Abstract
We examined the role of putative trafficking sequences in two GABAA receptor subunits: α4 and δ. These subunits assemble with a β subunit to form a subtype of GABAA receptor involved in generating the “tonic” outward current. Both α4 and δ subunits contain dibasic retention motifs in homologous positions. When basic residues are mutated to alanine in the α4 subunit, surface expression of epitope-tagged δ subunits is increased. When basic residues in homologous regions of the δ subunit are mutated, however, surface expression is reduced. We focused on the mutants that had the maximal effects to increase (in α4) or reduce (in δ) surface expression. The total expression of δ subunits is significantly decreased by the δ mutation, suggesting an effect on subunit maturation. We also examined surface expression of the β2 subunit. Expression of the mutated α4 subunit resulted in increased surface expression of β2 compared with wild-type α4, indicating enhanced forward trafficking. In contrast, mutated δ resulted in decreased surface expression of β2 compared with wild-type δ and to α4 and β2 in the absence of any δ. This observation suggests that the mutated δ incorporates into multimeric receptors and reduces the overall forward trafficking of receptors. These observations indicate that the roles of trafficking motifs are complex, even when located in homologous positions in related subunits. The physiologic properties of receptors containing mutated subunits were not significantly affected, indicating that the mutations in the α4 subunit will be useful to enhance surface expression.
Introduction
The delivery of multimeric proteins to the cell surface is a complex process, requiring appropriate insertion and assembly of subunits in intracellular compartments followed by forward trafficking to the plasma membrane. The inability of proteins to journey through the cell to their appropriate target location has long been known to result in disease, especially as it relates to protein folding aberrations and retention in the endoplasmic reticulum (ER) and other subcellular compartments (Lai and Jan, 2006; Marciniak and Ron, 2006). A number of signal sequences have been identified and characterized for their effects on protein subcellular localization and transport to and from the ER (Barlowe, 2003; Michelsen et al., 2005). Our work is concerned with one class of surface membrane protein, the family of pentameric ligand gated ion channels (pLGICs) that serve as receptors for neurotransmitters in the nervous system.
Several studies have examined the trafficking of receptors in the pLGIC family, which includes nicotinic, endoplasmic reticulum, GABAA, glycine, and serotonin receptors. Most studies have been directed toward the large intracellular loop portion of a subunit, between the third and fourth membrane-spanning helices. However, signals involved in trafficking have been reported in the first transmembrane domain (Wang et al., 2002) and in the short intracellular loop between the first and second transmembrane domain (Boyd et al., 2003). In the major intracellular loop, the ∼60 residues at the start of the loop have been most extensively studied. In this region, short stretches that contain multiple basic residues have been found in several pLGIC family members. Dibasic or multibasic signals are well known to both reduce export from the ER and increase transport back from the Golgi apparatus to the ER (Michelsen et al., 2005). Indeed, mutation of basic residues to remove the positive charge enhances surface expression of nicotinic (Keller et al., 2001; Srinivasan et al., 2011) and glycine (Melzer et al., 2010) receptors. There are also dihydrophobic signals that enhance forward trafficking (Barlowe, 2003). Two hydrophobic pairs have been identified in neuronal nicotinic α4 and β2 subunits that when mutated reduce surface expression (Ren et al., 2005). Mutation of the residues in this region of the rat nicotinic β2 subunit from leucine-phenylalanne-leucine to leucine-phenylalanine-methionine (LFM) to generate a forward trafficking motif (Mancias and Goldberg, 2008) also enhanced surface expression (Srinivasan et al., 2011). However, it should be noted that the effects of mutations can be complex or divergent between subunits. For example, mutations in the nicotinic α7 subunit in the region studied in β2 and α4 subunits reduce surface expression, but they also reduce total receptor assembly to an equal extent, suggesting that the mutation affects maturation rather than trafficking (Castelan et al., 2007; Mukherjee et al., 2009).
Our focus was on a specific subtype of GABAA receptor: that containing α4, β2, and δ subunits. This subtype contributes to the “tonic” GABA response that appears to be a major contributor to a steady inhibitory tone in several neuronal populations (Farrant and Nusser, 2005). Accordingly, there has been significant interest in expressing these receptors to examine their physiologic and pharmacologic properties. However, studies of this receptor have been hampered because surface expression is generally quite low. Numerous studies have examined the role of accessory proteins and chaperones in the trafficking of GABAA receptors (reviewed by Connolly, 2008), but little attention has been given to the role(s) of sequences in the main cytoplasmic loop of subunits. We examined the effect of putative signal sequences in the large cytoplasmic loop of the α4 and δ subunits, including dibasic retention sequences and an LFM forward trafficking motif. We find that mutations had opposite effects when made in the α4 subunit (increasing surface expression) and the δ subunit (no effect or decreasing expression).
Materials and Methods
cDNA Constructs and Mutagenesis.
Human α4 cDNA was obtained from Dr. Paul Whiting (Merck, Harlow, Essex, UK). Rat β2 was obtained from Dr. David Weiss (University of Texas Health Science Center, San Antonio, TX). Rat δ was obtained from Dr. Robert Macdonald (Vanderbilt University, Nashville, TN). Each of these cDNAs was transferred to the pcDNA3 expression vector (Life Technologies, Grand Island NY) before use or mutagenesis. Before introducing various mutations to the intracellular loop, the δ cDNA was mutated to include a FLAG tag near the N terminus of the mature δ peptide. Introduction of the FLAG tag reflects the following sequence: QPHHGARAMNDIGDYKDDDDKVGS, with the FLAG sequence underlined, where the first residue of the mature subunit is the Q shown.
