-30; higher score represents better function); Trails, Trail Making Tests (range, 0-300; higher score represents worse function).
Unless otherwise indicated, data are reported as number (percentage) of subjects.
P value for X2 test for categorical variables and 1-way analysis of variance for continuous variables.
Participants with no educational qualifications did not complete elementary school.
In logistic regression models adjusted only for baseline cognitive function, participants who were severely 25(OH)D deficient were more likely than those who were 25(OH)D sufficient to have substantial cognitive decline on the MMSE and Trails B test scores (Table 2). Significant linear trends between groups suggested a monotonic relationship. These associations were attenuated slightly but remained significant after full adjustment. Those who were severely 25(OH)D deficient were approximately 60% more likely than those who were 25(OH)D sufficient to experience substantial cognitive decline on the MMSE score and 31% more likely to have substantial decline on the Trails B score. There were no significant associations between 25(OH)D levels and performance on Trails A. The same pattern of associations was observed when we restricted the sample to participants who were nondemented at baseline (Table 3).
Table 2. Logistic Regression Models for Relative Risk of 6-Year Substantial Cognitive Decline in Older Persons by Serum 25(OH)D Level.
| Measure of Substantial Cognitive Declinea | Serum 25(OH)D, nmol/Lb |
P Value for Linear Trend | |||
|---|---|---|---|---|---|
| ≥75 | ≥50 to <75 | ≥25 to <50 | <25 | ||
| MMSE | |||||
| Unadjusted modelc | 1 [Reference] | 1.27 (0.93-1.62) | 1.26 (0.97-1.57) | 1.78 (1.43-2.09) | <.001 |
| Fully adjusted modeld | 1 [Reference] | 1.19 (0.84-1.58) | 1.09 (0.78-1.43) | 1.60 (1.19-2.00) | .02 |
| Trails A | |||||
| Unadjusted modelc | 1 [Reference] | 0.94 (0.56-1.47) | 1.30 (0.88-1.79) | 1.26 (0.78-1.86) | .12 |
| Fully adjusted modeld | 1 [Reference] | 0.95 (0.55-1.51) | 1.25 (0.75-1.71) | 1.16 (0.65-1.84) | .44 |
| Trails B | |||||
| Unadjusted modelc | 1 [Reference] | 0.94 (0.69-1.16) | 1.07 (0.86-1.26) | 1.27 (1.01-1.46) | .04 |
| Fully adjusted modeld | 1 [Reference] | 0.99 (0.74-1.23) | 1.11 (0.88-1.32) | 1.31 (1.03-1.51) | .04 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; MMSE, Mini-Mental State Examination29 (range, 0-30; higher score represents better function); Trails, Trail Making Tests (range, 0-300; higher score represents worse function).
Substantial cognitive decline was defined as 3 or more points on the MMSE and the worst 10% of cognitive decline or test discontinued for the Trails A and B.
Unless otherwise indicated, data are reported as relative risk (95% confidence interval).
Adjusted for baseline cognitive score only.
Adjusted for age, sex, education, baseline cognitive score, season tested, alcohol consumption, current smoking status, depressive symptoms, body mass index, total energy intake, serum vitamin E level (alpha tocopherol), and impaired mobility.
Table 3. Logistic Regression Models for Relative Risk of 6-Year Substantial Cognitive Decline in Nondemented Older Persons by Serum 25(OH)D Level.
| Measure of Substantial Cognitive Declinea | Serum 25(OH)D, nmol/Lb |
P Value for Linear Trend | |||
|---|---|---|---|---|---|
| ≥75 | ≥50 to <75 | ≥25 to <50 | <25 | ||
| MMSE | |||||
| Unadjusted modelc | 1 [Reference] | 1.28 (0.94-1.65) | 1.25 (0.94-1.65) | 1.85 (1.49-2.17) | <.001 |
| Fully adjusted modeld | 1 [Reference] | 1.22 (0.86-1.63) | 1.18 (0.80-1.47) | 1.64 (1.20-2.05) | .02 |
| Trails A | |||||
| Unadjusted modelc | 1 [Reference] | 0.91 (0.54-1.43) | 1.29 (0.88-1.78) | 1.27 (0.78-1.87) | .11 |
| Fully adjusted modeld | 1 [Reference] | 0.94 (0.54-1.50) | 1.19 (0.76-1.73) | 1.16 (0.65-1.85) | .41 |
| Trails B | |||||
| Unadjusted modelc | 1 [Reference] | 0.94 (0.69-1.17) | 1.07 (0.86-1.26) | 1.27 (1.01-1.46) | .04 |
| Fully adjusted modeld | 1 [Reference] | 0.99 (0.73-1.23) | 1.11 (0.87-1.32) | 1.32 (1.03-1.51) | .04 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; MMSE, Mini-Mental State Examination29 (range, 0-30; higher score represents better function); Trails, Trail Making Tests (range, 0-300; higher score represents worse function).
