Abstract
It is now well accepted that protease activated receptor (PAR) 1 and PAR4 have differential roles in platelet activation. PAR4, a low-affinity thrombin receptor in human platelets, participates in sustained platelet activation in a P2Y12-dependent manner; however, the mechanisms are not defined. Our previous studies demonstrated that thrombin induces the association of PAR4 with P2Y12, together with arrestin recruitment to the complex. Here we show that PAR4 and P2Y12 directly interact to coregulate Akt signaling after PAR4 activation. We observed direct and specific interaction of P2Y12 with PAR4 but not PAR1 by bioluminescent resonance energy transfer when the receptors were coexpressed in human embryonic kidney 293T cells. PAR4-P2Y12 dimerization was promoted by PAR4-AP and inhibited by P2Y12 antagonist. By using sequence comparison of the transmembrane domains of PAR1 and PAR4, we designed a mutant form of PAR4, "PAR4SFT," by replacing LGL194–196 at the base of transmembrane domain 4 with the corresponding aligned PAR1 residues SFT 220–222. PAR4SFT supported only 8.74% of PAR4-P2Y12 interaction, abolishing P2Y12-dependent arrestin recruitment to PAR4 and Akt activation. Nonetheless, PAR4SFT still supported homodimerization with PAR4. PAR4SFT failed to induce a calcium flux when expressed independently; however, coexpression of increasing concentrations of PAR4SFT, together with PAR4 potentiated PAR4-mediated calcium flux, suggested that PAR4 act as homodimers to signal to Gq-coupled calcium responses. In conclusion, PAR4 LGL (194–196) governs agonist-dependent association of PAR4 with P2Y12 and contributes to Gq-coupled calcium responses. PAR4-P2Y12 association supports arrestin-mediated sustained signaling to Akt. Hence, PAR4-P2Y12 dimerization is likely to be important for the PAR4-P2Y12 dependent stabilization of platelet thrombi.
Introduction
Platelets are key players in hemostatic plug formation at sites of vascular injury but can also precipitate thrombotic events under conditions such as atherosclerotic plaque rupture. Platelet accumulation is initiated at a site of injury by contact with extracellular matrix proteins and then extends because of activation by soluble agonists. All soluble platelet agonists bind to G protein–coupled receptors (GPCRs). Thrombin, the most potent platelet agonist, is a serine protease and cleaves the N-terminal exodomains of two human platelet GPCRs, protease activated receptor (PAR) 1 and PAR4, exposing tethered ligands that activate the receptors. Activated platelets also release ADP, which binds to two GPCRs on platelets P2Y12 and P2Y1. P2Y12 is the target of clinically effective antithrombotic drugs, such as clopidogrel and prasugrel, and functions by inhibiting cAMP levels and activating phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathways to extend and stabilize platelet plug formation (Foster et al., 2001; Niitsu et al., 2005; Savi et al., 2006).
Research in the last decade has increased our understanding of the regulatory control elicited by the dual thrombin receptor system of human platelets (PAR1 and PAR4). PAR1 requires lower amounts of thrombin for activation than PAR4 (Covic et al., 2000) and lowers the amount of thrombin required for PAR4 activation (Leger et al., 2006). The signaling events elicited by each receptor also reflect their differential roles in the thrombus. For example, PAR1 induces an acute and short-lived rise in cytosolic calcium and αIIbβ3 activation, whereas PAR4 causes a steady and sustained rise in calcium and αIIbβ3 activation that largely depends on P2Y12 (Shapiro et al., 2000; Holinstat et al., 2006; Tadokoro et al., 2011). There is a major difference in the mechanisms of cellular trafficking and recycling of PAR1 and PAR4, wherein activated PAR1 gets phosphorylated at serine residues in its cytoplasmic tail and is rapidly internalized independent of arrestins (Hoxie et al., 1993; Trejo and Coughlin, 1999); in contrast, PAR4 is not phosphorylated and is internalized rather slowly (Kahn et al., 1999; Shapiro et al., 2000). We previously showed that inhibition of P2Y12 reduces PAR4-mediated Akt activation and recruitment of arrestin-2 to PAR4 signaling complexes. We further showed that PAR4 and P2Y12 coimmunoprecipitate upon PAR4 activation in human platelets (Li et al., 2008, 2011). In this report, we investigate the possibility that direct association of P2Y12 and PAR4 mediates arrestin recruitment to PAR4 and contributes to the consequent Akt activation.