Mutations were made on the background of the α4 and δ GABAA subunit cDNA. Three regions, designated A, B, and C, referring to amino acid sequences on the α and δ peptides, were modified to assess the effect on receptor trafficking. Each region is located within the intracellular loop between the membrane spanning regions three and four. The locations of the regions and the mutations are shown in Fig. 1. The QuikChange (Agilent Technologies, Santa Clara, CA) mutagenesis method was used to introduce the appropriate base changes. Full-length sequencing of the cDNAs was done to validate the mutations and to show that no other changes were made.
Fig. 1.

Alignments of the initial portion of the large intracellular loops. This figure shows alignments of members of the pLGIC family, with residues emphasized that have been implicated in receptor trafficking. Basic residues are indicated by gray shading and possible dihydrophobic forward trafficking signals as italic and bold letters. (A) The first column identifies the subunit and the second the position in the mature subunit of the first residue shown. For all subunits, the third transmembrane region is predicted to end about six residues before the first residue shown. The first sequence is for the human nicotinic α1 subunit (NP_031415.2) with a dibasic signal (Keller et al., 2001). The second and third sequences show human nicotinic β1 (NP_033731.3) and δ (NP_067611.2) and basic residues (Rudell et al., 2014). The fourth and fifth sequences show human nicotinic α4 (NP_000735.1) and mouse β2 (NP_033732.2) subunits. The FL and LL forward trafficking motifs were studied by (Ren et al., 2005). In the β2 subunit, the gray highlighted basic region and the leucine-phenylalanne-leucine (LFL) motif (boxed and underlined; mutated to LFM) were studied by Srinivasan et al. (2011). The next row shows the human glycine α1 subunit (NP_001139512) with the basic residues studied by Melzer et al. (2010). The next two rows show the human GABAA α4 (NP_000800.2) and rat δ (NP_058985.1) subunits used in the present study, with potential trafficking motifs identified. (B) The mutations made, with mutations shown as large and bold letters. The abbreviation for the mutation is shown in the first column. The α4(AB) construct contained both the A and B mutations shown.
Cell Culture, Transfection, and Enzyme-Linked Immunosorbent Assay.
Cell culture conditions, using human embryonic kidney (HEK) 293 cells, have been described in detail previously (Steinbach and Akk, 2001; Akk et al., 2008). Transfections were done using the Effectene transfection reagent (Qiagen, Valencia, CA) and cDNAs at a ratio of 1:1:1 α4:β2:δ. The total amount of DNA was kept constant when a subunit was omitted by adding pcDNA3 to the mixture. Wild-type β2 was used in all experiments, whereas wild-type or mutated α4 and/or δ subunits were transfected as indicated in Results. HEK 293 cells (ATCC, Gaithersburg, MD) were plated at a density of about 50,000 cells/well in 24-well dishes treated with poly-d-lysine (Biocoat, Horsham, PA). Transfections were done 1 day after plating, and enzyme-linked immunosorbent assays (ELISA) were performed 2 days after transfection. ELISA assays were performed as previously reported (Bracamontes and Steinbach, 2008). In each experiment, for each set of subunits transfected, three wells were used for ELISA assays and two wells for a protein assay using a bicinchoninic acid method (Thermo Fisher Scientific, Waltham, MA). We indicate the subunits transfected with a dash (e.g., α4-β2-δ indicates that all three wild-type subunits were transfected).
The surface expression of the δ subunit was assayed by the binding of a monoclonal antibody to the FLAG epitope (M2 antibody; Sigma-Aldrich, St. Louis, MO), and a horseradish peroxidase–coupled IgG anti-mouse antibody (GE Healthcare Bio-Sciences, Piscataway, NJ). Surface expression of the β2 subunit was assayed in some experiments by an antibody recognizing β2 and β3 subunits (BD17-MAB341; Millipore, Billerica, MA) and a horseradish peroxidase IgG anti-mouse antibody (GE Healthcare Bio-Sciences). Cells were washed in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.3). The cells were blocked with 4% (w/v) powdered milk in PBS for 30 minutes at room temperature and then incubated with primary antibody for 60 minutes at room temperature. After three additional washes with milk-PBS, the cells were incubated with horseradish peroxidase conjugated secondary antibody (60 minutes), washed again with milk-PBS, and finally incubated with the horseradish peroxidase substrate 1-Step Ultra TMB (3,3′,5,5′ tetramethylbenzidine; Thermo Fisher Scientific). The absorbance of the supernatant was measured with a plate reader (iMark; Bio-Rad, Hercules CA).
In some experiments, the total expression of the FLAG epitope was assayed by permeabilizing lightly fixed cells. Cells were washed 1× with 2 ml PBS and then fixed with 0.5 ml of 3% paraformaldehyde in PBS for 10 minutes at room temperature. Fixing solution was aspirated, and cells were washed 3× with PBS and subsequently blocked with 4% milk-PBS, 30 minutes, at room temperature, then permeablized with 0.5% (v/v) NP40 in milk-PBS. All subsequent antibody incubations and washes contained 0.5% NP40. The ELISA assay was conducted as described for the surface expression assays.