Substantial cognitive decline was defined as 3 or more points on the MMSE and the worst 10% of cognitive decline or test discontinued for the Trails A and B.
Unless otherwise indicated, data are reported as relative risk (95% confidence interval).
Adjusted for baseline cognitive score only.
Adjusted for age, sex, education, baseline cognitive score, season tested, alcohol consumption, current smoking status, depressive symptoms, body mass index, total energy intake, serum vitamin E level (alpha tocopherol), and impaired mobility.
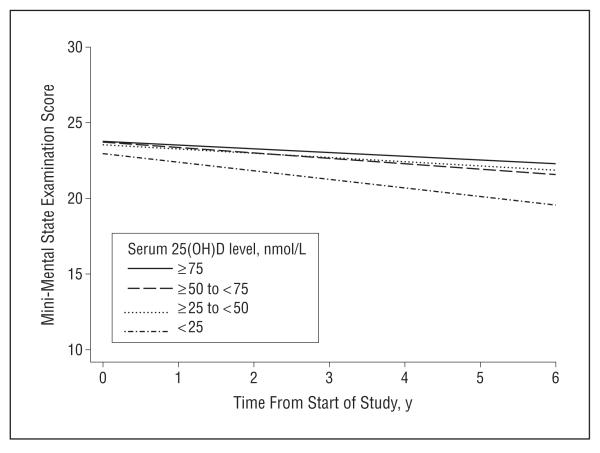
Lower levels of serum 25(OH)D were associated with greater year-on-year decline in cognitive function (Table 4). In random-effects models adjusted for baseline cognitive function only, those who were severely 25(OH)D deficient declined by an additional 0.7 MMSE points per year compared with those who were 25(OH)D sufficient. In fully adjusted models, participants who were severely 25(OH)D deficient declined by 0.3 MMSE points per year more than participants who were 25(OH)D sufficient. The increased rate of decline for those who were severely 25(OH)D deficient was statistically significant, as was the linear trend across groups. The Figure shows the estimated mean MMSE trajectory for each of the different 25(OH)D groups. Excluding those who were demented at baseline had little effect on the results.
Table 4. Random-Effects Models Illustrating Change in MMSE-Measured Cognitive Function by Serum 25(OH)D Levela.
| Serum 25(OH)D Level, nmol/L | All Participants (n=858) |
Nondemented Participants Only (n=829) |
||
|---|---|---|---|---|
| Unadjusted Modelb | Fully Adjusted Modelc | Unadjusted Modelb | Fully Adjusted Modelc | |
| ≥75 | 0.0 [Reference] | 0.0 [Reference] | 0.0 [Reference] | 0.0 [Reference] |
| ≥50 to <75 | −0.085 (0.169) | −0.111 (0.115) | −0.117 (0.174) | −0.130 (0.114) |
| ≥25 to <50 | −0.139 (0.146) | −0.035 (0.095) | −0.154 (0.153) | −0.051 (0.096) |
| <25 | −0.664 (0.146) | −0.321 (0.109) | −0.684 (0.153) | −0.310 (0.109) |
| P value for linear trend | <.001 | .03 | <.001 | .04 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; MMSE, Mini-Mental State Examination29 (range, 0-30; higher score represents better function).
All data are reported as estimated (SE) β values.
Adjusted for baseline cognitive score only.
Adjusted for age, sex, education, baseline cognitive score, season tested, body mass index, impaired mobility, diabetes, and stroke.
Figure.
Change in cognitive function for 858 older persons by serum 25-hydroxyvitamin D (25[OH]D) concentration. Results are based on a random-effects model with multivariate adjustment for age, sex, education, baseline cognitive score, season tested, body mass index, impaired mobility, diabetes, and stroke.
No significant 2-way interactions between 25(OH)D level and baseline cognitive function were observed for the MMSE (P=.76), Trails A (P=.40), or Trails B (P = .91) tests. Additional adjustment for stroke, diabetes, and hypertension did not change the pattern of results observed (Table 5), suggesting that these conditions are not likely to mediate the observed associations. Random-effects models incorporating log-transformed levels of 25(OH)D rather than predefined categories also indicated that baseline levels of 25(OH)D were associated with subsequent cognitive decline. In fully adjusted models, the association between log-transformed 25(OH)D level and performance on the MMSE test was significant for all participants (β=0.143, SE=0.051, and P=.005) and for participants who were nondemented at baseline (β=0.134, SE=0.050, and P=.008).
Table 5.