Dimer- or oligomerization of GPCRs has increasingly been reported in platelets (Leger et al., 2006; Savi et al., 2006; de la Fuente et al., 2012; Frey et al., 2013) and other cell types (Angers et al., 2002; Rozenfeld and Devi, 2011). Structure/function studies of GPCR oligomerization have demonstrated that the transmembrane helices (TM), most commonly TM4–6 of GPCRs, provide the interfaces for dimerization of many GPCRs (Filizola and Weinstein, 2005). Homo- or heterotypic interaction of GPCRs affects their pharmacological properties, interactions with scaffolding molecules, and downstream signaling events. For example, it was previously shown that paired activation of both components of M3 receptor dimers is required for β-arrestin recruitment to the receptor (Novi et al., 2005). More recently, PAR1–PAR2 heterodimers were shown to elicit arrestin-dependent signaling to endosomes (Lin and Trejo, 2013). With these models in mind, we expressed fluorescent and luciferase-tagged forms of PAR4 and P2Y12 in a heterologous expression system to determine whether the receptors directly interact using bioluminescent resonance energy transfer (BRET) and to determine whether signaling responses in the cells were dependent upon their direct interaction. We demonstrate that PAR4 and P2Y12 specifically and directly interact, whereas PAR1 and P2Y12 do not. PAR1 and PAR4 show specific sequence divergence at the base of TM4. Interestingly, TM4 of PAR4 has been reported to be necessary for its homodimerization (de la Fuente et al., 2012) and could potentially regulate heterodimer formation with other receptors. Hence, using a site-directed mutagenic approach, we designed a mutant variant of PAR4 (PAR4SFT) containing a 3-amino acid substitution at the base of TM4 (based upon PAR1 sequence at this location). We show that PAR4SFT does not interact with P2Y12 and fails to support P2Y12-dependent arrestin recruitment and Akt activation. Interestingly, PAR4SFT also fails to support Gq-mediated calcium mobilization when expressed on its own, but this response could be rescued by its dimerization with (wild-type) PAR4. We conclude that the agonist-dependent specific interaction of PAR4 with P2Y12 supports sustained signaling to Akt that is partially dependent on recruitment of arrestin-2 to PAR4, and this mechanism is likely important for the PAR4- and P2Y12-dependent stabilization of platelet thrombi.
Materials and Methods
Unless otherwise specified, reagents were from Sigma-Aldrich (St. Louis, MO). ADP and human thrombin was from Chronolog Corp (Havertown, PA). AYPGKF (Ala-Tyr-Pro-Gly-Lys-Phe) was from Biopeptek (Malvern, PA). Antibodies were from Cell Signaling Technology (anti-Akt, phospho-Akt-Ser473, Pan-actin, Alexa 488–conjugated anti-rabbit and anti-mouse; Boston, MA), Santa Cruz Biotechnology (anti–arrestin-2, PAR4 and P2Y12; Santa Cruz, CA), Abcam (anti-FLAG; Cambridge, MA), and MBL (anti–green fluorescent protein [GFP]; Woburn, MA). Other reagents were from Invitrogen (BAPTA-AM [1,2-bis(oaminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid], Fluo4-AM and Pluronic Acid F-127; Grand Island, NY).
Plasmid Constructs and Cell Culture.
Rluc-PAR4 and Rluc-P2Y12 were generated by subcloning cDNAs for human PAR4 and human P2Y12 into the codon-humanized Renilla luciferase vectors pRLuc N1 (gift from Emer Smyth, University of Pennsylvania, Philadelphia, PA). Enhanced yellow fluorescent protein (EYFP)-PAR4 (PAR4-EYFP), PAR4SFT-EYFP, PAR1-EYFP, and P2Y12-EYFP were generated by cloning cDNA for PAR4, PAR4SFT, PAR1, and P2Y12 into the expression vectors pEYFP N1 (gift from Emer Smyth). The hemagglutinin (HA)- or FLAG-tagged human PAR4, PAR4SFT, PAR1, and P2Y12 constructs were obtained by subcloning human PAR1, PAR4, PAR4SFT, and P2Y12 into HA pcDNA 3.0 or FLAG pcDNA 3.0 vector (gift from Ji-Fang Zhang, Thomas Jefferson University, Philadelphia, PA). V5-PAR4-GFP was a gift from Dr. Marvin Nieman (Case Western Reserve University, Cleveland, OH). HA-PARSFT-GFP was constructed by subcloning HA-PARSFT into codon-humanized pGFP2-N2 vector (gift from Marvin Nieman).
Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum and 1% penicillin/streptomycin at 37°C and 5% CO2. Cells were transfected with Fugene6 or TransIT293 transfection reagent (Mirus Bio LLC, Madison, WI).
Generation of Stable Cell Lines.
HEK293T cells were cotransfected with FLAG tagged P2Y12 pcDNA 3.0 and HA-tagged PAR4 or HA-tagged PAR4SFT pcDNA 3.0 construct using Fugene6 (Promega, Madison, WI) and selected using 400 μg/ml Hygromycin (for P2Y12) and 1000 μg/ml Neomycin (for PAR4 and PAR4SFT) for 2 weeks. Stable cells were maintained in 200 μg/ml Hygromycin (Mediatech Inc., Manassas, VA) and 500 μg/ml Geneticin (Life Technologies, Grand Island, NY).
Bioluminescence Resonance Energy Transfer Assay.