ELISA results were analyzed by subtracting machine background, then normalizing the ELISA optical density signal to the cell protein. We excluded the results from any transfection in which the normalized ELISA signal for the positive control (wild-type subunits) did not differ significantly from that for the negative control (pcDNA3) (P > 0.05, two-tailed t test) because we judged that the transfection had failed. All data from that set of transfections were discarded. We then subtracted the mean normalized signal from the negative control (pcDNA3) from all values. To control for variability in transfection, all the data were then normalized to the relative ELISA for the positive control obtained for that transfection. The end result is a number giving the expression relative to that for wild-type receptors, where 1 means equivalent expression and 0 expression equivalent to the negative control.
Electrophysiology.
Electrophysiologic experiments were performed 48 to 72 hours after transfection. HEK 293 cells expressing GABAA receptors were identified using a bead-binding technique (Ueno et al., 1996). Surface expression of the FLAG peptide was determined using a mouse monoclonal antibody to the FLAG epitope, which had been adsorbed to immunobeads with a covalently attached goat anti-mouse IgG antibody (Life Technologies, Carlsbad, CA).
The experiments were conducted using standard whole-cell voltage clamp techniques. The bath solution contained (in mM) 140 sodium chloride (NaCl), 5 potassium chloride (KCl), 1 magnesium chloride (MgCl2), 2 calcium chloride (CaCl2), 10 d-glucose, and 10 HEPES, pH 7.4. The pipette solution contained (in mM) 140 caesium chloride (CsCl), 4 NaCl, 4 MgCl2, 0.5 CaCl2, 5 EGTA, 10 HEPES, pH 7.4. The agonist and modulator were applied to a cell using an SF-77B fast perfusion stepper system (Warner Instruments, Hamden, CT). The whole-cell superperfusion time course was ∼50–100 milliseconds (10–90 time) (data not shown). The cells were clamped at −60 mV. The currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Union City, CA), low-pass filtered at 2 kHz, and digitized with a Digidata 1320 series interface (Molecular Devices) at 10 kHz. The data were typically low-pass filtered at 100 Hz before current analysis using the pClamp 9.0 software package (Molecular Devices), and no apparent distortion in the response was introduced by filtering.
The electrophysiologic experiments were aimed at determining the peak and steady-state amplitudes to saturating GABA, the GABA concentration-response relationship, potentiation of responses to GABA by pentobarbital (PEB), and the steroid allopregnanolone (3α5αP), and direct activation by PEB.
The peak response was determined by measuring the maximal current response. The steady-state response was measured as the mean current response at the end of an 8 second application of GABA.
The concentration-response profile was determined as follows. A cell was exposed to 0.5–50 µM GABA. Each drug application lasted for 8 seconds and was followed by a 30-second washout period. The concentration-response curves for each cell were fitted to the Hill equation (eq. 1):
| (1) |
where Ymax is the maximal fitted response, EC50 is the concentration producing the half-maximal effect, and n is the Hill coefficient. The fitting was conducted using the program NFIT (The University of Texas Medical Branch at Galveston, TX). Data averaged over several cells are presented in the manuscript as mean EC50 estimate.
Potentiation by PEB (100 μM) or the steroid 3α5αP (1 μM) was examined by comparing the peak current in the presence of 50 µM GABA (a saturating concentration) plus potentiator to the response to 50 µM GABA alone. Direct activation by PEB was estimated by comparing the tail response to 1 mM pentobarbital with the peak response to 50 µM GABA.
Statistical analysis was done using the Systat 7.0 (SPSS Inc., Chicago, IL) software package or Excel (Microsoft, Redmond, WA).
Drugs.
The drugs used in the study were obtained from Sigma-Aldrich except for the δ subunit specific potentiator, DS2 (4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide; R&D Systems, Minneapolis, MN). The stock solution of GABA (500 mM) was made in bath solution and stored at −20°C. The stock solution of 3α5αP was made at 10 mM in dimethylsulfoxide and stored at room temperature. The stock solution of pentobarbital was made at 3 mM in bath solution on the day of experiment and kept at room temperature. The stock solutions were further diluted on the day of experiment.
Results
Surface Expression.
We estimated the surface expression of receptors by an intact cell ELISA approach using a FLAG epitope inserted into the extracellular domain of the δ subunit. We chose to follow surface expression of the δ subunit because δ does not express on the surface in the absence of α and β subunits, whereas the α4 and β2 subunits can form surface receptors when expressed together in the absence of δ. Receptors containing only α4 and δ or β2 and δ subunits express poorly (Table 1). Accordingly the ELISA signal represents the surface expression of receptors containing all three subunits: α4, β2, and δ.
TABLE 1.
Surface expression of δ subunits for receptors lacking one or more subunits
The surface ELISA signal relative to wild-type [mean ± S.E. (no. of transfections)] is shown for the subunit combinations shown in the first column. Two significance levels are shown, evaluated by one-way analysis of variance with Bonferroni correction over a set including negative control (pcDNA3), positive control (wild-type), and the subunit combinations shown. The first is the difference to wild-type and the second the difference to pcDNA3. Note that δ alone expressed at background, but both β2-δ and α4-δ showed significant expression compared with pcDNA3 albeit much less than α4-β2-δ.
| Subunits Expressed | Surface Expression |
|---|---|
| δ | 0.03 ± 0.02 (5)*** (ns) |
| β2-δ | 0.22 ± 0.07 (3)*** |
| α4-δ | 0.08 ± 0.06 (3)*** |
ns, P > 0.05; ***P < 0.001.
The goal of the experiments was to identify mutations that enhance overall surface expression. We selected regions that previous studies of nicotinic receptor subunits had suggested would affect expression (Fig. 1A). Figure 1B shows the mutations made for this study.