Fully Adjusted Logistic Regression Models for Relative Risk of 6-Year Substantial Cognitive Decline in Older Persons by Serum 25(OH)D Level, Including Adjustment for Potential Mediatorsa
| Measure of Substantial Cognitive Declineb | Serum 25(OH)D Level, nmol/L |
P Value for Linear Trend | |||
|---|---|---|---|---|---|
| ≥75 | ≥50 to <75 | ≥25 to <50 | <25 | ||
| MMSE | 1 [Reference] | 1.24 (0.88-1.63) | 1.09 (0.78-1.44) | 1.61 (1.19-2.01) | .02 |
| Trails A | 1 [Reference] | 0.98 (0.57-1.55) | 1.18 (0.75-1.72) | 1.16 (0.65-1.72) | .44 |
| Trails B | 1 [Reference] | 1.00 (0.74-1.24) | 1.11 (0.87-1.31) | 1.32 (1.03-1.51) | .05 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiologic Studies Depression Scale30; MMSE, Mini-Mental State Examination29 (range, 0-30; higher score represents better function); Trails, Trail Making Tests (range, 0-300; higher score represents worse function).
Unless otherwise indicated, data are reported as relative risk (95% confidence interval). All analyses have been adjusted for age, sex, education, baseline cognitive score, season tested, alcohol consumption, current smoking status, depressive symptoms, body mass index, total energy intake, serum vitamin E level (alpha tocopherol), and impaired mobility plus potential mediators (stroke, hypertension, and diabetes).
Substantial cognitive decline was defined as 3 or more points on the MMSE and the worst 10% of cognitive decline or test discontinued for Trails A and B.
COMMENT
In this population-based prospective study, we found that elderly people with low levels of 25(OH)D were at increased risk of cognitive decline over 6 years, and there was evidence of a monotonic relationship. The association remained significant after adjustment for a wide range of potential confounders and when analyses were restricted to elderly subjects who were nondemented at baseline. To our knowledge, this is the first prospective study to show that low levels of 25(OH)D are associated with elevated risk of cognitive decline.
The strengths of this study include that we were able to adjust statistically for a wide range of potentially confounding variables such as sociodemographic characteristics, clinical status, health behaviors, and dietary factors. Response rates at each wave of the InCHIANTI study were high, and minimal bias is likely due to attrition. Low levels of 25(OH)D at baseline may reflect the limited physical or outdoor activity of participants who already had dementia and were thus susceptible to further cognitive decline. However, we were able to conduct analyses excluding those with dementia at baseline, and no 2-way interactions were observed between 25(OH)D levels and baseline cognitive function. Taken together with the prospective study design, this fact allows us to be more confident that the association observed was not due to reverse causation. We also adjusted for impaired mobility, which did not greatly attenuate the association. Assessment of cognitive performance included the MMSE, the most widely used measure of cognitive function, and Trails A and B, which are also commonly used.
Our study had some limitations that should be considered when interpreting the results. While the InCHIANTI study is population based, it incorporates participants from a geographically confined area, and further research is needed to examine whether our findings generalize to other regions. Similarly, participants were all of white European origin, and we were not able to assess whether the association was similar in other ethnic groups. While attrition was minimal for a study of this kind, it is still possible that attrition biased our results. However, we conducted weighted analyses to allow for nonrandom attrition, and these gave highly similar results. Finally, the underlying cause of the cognitive changes observed was not assessed, and we were unable to evaluate which of the possible pathophysiologic mechanisms are important on a population level.
It has long been known that calcitriol (1,25-dihydroxycholecalciferol), the bioactive form of 25 (OH)D, plays a crucial role in phosphate homeostasis, bone mineralization and regulating levels of calcium. However, accumulating evidence suggests previously unsuspected roles for vitamin D in brain development and neuroprotection. Low levels of serum 25(OH)D may be associated with an increased risk of neurologic diseases such as multiple sclerosis,38 and Parkinson disease.39 Vitamin D receptors are present in a wide variety of cells, including neurons and glial cells, and genes encoding the enzymes involved in the metabolism of vitamin D are also expressed in the brain.9 In a recent review, Buell and Dawson-Hughes10 emphasize that vitamin D may be neuroprotective through antioxidative mechanisms, immunomodulation, neuronal calcium regulation, detoxification mechanisms, and enhanced nerve conduction.10 Vitamin D may play a role in brain detoxification pathways by reducing cellular calcium, inhibiting the synthesis of inducible nitric oxide synthase, and increasing levels of the antioxidant glutathione.12 Vitamin D stimulates neurogenesis and regulates the synthesis of neurotrophic factors, which are important for cell differentiation and survival.9,40 Vitamin D is also an immunosuppressor and may inhibit autoimmune damage to the nervous system.12 Calcitriol stimulates β-amyloid phagocytosis and clearance while protecting against apoptosis.11
Results from small clinical studies assessing the relationship between vitamin D and cognitive function that incorporate highly selected samples and limited adjustment for potential confounders have been equivocal.14-20 Four large population-based cross-sectional studies have examined the association between levels of serum 25(OH)D and cognitive dysfunction. The first of these, by McGrath and colleagues,21 found no association with a brief measure of verbal memory. However, Llewellyn and colleagues22 observed a significant association between low levels of 25(OH)D and increased odds of cognitive impairment. Similarly, Buell and colleagues24 observed a positive association between 25(OH)D levels and tests of executive function and processing speed, but not memory. Finally, Lee and colleagues23 observed a significant positive association between 25(OH)D levels and a test of sustained attention, but not with memory or visuospatial ability. Two small trials also suggest that vitamin D supplementation may be associated with improved cognitive function over short periods.25,26 Taken together with these findings, our results suggest that low serum 25(OH)D level is associated with cognitive dysfunction. Low levels of 25(OH)D may be particularly detrimental to executive functions, whereas other cognitive domains such memory may be relatively preserved, as previously hypothesized.22 Future trials of vitamin D supplementation in the elderly population could usefully include tests of cognitive function.