HEK293T cells were grown in 6-cm dishes and transiently transfected with Rluc donor plasmid (0.18–0.36 μg/dish) and EYFP-acceptor (varying from 0.18 to 7.2 μg/dish to achieve the required ratio of acceptor:donor from 1:1 to 20:1) and balanced with an empty pcDNA3 vector to maintain equal amount of DNA transfected per dish (7.2 µg) using FuGENE 6 transfection reagent (Promega). For optimization of BRET conditions, the acceptor-donor ratio was varied. The optimum ratio of 1:1 to 20:1 of cotransfection was used for the subsequent transfections. Cells were detached using Hanks' balanced salt solution-EDTA 48 hours after transfection, washed, and resuspended in PBS containing 0.1% glucose and 1 mM Ca2+ and Mg2+, then transferred into 96-well black optiplates (PerkinElmer, Waltham, MA) (1× 105 cells/well/50 μl) to assess EYFP expression by measuring EYFP emission using fluorometry (excitation at 485 nm and emission at 535 nm) on a Victor 3 multilabel plate reader (PerkinElmer) without the addition of Coelenterazine H. Fold over basal (FOB) YFP emission was plotted against ratio YFP:Rluc of amount of constructs used to cotransfect per dish to check for increase in YFP expression from ratio 1:1 to 20:1 (see Supplemental Fig. 1). For BRET, 48 hours after transfection cells were collected posttreatment with agonist or antagonist and then transferred to 96-well white plates (PerkinElmer). Coelenterazine H (Invitrogen) was added in PBS to a final concentration of 5 μM, and donor (475 nm) and acceptor (535 nm) readings were collected immediately using Victor3 (PerkinElmer), which allows simultaneous dual emission detection. Cells were kept at 37°C throughout the BRET measurements. The BRET ratio was calculated as (535 nm emission/475 nm in test cells) − (535 nm emission/475 nm in control cells transfected with Rluc donor alone) and multiplied by 1000 to calculate the milli-BRET units (mBU). The milli BRET units were plotted as a function of YFP FOB values to assess saturable interaction between the donor and acceptor (data not shown for each assay, see Supplemental Fig. 1). The mBU values were then expressed as a percent of the maximal BRET reached (maximal mBU) and plotted as a function of ratio of YFP:Rluc of amount of constructs used for cotransfections per dish (1:1 to 20:1).
Immunoblotting.
HEK293T cells stably expressing PAR4 and P2Y12 (2 × 106 cells/ml) were grown to 70% confluency and starved for 12 hours. They were then treated with agonist for 10 minutes at 37°C or antagonist for 10 minutes at room temperature; lysed in 1× lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, 0.5% deoxycholic acid, 5 mM EDTA, 0.5 mM phenylmethanesulfonyl fluoride [PMSF], pH 7.4) with freshly added 1% Triton X-100, 1× protease inhibitor, and 1× phosphatase inhibitor (Sigma-Aldrich). Lysates were resolved on 10% SDS-PAGE and immunoblotted with an antibody to phospho-Akt-Ser473 or Pan-actin (Cell Signaling Technology, Beverly, MA), PAR4 or P2Y12 (Santa Cruz Biotechnology) at a 1:1000 dilution, then anti-rabbit or anti-goat horseradish peroxidase and exposed on a film.
Immunoprecipitation.
Samples (8 × 108 platelets/ml or 8–10 × 106 HEK293T cells/ml) were treated with antagonist MeSAMP (2-methylthioadenosine 5′-monophosphate triethylammonium salt) for 10 minutes at room temperature or 20 μM BAPTA-AM for 10 minutes at 37°C. Agonist was added in a 5 μl volume to 500 μl platelets per sample and incubated for 10 minutes at 37°C; platelets were lysed by addition of 2× immunoprecipitation (IP) buffer (1% NP40, 150 mM NaCl, 10 mM Tris, 1 mM Na3V04, 5 mM EDTA, 0.5 mM PMSF, pH 7.4) containing a cocktail of protease inhibitors (Sigma-Aldrich). HEK 293T cells were lysed by addition of 1× IP buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, 0.5% deoxycholic acid, 5 mM EDTA, 0.5 mM PMSF, pH 7.4) containing a cocktail of protease inhibitors (Sigma-Aldrich). Lysates were rotated at 4°C for 30 minutes and spun 30 minutes at 12,000g. Antibodies or control IgG were added to lysates (2 μg/per sample) and rotated at 4°C overnight followed by protein A/G agarose 15 μl/ml at 4°C for 2 hours. Samples were washed with 1× IP buffer three times and applied in Laemmli buffer to 10% SDS-PAGE for immunoblotting.
Calcium Mobilization Assay.
HEK293T cells were transiently transfected and/or cotranfected with V5-PAR4-GFP and HA-PAR4SFT-GFP. Cotransfections were done as PAR4:PAR4SFT in increasing ratios 1:1 and 1:2, where 0.1 μg of PAR4 was used, because it gave the smallest detectable calcium flux. Forty-eight hours post-transfection cells were removed from plates with phenol red–free Hanks' balanced salt solution containing 0.02% EDTA, washed, and resuspended in Tyrode's buffer. Cells (2.0 × 105 cells/sample) were loaded with 10 μM Fluo4-AM (Invitrogen) in Tyrode’s buffer (137 mM NaCl, 5.6 mM glucose, 1 g/l bovine serum albumin, 1 mM MgCl2, 2.7 mM KCl, 3.3 mM NaH2PO4) in the presence of Pluronic acid F-127 (Invitrogen) 0.01% for 20 minutes at 37°C. This was diluted 1:1 with Tyrode's buffer containing 1 mM calcium. Basal levels of cytosolic calcium were recorded on AccuriC6 flowcytometer (BD Biosciences, San Jose, CA) for 1 minute and then PAR4-AP:AYPGKF (2 mM) was added without interrupting the real-time calcium flux measurement to record the response for 10 minutes. The data were analyzed using FCS Express Ruo version 4 (De Novo Software, Los Angeles, CA) where the Fluo4-AM intensity changes over time were converted into percent above threshold by gating the calcium levels pre- and postagonist treatment.