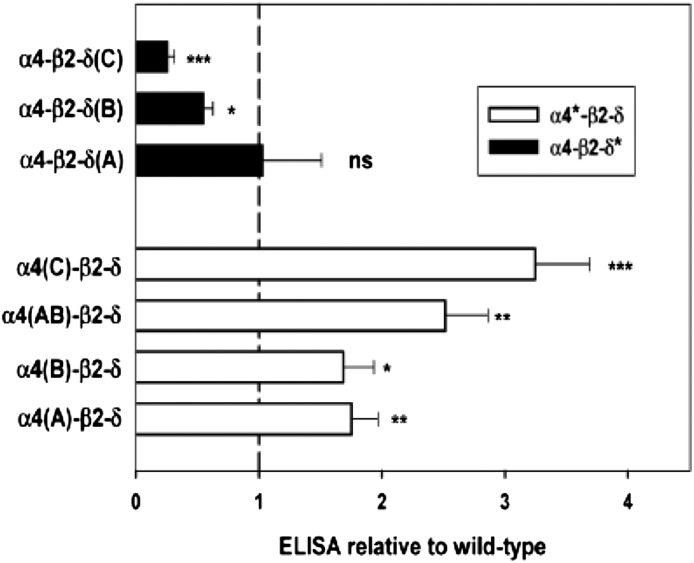
In the α4 subunit, the effects of introducing an LFM forward trafficking signal were examined in the A construct, the consequences of removing the first basic retention sequence in the B construct, and the combined effect of adding LFM and removing the second basic sequence in the C construct. As shown in Fig. 2 and Table 2, each of these changes enhanced surface expression. The combination of the A and B mutations [α4(AB)] provided slightly but not significantly more expression than A or B alone. On the other hand, combining the LFM mutation with removal of the second basic retention signal [α4(C)] did provide significantly more surface expression than the A mutation alone [one-way analysis of variance (ANOVA) with Bonferroni correction over wild-type and α4 constructs, P < 0.001).
Fig. 2.
Effects of constructs on surface expression. The relative surface expression of the δ subunit (assayed by FLAG expression on the surface) is shown for receptors containing different constructs (all relative to receptors with wild-type subunits; value of 1 indicated by dashed line). *Significance of difference to wild-type for mutations of the α4 or of the δ subunit (one-way ANOVA with Dunnett’s correction; ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001).
TABLE 2.
Surface expression of δ subunits for receptors containing mutated subunits
The surface enzyme-linked immunosorbent assay signal relative to wild-type [mean ± S.E. (no. of transfections)] is shown for the subunit combinations shown in the first column. In each group, significance levels were computed using a one-way analysis of variance with Bonferroni correction over positive control (wild-type), and the three or four subunit combinations shown. Results are shown for the difference to wild-type expression. The receptors containing both mutated α4 and δ subunits were compared as described in Results.
| Subunits Expressed | Surface Expression |
|---|---|
| α4(A)-β2-δ | 1.75 ± 0.21 (16)** |
| α4(B)-β2-δ | 1.69 ± 0.25 (14)* |
| α4(AB)-β2-δ | 2.51 ± 0.35 (4)** |
| α4(C)-β2-δ | 3.25 ± 0.44 (8)*** |
| α4-β2-δ(A) | 1.03 ± 0.48 (5) (ns) |
| α4-β2-δ(B) | 0.55 ± 0.08 (6)* |
| α4-β2-δ(C) | 0.26 ± 0.06 (5)*** |
| α4(B)-β2-δ(B) | 0.53 ± 0.27 (3) |
| α4(C)-β2-δ(C) | 1.49 ± 0.16 (6) |
ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
In the δ subunit, two regions of basic residues were studied. In contrast to effects on the α4 subunit, mutation of basic residues to alanine either had no effect on surface expression [δ(A)] or had significantly reduced surface expression [δ(B) and δ(C)] (Fig. 2; Table 2).
We tested two combinations of mutations in both α4 and δ subunits. One was α4(B)-β2-δ(B). Expression of α4-β2-δ(B) receptors was reduced to 55% ± 8% (six transfections) of the level for α4-β2-δ; expression of receptors containing α4(B)-β2-δ(B) was reduced to 28% ± 19% (three cases in which both α4-β2-δ(B) and α4(B)-β2-δ(B) were directly compared) the level of α4(B)-β2-δ receptors. The reductions did not differ significantly (P = 0.28, 2-tailed t test). (Note that the data in Table 2 have been normalized so that the ratio of the mean effects would be {α4(B)-β2-δ(B)/α4-β2-δ}/{α4-β2-δ(B)/α4-β2-δ} = {0.53/1.69} = 0.31.) We also examined α4(C)-β2-δ(C). Expression of α4-β2-δ(C) receptors was reduced to 26% ± 6% of α4-β2-δ receptors five transfections) and α4(C)-β2-δ(C) to 46 ± 2% of the level for α4(C)-β2-δ (six comparisons; P = 0.02, two-tailed t test). Because the inclusion of altered δ constructs did not result in major or consistent differences in expression between receptors containing wild-type or mutated α4 subunits, we conclude that the effects of mutations in the two subunits are largely independent of each other.