We found that elderly subjects with low levels of 25(OH)D had a higher relative risk of substantial cognitive decline over a 6-year period and that this association remained after adjusting for potential confounders. If future prospective studies and randomized controlled trials confirm that vitamin D deficiency is causally related to cognitive decline, then this would open up important new possibilities for treatment and prevention.
Acknowledgments
Funding/Support: The InCHIANTI study is supported in part by the Italian Ministry of Health and by the United States National Institute on Aging (NIA). Dr Langa was supported by NIA grant R01 AG027010. Dr Lang is an Academic Speciality Registrar in Public Health supported by the National Health Service South-West Region Public Health Training Scheme. Dr Muniz-Terrera is funded by an MRC Career Development Award in Biostatistics.
Footnotes
Financial Disclosure: None reported.
Disclaimer: The sponsors played no part in the study design; collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.van der Wielen RP, Lowik MR, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346(8969):207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 3.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 5.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3(5):1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferri CP, Prince M, Brayne C, et al. Alzheimer’s Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 10.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29(6):415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masoumi A, Goldenson B, Ghirmai S, et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17(3):703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 12.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 13.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain, I: micronutrients. J Nutr Health Aging. 2006;10(5):377–385. [PubMed] [Google Scholar]
- 14.Aung K, Burnett J, Smith SM, Dyer CB. Vitamin D deficiency associated with self-neglect in the elderly. J Elder Abuse Negl. 2006;18(4):63–78. doi: 10.1300/j084v18n04_07. [DOI] [PubMed] [Google Scholar]
- 15.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels: the Tromso study. J Neurol. 2006;253(4):464–470. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 16.Kipen E, Helme RD, Wark JD, Flicker L. Bone density, vitamin D nutrition, and parathyroid hormone levels in women with dementia. J Am Geriatr Soc. 1995;43(10):1088–1091. doi: 10.1111/j.1532-5415.1995.tb07005.x. [DOI] [PubMed] [Google Scholar]
- 17.Nes M, Sem SW, Rousseau B, et al. Dietaryintakesandnutritionalstatusofoldpeople with dementia living at home in Oslo. Eur J Clin Nutr. 1988;42(7):581–593. [PubMed] [Google Scholar]
- 18.Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 19.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? a positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 21.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29(1-2):49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 22.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin d concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22(3):188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DM, Tajar A, Ulubaev A, et al. EMAS study group Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722–729. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- 24.Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64(8):888–895. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33(6):589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 26.Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19(11):1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Maggio D, Cherubini A, Lauretani F, et al. OH)D serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyper-parathyroidism in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(11):1414–1419. doi: 10.1093/gerona/60.11.1414. [DOI] [PubMed] [Google Scholar]
- 30.Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol. 1983;39(2):249–251. doi: 10.1002/1097-4679(198303)39:2<249::aid-jclp2270390218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 32.Cherubini A, Martin A, Andres-Lacueva C, et al. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging. 2005;26(7):987–994. doi: 10.1016/j.neurobiolaging.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Melzer D. Neighbourhood deprivation and incident mobility disability in older adults. Age Ageing. 2008;37(4):403–410. doi: 10.1093/ageing/afn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 35.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 36.Schwarz GE. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 37.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 38.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 39.Newmark HL, Newmark J. Vitamin D and Parkinson’s disease–a hypothesis. Mov Disord. 2007;22(4):461–468. doi: 10.1002/mds.21317. [DOI] [PubMed] [Google Scholar]
- 40.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]