Flow Cytometry to Assess Surface Expression of Receptors.
HEK293T cells stably/transiently expressing FLAG-P2Y12 or HA-PAR4 or HA-PAR4SFT were collected in Versene and washed and resuspended in DMEM + 10% fetal bovine serum (FBS). Cells (1 × 106) per sample were incubated in anti-FLAG (1:100), or anti-HA (1:50) for 1 hour on ice with intermittent mixing. Cells were washed with DMEM + 5% FBS twice and then incubated with Alexa 488–tagged rabbit or mouse secondary antibodies (Cell Signaling Technology) to detect FLAG and HA tag, respectively, for 1 hour on ice with intermittent mixing. Cells were then washed twice with DMEM + 5% FBS, fixed with 2% paraformaldehyde for 10 minutes at room temperature, and read on FACS caliber (BD Biosciences).
Platelet Isolation and Preparation of Human Blood.
Blood for biochemical studies of human platelets was collected by venipuncture from adult human volunteers after providing written informed consent as approved by the Institutional Review Board at University of Delaware. Blood was collected into a 60-ml syringe containing ACD (65 mM trisodium citrate, 70 mM citric acid, 100 mM dextrose, pH 4.4) at a ratio of 1:6 parts ACD/blood. Anticoagulated blood was spun by centrifugation at 250g, and the supernatant containing platelet-rich plasma was then pelleted at 750g (10 minutes), washed once in HEN buffer (10 mM HEPES, pH 6.5, 1 mM EDTA, 150 mM NaCl) containing 0.05 U/ml apyrase and platelets resuspended with HEPES-Tyrode’s buffer (137 mM NaCl, 20 mM HEPES, 5.6 mM glucose, 1 g/l bovine serum albumin, 1 mM MgCl2, 2.7 mM KCl, 3.3 mM NaH2PO4) at a concentration of 4 × 108 platelets/ml in HEPES-Tyrode’s buffer containing 0.05 U/ml apyrase for immunoblotting and immunoprecipitation.
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). For pairwise comparisons, unpaired t tests were performed. For comparisons across three groups or larger, one-way analysis of variance followed by post hoc tests (Tukey Kramer) were performed. Data are shown as means ± S.E.M.
Results
PAR4 Directly Associates with P2Y12 upon Receptor Activation.
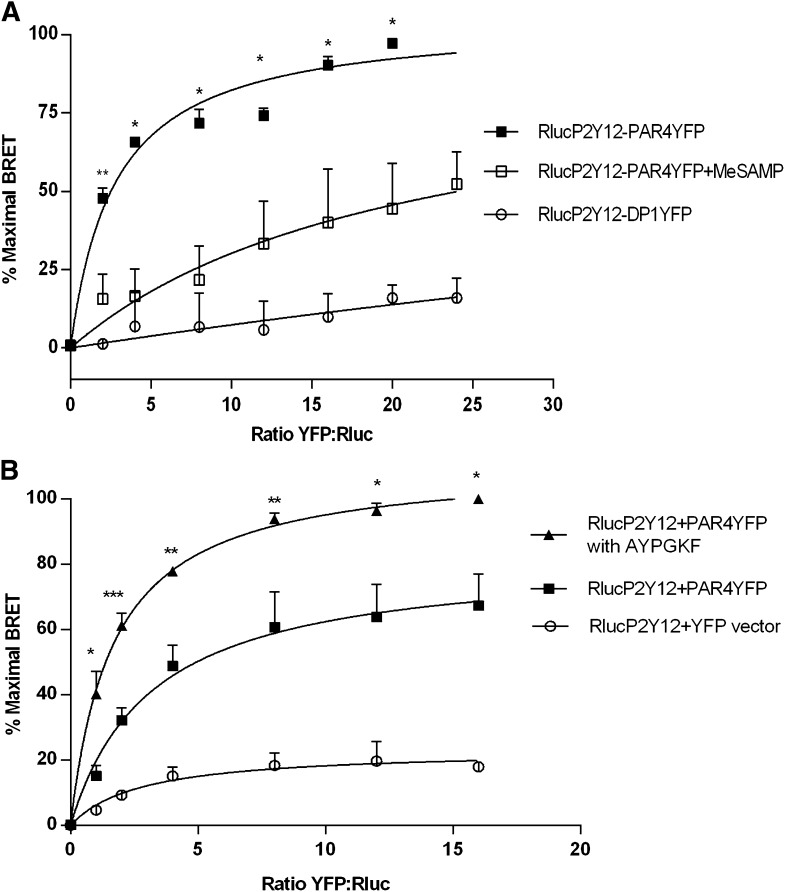
We have previously shown that PAR4 and P2Y12 coimmunoprecipitate upon thrombin stimulation of human platelets and that this interaction, as well as PAR4 recruitment of arrestin-2 and signaling to Akt, is prevented in the presence of P2Y12 antagonists (Li et al., 2011). To determine whether physical association of the two receptors may contribute to their signaling responses, we first determined whether physical association of the two receptors is detected in a heterologous expression system. HEK293T cells were transfected with Rluc-P2Y12 (0.36 μg of plasmid per dish) in the presence of increasing amounts of PAR4-YFP in ratios 1:1 to 20:1 (0.36–7.2 μg of PAR4-YFP). The direct interaction between P2Y12 and PAR4 was assessed using BRET. Briefly, BRET is a measurement of the energy transfer from the donor enzyme (Rluc), which oxidizes its substrate coelenterazine to coelenteramide, which in turn results in a nonradiative emission at 485 nm. When EYFP, the acceptor fluorophore