To determine whether the effects of mutations were largely on subunit translation and assembly or on trafficking, two experiments were performed using the most highly expressed [α4(C)-β2-δ] and poorly expressed [α4-β2-δ(C)] receptors. The first experiment was to follow the surface expression of the β2 subunit, using the monoclonal antibody BD17. Our reasoning was that if the mutated subunit were incorporated and enhanced trafficking, then surface expression of the β2 subunit would be enhanced, whereas, if trafficking were reduced, then it would be reduced. Expression of the α4(C) construct increased the signal using either antibody: with the FLAG antibody to the epitope on δ, surface expression was 3.3 ± 0.4 times the signal with wild-type α4, and with the BD17 antibody to an epitope on β2 surface expression was 2.3 ± 0.2 (six transfections). In contrast, on inclusion of the δ(C) construct, the FLAG signal (0.26 ± 0.06 times wild-type) was reduced more than BD17 (0.51 ± 0.11; six transfections).
We also examined the relative surface expression of β2 subunits when expressed in the absence of any δ subunit. Surface expression of β2 was not affected by coexpression of δ (the signal for α4-β2 receptors was 1.14 ± 0.08 times the expression of α4-β2-δ, which did not differ significantly from a ratio of 1; three transfections). Inclusion of α4(C) increased surface expression to 2.3 ± 0.2 times wild-type δ. This level was significantly larger than the expression seen for either α4-β2 or α4-β2-δ (P < 0.001, one-way ANOVA with Bonferroni correction; six transfections). Inclusion of δ(C), on the other hand, reduced surface expression of β2 to 0.51 ± 0.11 of the level for α4-β2-δ (P < 0.001 for both comparisons; six transfections). These results suggest that in the case of α4(C) assembly is normal (or enhanced) and forward transport of assembled receptors is enhanced. In addition, the observation that surface expression of β2 is reduced below the level when only α4 and β2 subunits are expressed indicates that δ(C) assembles with other subunits, but forward trafficking is reduced.
The second approach was to assay total FLAG epitope expression in cultures fixed and permeabilized with NP40. Our reasoning was that if the total level of δ subunit expression were reduced, it was likely that the mutations resulted in a reduction in maturation of the subunit. In the case of the α4(C)-β2-δ receptors, the total signal was indistinguishable from wild-type (109% ± 13% of wild type, P = 0.7 by one-way ANOVA with Bonferroni correction), whereas for α4-β2-δ(C) receptors, it was significantly reduced (53% ± 5%, P < 0.001). For all receptors, the signal was significantly greater than the negative control (P < 0.001 for all comparisons). These results suggest that the δ(C) construct impairs maturation or stability of the δ subunit.
Physiologic and Pharmacologic Properties of the Receptors.
We recorded currents from HEK cells expressing receptors containing wild-type β2 subunits and wild-type or mutated α4 and δ subunits. Because of an indication that β2-δ receptors are expressed on the surface (see preceding), we tested 15 bead-binding cells from two transfections with just β2 and δ subunits and did not record measurable responses to high concentrations of GABA (data not shown). Accordingly, the results do not reflect contributions from receptors that contain only α4 and δ subunits. We were also concerned that the low surface expression levels of α4-β2-δ(C) receptors might indicate that few receptors actually contained the δ subunit. We tested this using the δ subunit specific potentiator, DS2 (Wafford et al., 2009). DS2 was equally effective at potentiating responses from cells expressing wild-type receptors (1.58 ± 0.11 fold, n = 5) and α4-β2-δ(C) receptors (1.59 ± 0.10 fold, n = 5). In contrast, DS2 did not potentiate α4-β2 receptors (1.00 ± 0.06 fold, n = 4).
The receptors were all quite sensitive to GABA, with the concentration of GABA required to evoke a half-maximal response (EC50) in the range of 2–5 μM (Table 3). The EC50 values did not differ significantly from that for wild-type receptors (one-way ANOVA with Dunnett’s correction). We note that responses to high concentrations of GABA showed a peak followed by a relatively rapid decay to a steady level (Fig. 3). There have been reports that responses of receptors containing the δ subunit are nondesensitizing (Feng et al., 2006), although others have reported that responses show desensitization (Mortensen et al., 2010; Bright et al., 2011). In any case, there was no difference in the ratio of the steady-state to peak response for receptors containing any of the constructs (Table 3). Overall, we infer that the mutations do not significantly affect activation by GABA.
TABLE 3.
Effects of mutations on responses to GABA
Responses were elicited with GABA concentrations from 0.5 to 50 μM. The first column shows the subunits expressed. Concentration-response data were fit with the Hill equation (see Materials and Methods). The second and third columns show the average EC50s and Hill coefficient for activation by GABA. The fourth column shows the maximal response to GABA (50 μM), and the fifth column shows the ratio of the steady-state response (after 8-second application) to the peak response for responses to 50 μM GABA. The significance of differences to wild-type receptors was assessed by one-way analysis of variance with Dunnett’s post hoc correction. The only result with a significant difference (P < 0.05) was for the maximal response to saturating (GABA) for α4(C)-β2-δ receptors. Data are shown as mean ± S.E. (no. of cells).