is within 10 nm of the Rluc, emission from the coelenteramide at 485 nm in turn excites the EYFP on the neighboring acceptor molecule (Gandia et al., 2008) and results in emission at a peak of 527 nm. The interaction between Rluc-P2Y12 and PAR4-YFP is specific and saturable, as no BRET was detected because of coexpression of Rluc-P2Y12 with either vector expressing YFP alone (Fig. 1B) or with the prostaglandin D2 receptor 1-YFP (Fig. 1A). For each BRET assay, a duplicate black 96-well optiplate was used to measure the YFP emission at 535 nm in the absence of coelenterazine to assess YFP FOB expression for 1:1 to 24:1 YFP:Rluc ratios of transfection. mBU were plotted as a function of YFP FOB to see saturable interaction between PAR4 and P2Y12 (representative graph shown in Supplemental Fig. 1). Percent maximal BRET was calculated as a percent of the maximal mBU achieved per assay and plotted against YFP:Rluc ratio of amount of cotransfected plasmid. Saturation plots showed no difference in the binding curve irrespective of whether YFP:Rluc ratio or FOB axes were used (data not shown), hence percent maximal BRET was plotted against YFP:Rluc ratio for all BRET assays. Interaction appears to require activation of both PAR4 and P2Y12, because percent maximal BRET was reduced by 62.5% when P2Y12 was inhibited by P2Y12 antagonist (MeSAMP) (100 μM) (Fig. 1A) and increased by 40% in the presence PAR4 agonist (AYPGKF) (200 μM) (Fig. 1B). Basal interaction of PAR4 with P2Y12 is probably due to their constitutive activity, because increased constitutive activity of diverse GPCRs has been demonstrated by measuring secondary messenger activities after expression in heterologous systems (Tiberi and Caron, 1994; Milligan et al., 1995; Daeffler and Landry, 2000). A significant increase in net BRET signal after PAR4 activation and decrease in the presence of P2Y12 antagonist suggests that association of PAR4 with P2Y12 is driven by the active states of both receptors.
Fig. 1.
Saturation BRET of PAR4 and P2Y12 in the presence and absence of PAR4-AP (AYPGKF) and P2Y12 antagonist (MeSAMP). HEK293T cells were transiently cotransfected with 0.36 μg of Rluc-P2Y12 and varying amounts (0.36–8.24 μg) of PAR4-YFP, prostaglandin D2 receptor 1 (DP1)-YFP, or YFP-expressing vector control to achieve YFP:Rluc ratios from 1:1–24:1. BRET is expressed in relative % maximal BRET units as described in Materials and Methods. (A) P2Y12 antagonist MeSAMP (100 μM) treatment of 10 minutes at room temperature, reduced the % maximal BRET of Rluc-P2Y12 and PAR4-YFP by 62.5 ± 17% (P = 0.021). The average maximum mBU achieved for RlucP2Y12:PAR4YFP was 50.40 units (N = 3) at a ratio of 24:1, which was defined as 100% BRET. Shown is the average ± S.E.M. of 3 independent experiments. (B) PAR4 agonist AYPGKF (200 μM) treatment of 10 minutes at 37°C increased the % maximal BRET of Rluc-P2Y12 and PAR4-YFP by 40 ± 11.06% (P = 0.019) with an average maximum 475.49 mBU (N = 4). Shown is the mean ± S.E.M. of 4 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
P2Y12 Directly Associates with PAR4 but Not PAR1.
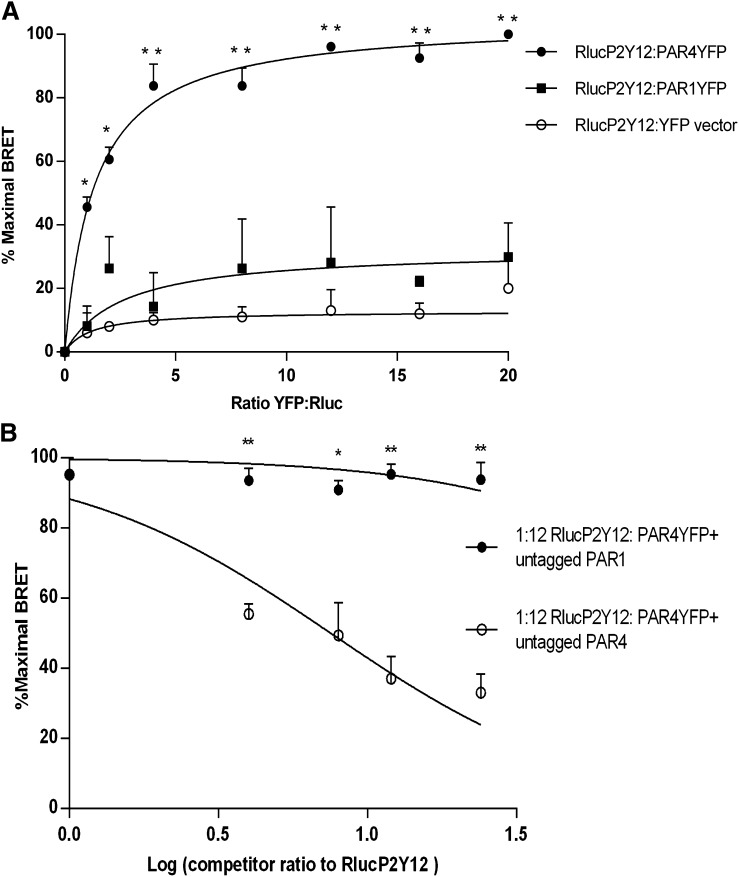
To determine whether the agonist-dependent association of P2Y12 with PAR4 is specific, we compared the interaction of P2Y12 with PAR4 and its ability to associate with PAR1. Comparative saturation BRET assays demonstrate that the BRET50 for PAR4-P2Y12 interaction is 3.1 ± 0.4; however, PAR1-YFP did not demonstrate any detectable saturable BRET with Rluc-P2Y12 and did not differ significantly from BRET with YFP vector control, indicating that PAR1 and P2Y12 do not interact using this system (Fig. 2A). To confirm the relative affinity of PAR4 versus PAR1 for interaction with P2Y12, we compared the ability of untagged PAR4 or PAR1 to compete with the BRET interaction of PAR4-YFP and Rluc-P2Y12. Untagged PAR4 reduced BRET between PAR4-YFP and Rluc-P2Y12 in a dose-dependent fashion to a maximal reduction of 61%; however, untagged PAR1 was unable to compete with the PAR4-P2Y12 interaction at even the highest expression level tested (Fig. 2B). These results suggest that PAR1 is unable to interact directly with P2Y12, at least not with an affinity that can compete for the interaction of PAR4 with P2Y12.
Fig. 2.
PAR4 and P2Y12 heterodimerize in BRET but PAR1 P2Y12 do not. (A) HEK293T cells were transiently cotransfected with (0.18 μg) of Rluc-P2Y12 and varying amounts (0.18–2.88 μg) of PAR4-YFP or PAR1-YFP or YFP-expressing vector control to achieve YFP:Rluc ratios from 1:1 to 16:1. BRET is expressed in relative % maximal BRET as described in Materials and Methods. PAR4YFP:RlucP2Y12 reached an average maximum mBU of 1000 units compared with only 270 mBU for PAR1YFP:RlucP2y12 (N = 3). (B) HEK293T cells were transiently cotransfected with Rluc-P2Y12 (0.18 μg) and PAR4-YFP (2.16 μg) at a saturating ratio of 12:1 (YFP:Rluc) (max mBU = 30.72) and increasing amounts of HA tagged PAR4 (○) or PAR1(●) from ratio 4:1 to 24:1 of competitor:Rluc. HA-PAR4 was able to compete off Rluc-P2Y12 and PAR4-YFP dimerization and reduced the % maximal BRET in a dose-dependent manner but HA-PAR1 could not. Shown is the mean ± S.E.M. of 3 independent experiments. *P < 0.05; **P < 0.01.
Residues at the Base of PAR4 Transmembrane Helix 4 Are Required to Promote Interaction with P2Y12 but Not with Itself.
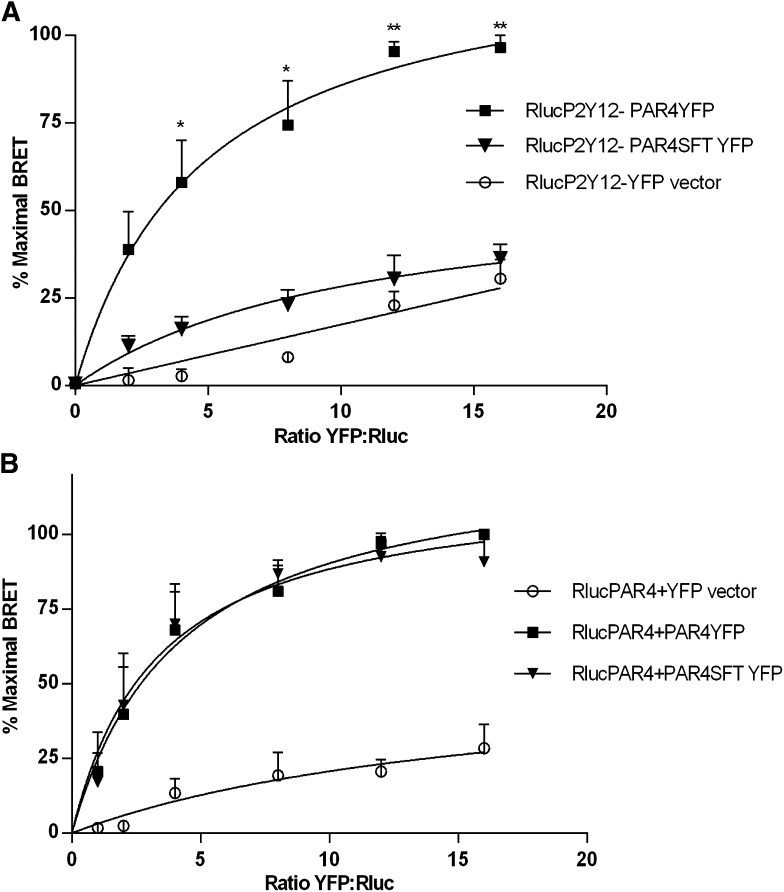
Because PAR4 associates specifically with P2Y12 but PAR1 does not (Fig. 2A), we sought to determine residues in PAR4 that are required for the specificity of its interaction with P2Y12 relative to PAR1. Accordingly, we examined the sequence of PAR4 and PAR1 in their transmembrane domains, a common site of GPCR heterodimerization, to determine whether there are specific sites of amino acid sequence divergence between the two receptors. We identified three amino acids at the base of the TM4 of PAR4 (LGL 194-196) that differ significantly from the SFT (220–222) expressed at the aligned sequence of TM4 in PAR1. Based upon the sequence comparison, we designed a variant of PAR4, termed PAR4SFT, that is mutated at these three residues (LGL194–196) to contain the corresponding PAR1 residues at the aligned position (SFT 220–222). We found that PAR4SFT fails to interact with P2Y12 when assessed by saturation BRET (Fig. 3A). Notably, residues in the 4th transmembrane domain have also been demonstrated to promote PAR4 homodimer formation (de la Fuente et al., 2012). To determine whether residues LGL (194–196) at the base of TM4 also contribute to PAR4 homodimerization, we tested whether PAR4SFT could homodimerize with (wild-type) PAR4 when the two receptors were coexpressed in HEK293T cells and assessed using saturation BRET (Fig. 3B). The results show that luciferase-tagged PAR4 interacted equally well with PAR4SFT-YFP as with PAR4-YFP, indicating that these residues specifically support association with P2Y12 but are not required for the homodimeric interaction.
Fig. 3.
PAR4SFT fails to dimerize with P2Y12 in saturation BRET but still dimerizes with wild-type PAR4. HEK293T cells were transiently cotransfected with (0.18 μg) of Rluc-P2Y12 (A) Rluc-PAR4 (B) and varying (amounts) of PAR4-YFP or PAR4SFT-YFP or YFP-expressing vector control to achieve YFP:Rluc ratios from 1:1 to 16:1 (0.18–2.88 μg). BRET is expressed in relative % maximal BRET. (A) PAR4SFT supported only 8.74% of PAR4 P2Y12 dimerization in saturation BRET, where PAR4YFP:RlucP2Y12 achieved an average maximum 104.40 mBU at a ratio of 16:1. (B) PAR4SFT-YFP homodimerized equally well with Rluc-PAR4, like PAR4-YFP, with an average max 268.08 mBU for PAR4YFP:RlucPAR4 and 260.24 mBU for PAR4SFTYFP:RlucPAR4 at a 16:1 ratio. Shown are the mean ± S.E.M. of 3 and 4 independent experiments, respectively. *P < 0.05; **P < 0.01.
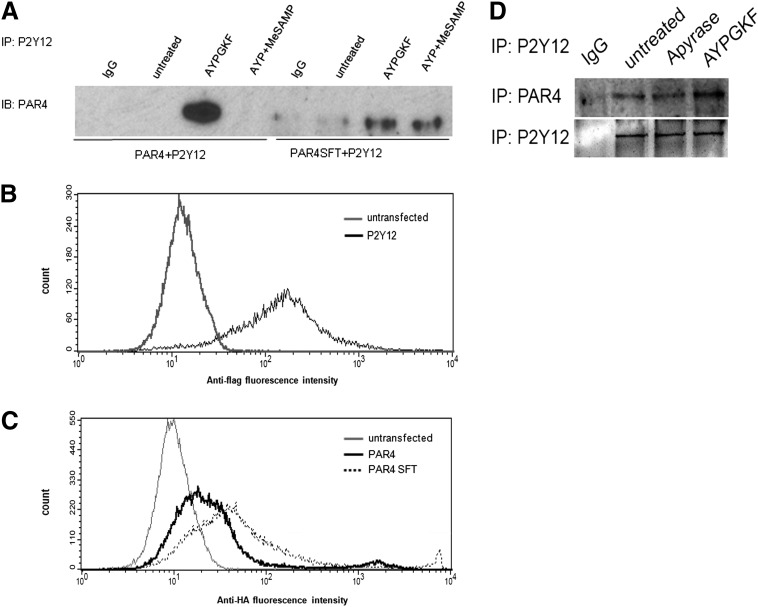
When expressed in HEK293T cells, coimmunoprecipitation of PAR4 or PAR4SFT with P2Y12 also supports this finding (Fig. 3A), with an observed increase in PAR4-P2Y12 receptor coimmunoprecipitation upon agonist treatment and a comparatively less coimmunoprecipitation of P2Y12 with PAR4SFT relative to PAR4 (Fig. 4A). All the receptors, P2Y12, PAR4, and PAR4SFT, were expressed on the HEK293T cell membranes; hence the reduction in coimmunoprecipitation of PAR4SFT and P2Y12 was not due to abnormal receptor trafficking to the membrane (Fig. 4, B and C). To determine if endogenous ADP levels in the media could promote the basal PAR4-P2Y12 association observed, coimmunoprecipitation studies were conducted in the presence and absence of apyrase. Figure 4D shows that apyrase treatment does not alter the basal level of PAR4 and P2Y12 association.