| Receptor | EC50 | nHill | Imaximal | Isteady-state/Ipeak |
|---|---|---|---|---|
| µM | −pA | |||
| α4-β2-δ | 3.4 ± 1.0 (4) | 1.1 ± 0.3 (4) | 513 ± 62 (7) | 0.6 ± 0.1 (7) |
| α4(B)-β2-δ | 3.2 ± 0.9 (5) | 1.3 ± 0.3 (5) | 292 ± 46 (9) | 0.6 ± 0.1 (9) |
| α4(AB)-β2-δ | 3.8 ± 0.9 (4) | 1.2 ± 0.2 (4) | 360 ± 55 (6) | 0.5 ± 0.1 (6) |
| α4(C)-β2-δ | 1.7 ± 0.2 (7) | 1.7 ± 0.2 (7) | 1074 ± 272 (6)* | 0.8 ± 0.0 (6) |
| α4-β2-δ(B) | 4.8 ± 0.9 (4) | 1.3 ± 0.2 (4) | 456 ± 111 (7) | 0.7 ± 0.1 (7) |
| α4-β2-δ(C) | 2.1 ± 0.2 (8) | 1.4 ± 0.1 (8) | 461 ± 122 (7) | 0.7 ± 0.1 (7) |
| α4(B)-β2-δ(B) | 4.5 ± 0.6 (3) | 1.2 ± 0.1 (3) | 455 ± 201 (7) | 0.6 ± 0.1 (7) |
| α4(C)-β2-δ(C) | 1.5 ± 0.2 (7) | 1.7 ± 0.2 (7) | 637 ± 171 (6) | 0.7 ± 0.1 (6) |
P < 0.05
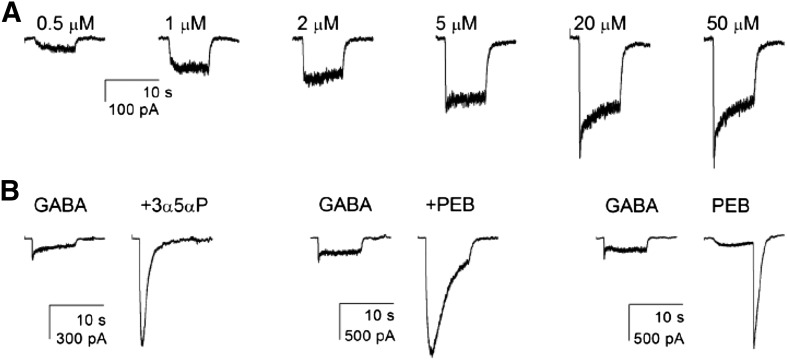
Fig. 3.
Summary of physiologic and pharmacologic responses. All recordings are from cells expressing wild-type α4-β2-δ receptors. (A) The response of one cell to increasing concentrations of GABA. The half-maximal response is between 2 and 5 μM. (B) Responses of three different cells. In the left pair, 50 μM GABA was first applied alone then in the presence of 1 μM of the neurosteroid 3α5αP. In the middle pair, 50 μM GABA was applied alone or in the presence of a low (potentiating) concentration of PEB (100 μM). The right pair shows the response of a cell to a maximal concentration of GABA (50 μM) and to 1 mM PEB. At this higher concentration of PEB, the response in the presence of PEB is reduced by channel block, whereas the large “tail” of current after removal of PEB shows the response of activated channels that have unblocked. Note that the tail is much larger than the maximal response to GABA. See Tables 3 and 4 for average values for all receptor types studied.
We measured the maximal response to GABA as an assay for the number of functional receptors on the cell surface. As shown in Table 3, the only construct whose average maximal response differed significantly from that for wild-type receptors was the α4(C)-β2-δ receptor, which had a larger average response. We note, however, that the cells we studied electrophysiologically were selected to express a large number of surface receptors (see Materials and Methods) and so would be expected to have more comparable numbers of receptors. A linear regression of relative maximal response on relative expression had a positive slope, which was not significantly different from zero (P = 0.16; data not shown).
To establish the effect of these mutations on the basic pharmacologic properties of α4-β2-δ receptors, we examined the modulation of GABA-activated receptors by pentobarbital (PEB) and the steroid 3α5αP. A saturating concentration of GABA (50 μM) was used to activate the receptors, as previous work has found that GABA is a relatively inefficacious activator of α4-β2-δ receptors (Akk et al., 2004). Using a maximal concentration of GABA removes complications resulting from possible differences in fractional activation by a given concentration GABA in the absence of potentiator. Cells expressing receptors were exposed to 50 µM GABA in the absence and presence of 100 µM PEB or 1 µM 3α5αP. The effect of the modulator was evaluated from the ratio of the response plus modulator to that minus modulator, so that the absence of potentiation would result in a ratio of 1. Both drugs are efficacious potentiators of the receptor. The effect of PEB ranged from 5- to 9-fold, and that of 3α5αP ranged from 5- to 11-fold. However, the differences among receptors types were not statistically significant. We infer that the mutations do not affect receptor modulation by these drugs. A summary of the data is given in Table 4.
TABLE 4.
Effects of mutations on GABA receptor pharmacology
The first column shows the receptor composition. The second and third columns show the ability of two compounds to enhance the response to GABA. Responses were elicited with a saturating concentration of GABA (50 μM) in the absence and presence of the potentiators 3α5αP and PEB and the ratio of the responses computed. The final column shows the relative ability of PEB to activate the receptor compared with the maximal response to GABA. This was determined from the ratio of the peak “tail” response to 1 mM PEB (see Fig. 3) to the peak response to 50 μM GABA in the same cell. The significance of differences to wild-type receptors was assessed by analysis of variance with Dunnett's post hoc correction. No results were significantly different. Data are shown as mean ± S.E. (no. of cells).
| Receptor | Potentiation by 1 μM 3α5αP | Potentiation by 100 μM PEB | Activation by 1 mM PEB |
|---|---|---|---|
| α4-β2-δ | 6.7 ± 0.7 (7) | 4.8 ± 0.6 (7) | 5.2 ± 1.1 (7) |
| α4(B)-β2-δ | 8.9 ± 2.1 (8) | 7.2 ± 1.2 (7) | 8.3 ± 2.2 (9) |
| α4(AB)-β2-δ | 8.5 ± 1.5 (5) | 5.6 ± 1.6 (6) | 3.9 ± 0.7 (6) |
| α4(C)-β2-δ | 4.9 ± 0.9 (6) | 6.1 ± 1.3 (6) | 6.0 ± 1.3 (6) |
| α4-β2-δ(B) | 8.3 ± 2.2 (6) | 5.5 ± 1.3 (6) | 4.7 ± 1.1 (7) |
| α4-β2-δ(C) | 10.1 ± 1.6 (6) | 9.2 ± 1.1 (15) | 10.3 ± 1.8 (7) |
| α4(B)-β2-δ(B) | 11.0 ± 3.3 (6) | 7.7 ± 2.2 (7) | 9.2 ± 3.3 (7) |
| α4(C)-β2-δ(C) | 5.8 ± 1.0 (6) | 8.2 ± 1.0 (6) | 7.7 ± 1.0 (6) |
We also probed direct activation by 1 mM PEB. At this concentration, PEB blocks GABAA receptors, but block is rapidly reversible so that a prominent “tail” of current appears when PEB is removed (Fig. 3). Accordingly, we determined the ratio of the peak tail current elicited by 1 mM PEB to the peak current to 50 μM GABA in the same cell. This provides an indication of the relative efficacy of PEB as agonist compared with GABA. As shown in Table 4, the ratio ranged from 4- to 10-fold, but no values were significantly different from wild-type.
The observation that PEB is much more efficacious than GABA is consistent with the ability of PEB and 3α5αP to potentiate the maximal response to GABA. The high efficacy of PEB shows that even at a saturating concentration of GABA, only a relatively small proportion of receptors have open channels since PEB can elicit a much larger maximal response. A previous study of α4-β2-δ receptors estimated that when activated by PEB, they have a maximal probability of being open (Popen) of at least 0.7, whereas the maximal Popen for GABA was too low to be reliably estimated (Akk et al., 2004). The present data on relative efficacy of PEB and potentiation of maximal GABA response suggest that the true maximal response is between 4- and 11-fold larger than the maximal response to GABA alone. If PEB is equally efficacious on all the receptor types studied (maximal Popen ∼0.7), then the estimated maximal Popen for GABA in the absence of potentiators would be in the range of 0.1–0.2 of that value (0.7), or ∼0.1.
Discussion
The immediate goal of our study was to determine whether the surface expression of α4-β2-δ GABAA receptors could be enhanced by mutations of trafficking signals in the large intracellular loops of subunits. Using sequence analysis, we identified possible trafficking signals in the GABAA α4 and δ subunits; most were dibasic or multibasic retention motifs. Similar to results seen with other LGICs, mutation of basic residues in the GABAA α4 subunit increased surface expression. However, mutation of basic residues in the δ subunit resulted in either no change in surface expression or a significant reduction. This observation was surprising to us, in particular because the residues mutated are in similar positions with respect to the end of the third transmembrane region of the two subunits and hence might be expected to be positioned to interact in a similar fashion with trafficking proteins.
To probe the possible mechanisms of these effects, we focused on the constructs with the largest effects to increase or reduce surface expression. In the case of the α4 subunit, the data indicate that the α4(C) mutations enhance surface expression by enhancing the forward trafficking of assembled receptors. In the case of the δ subunit, it seems most likely that the δ(C) mutations have more than one effect. The reduction in the total amount of δ subunit suggests that subunit maturation is reduced. When we examined the surface expression of both the δ and β2 subunits, surface expression of δ was reduced to a greater extent than expression of β2, suggesting that there might be some expression of receptors that lack the δ subunit. We tested the possibility that most of the surface receptors lacked the δ subunit by using the δ-specific modulator DS2, and the results indicate that a large fraction of the functional receptors contain the δ subunit. The difference in expression between δ and β2 subunits might be consistent with either reduced levels of the mutated δ subunit or a reduced assembly. However, the level of expression of β2 was significantly lower than that seen when no δ subunit was expressed at all. This observation indicates that the mutated δ subunit does associate with other subunits but is very ineffective either at forward trafficking or at producing a fully assembled pentamer. In any case, the results are not consistent with a simple role of these putative basic retention signals in the δ subunit and may indicate that, in some situations, removal of basic residues actually increases retention.
These observations are reminiscent of the results of studies of neuronal nicotinic subunits. Removal of dihydrophobic (forward trafficking) motifs in the α4 or β2 subunits reduced surface expression by reducing forward trafficking (Ren et al., 2005). In contrast, similar mutations in the α7 subunit reduced surface expression but apparently by reducing subunit maturation (Castelan et al., 2007; Mukherjee et al., 2009). The present results differ, however, because removing dibasic signals from the GABAA α4 subunit enhances surface expression, likely by enhancing trafficking, whereas mutations to homologous residues in the δ subunit actually reduce surface expression, likely by reducing maturation and trafficking. These observations demonstrate the context specificity of possible trafficking signals, even when they are located in homologous positions in closely related proteins.
None of the constructs had significant effects on the physiologic or pharmacologic properties we examined for the expressed GABAA receptors, so receptors containing these mutations are functionally normal. This, perhaps, is not surprising, as the mutations are not in any region shown, or considered likely, to be functionally relevant. We succeeded in reaching our immediate goal, to enhance the level of assembled α4-β2-δ receptors on the surface, and the increased expression level will facilitate further studies of the α4-β2-δ class of tonic GABAA receptor. The α4(C)-β2-δ receptor also showed an increased average maximal response to GABA consistent with the increased surface expression seen with the surface ELISA. These results indicate that mutation of putative trafficking signals can result in increased surface expression of GABAA receptors. However, the disparity between results with α4 and δ subunits indicates that the approach is not universal in applicability.
In summary, we have identified mutations of residues in the main cytoplasmic loop of the GABAA α4 subunit that significantly enhance surface expression of α4-β2-δ receptors. We also have found that similar mutations in the δ subunit reduce surface expression. The results indicate that putative trafficking signals may have more complex roles in the assembly and trafficking of multimeric membrane proteins.
Abbreviations
- 3α5αP
allopregnanolone
- ANOVA
analysis of variance
- DS2
4-chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- HEK
human embryonic kidney
- LFM
leucine-phenylalanine-methionine
- PBS
phosphate-buffered saline
- PEB
pentobarbital
- pLGIC
pentameric ligand gated ion channels
- Popen
probability of being open
Authorship Contributions
Participated in research design: Bracamontes, Akk, Steinbach.
Conducted experiments: Bracamontes, Li.
Contributed new reagents or analytic tools: Bracamontes.
Performed data analysis: Bracamontes, Li, Akk, Steinbach.
Wrote or contributed to the writing of the manuscript: Bracamontes, Li, Akk, Steinbach.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant P01-GM47969]. J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology.
References
- Akk G, Bracamontes J, Steinbach JH. (2004) Activation of GABA(A) receptors containing the α4 subunit by GABA and pentobarbital. J Physiol 556:387–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. (2008) Mutations of the GABA-A receptor α1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol 74:614–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. (2003) Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol 13:295–300 [DOI] [PubMed] [Google Scholar]
- Boyd GW, Doward AI, Kirkness EF, Millar NS, Connolly CN. (2003) Cell surface expression of 5-hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J Biol Chem 278:27681–27687 [DOI] [PubMed] [Google Scholar]
- Bracamontes JR, Steinbach JH. (2008) Multiple modes for conferring surface expression of homomeric β1 GABAA receptors. J Biol Chem 283:26128–26136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, Farrant M, Brickley SG. (2011) Profound desensitization by ambient GABA limits activation of δ-containing GABAA receptors during spillover. J Neurosci 31:753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelán F, Mulet J, Aldea M, Sala S, Sala F, Criado M. (2007) Cytoplasmic regions adjacent to the M3 and M4 transmembrane segments influence expression and function of α7 nicotinic acetylcholine receptors. A study with single amino acid mutants. J Neurochem 100:406–415 [DOI] [PubMed] [Google Scholar]
- Connolly CN. (2008) Trafficking of 5-HT(3) and GABAA receptors. Mol Membr Biol 25:293–301 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229 [DOI] [PubMed] [Google Scholar]
- Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL. (2006) δ subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of α4β2δ GABAA receptors. J Neurosci 26:1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SH, Lindstrom J, Ellisman M, Taylor P. (2001) Adjacent basic amino acid residues recognized by the COP I complex and ubiquitination govern endoplasmic reticulum to cell surface trafficking of the nicotinic acetylcholine receptor α-Subunit. J Biol Chem 276:18384–18391 [DOI] [PubMed] [Google Scholar]
- Lai HC, Jan LY. (2006) The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci 7:548–562 [DOI] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J. (2008) Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J 27:2918–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Ron D. (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149 [DOI] [PubMed] [Google Scholar]
- Melzer N, Villmann C, Becker K, Harvey K, Harvey RJ, Vogel N, Kluck CJ, Kneussel M, Becker CM. (2010) Multifunctional basic motif in the glycine receptor intracellular domain induces subunit-specific sorting. J Biol Chem 285:3730–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen K, Yuan H, Schwappach B. (2005) Hide and run: arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep 6:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. (2010) Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol 588:1251–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kuryatov A, Moss SJ, Lindstrom JM, Anand R. (2009) Mutations of cytosolic loop residues impair assembly and maturation of α7 nicotinic acetylcholine receptors. J Neurochem 110:1885–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XQ, Cheng SB, Treuil MW, Mukherjee J, Rao J, Braunewell KH, Lindstrom JM, Anand R. (2005) Structural determinants of α4β2 nicotinic acetylcholine receptor trafficking. J Neurosci 25:6676–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudell JC, Borges LS, Rudell JB, Beck KA, Ferns MJ. (2014) Determinants in the β and δ subunit cytoplasmic loop regulate golgi trafficking and surface expression of the muscle acetylcholine receptor. J Biol Chem 289:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. (2011) Nicotine up-regulates α4β2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol 137:59–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach JH, Akk G. (2001) Modulation of GABA(A) receptor channel gating by pentobarbital. J Physiol 537:715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Zorumski C, Bracamontes J, Steinbach JH. (1996) Endogenous subunits can cause ambiguities in the pharmacology of exogenous γ-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol 50:931–938 [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. (2009) Novel compounds selectively enhance δ subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology 56:182–189 [DOI] [PubMed] [Google Scholar]
- Wang JM, Zhang L, Yao Y, Viroonchatapan N, Rothe E, Wang ZZ. (2002) A transmembrane motif governs the surface trafficking of nicotinic acetylcholine receptors. Nat Neurosci 5:963–970 [DOI] [PubMed] [Google Scholar]