Fig. 4.
PAR4 coimmunoprecipitates with P2Y12 upon PAR4-AP stimulation, but PAR4SFT does not in HEK293T cells. (A) HEK293T cells stably expressing FLAG-tagged P2Y12 were transiently transfected with V5-PAR4-GFP or HA-PAR4SFT-GFP. Forty-eight hours post-transfection, cells were left untreated or stimulated with AYPGKF (200 μM) 10 minutes at 37°C with and without MeSAMP (150 μM) 10 minutes at room temperature. Cell were lysed, immunoprecipitated with anti-FLAG antibody (Abcam; 1:1000) and immunoblotted (IB) for antibody to GFP (MBL; 1:500). (B and C) Surface expression of P2Y12, PAR4, and PAR4SFT in HEK293T cells. HEK293T cells stably expressing FLAG-P2Y12 (B) and transiently expressing HA-PAR4 or HA-PAR4SF (C) were harvested in Hanks' balanced salt solution + 0.02% EDTA, washed with 1× PBS, and resuspended to be fixed in DMEM + 5% fetal bovine serum, stained with anti-FLAG and anti-HA, and probed with Alexa 488 secondary antibody to test the membrane expression of these receptors; cells were fixed poststaining. (D) HEK293T cells transiently expressing V5-PAR4-GFP and FLAG-P2Y12 were treated with apyrase (2 U/ml) and AYPGKF (200 μM), lysed, and immunoprecipated with anti-FLAG (2 μg/ml) and immunoblotted with anti V5 (1:1000).
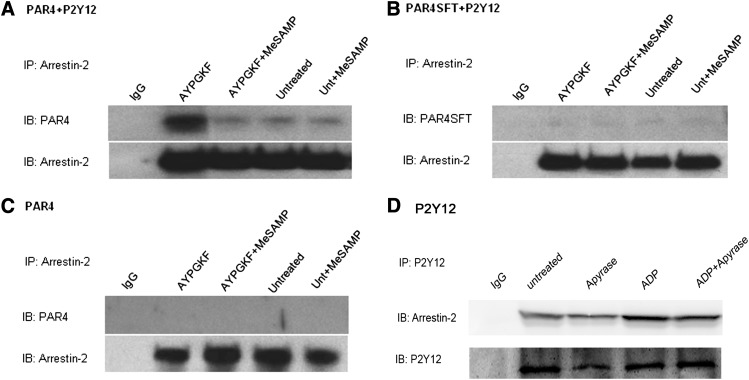
Association of PAR4 with P2Y12 Is Required for PAR4-Dependent Recruitment of Arrestin-2 and Akt Activation.
We previously reported that stimulation of mouse platelets with thrombin or PAR4-AP causes arrestin-2 recruitment to PAR4 in a P2Y12-dependent manner and that PAR4-mediated Akt activation is partially governed by arrestin signaling (Li et al., 2011). To determine whether association of P2Y12 with PAR4 governs the recruitment of arrestin to PAR4 and, thus, influences the maximal Akt activation observed in response to PAR4 activation, we compared PAR4-dependent arrestin recruitment and Akt activation in cells coexpressing PAR4 with P2Y12 or coexpressing PAR4SFT with P2Y12. HEK293T cells were selected to stably express either HA-tagged PAR4 or HA-tagged PAR4SFT alone or together with FLAG-tagged P2Y12. PAR4- or PAR4SFT-expressing HEK293T cells were then stimulated with AYPGKF in the presence or absence of the P2Y12 antagonist MeSAMP. Lysates of PAR4-stimulated cells were immunoprecipitated with antibody to arrestin-2 and immunoblotted for PAR4 (Fig. 5). We observed that PAR4 coimmunoprecipitates with arrestin-2 upon activation and only in the presence of P2Y12 expression (Fig. 5, A and C). When PAR4SFT was coexpressed with P2Y12, it failed to associate with arrestin even after PAR4 activation (Fig. 5B). However, P2Y12 can associate with arrestin by itself upon ADP stimulation (Fig. 5D), suggesting that PAR4-P2Y12 dimerization assists arrestin recruitment to PAR4 with P2Y12 as a mediator.
Fig. 5.
Arrestin coimmunoprecipitates with PAR4 only in the presence of P2Y12 and depends on PAR4 P2Y12 dimerization. HEK293T cells stably expressing HA-PAR4 or HA-PAR4SFT or coexpressing it along with FLAG-P2Y12 were left untreated or stimulated with AYPGKF (200 μM) 10 minutes at 37°C with and without MeSAMP (150 μM) 10 minutes at room temperature. Cell were lysed, immunoprecipitated with antibodies to arrestin-2 (2 μg/ml; Santa Cruz Biotechnology), and immunoblotted (IB) for PAR4 (1:1000; Santa Cruz Biotechnology) (A–C) . P2Y12 stable cells were treated as shown and immunoprecipated with anti-FLAG (2 μg/ml) and immunoblotted for arrestin-2 (1:1000; Santa Cruz Biotechnology) (D).
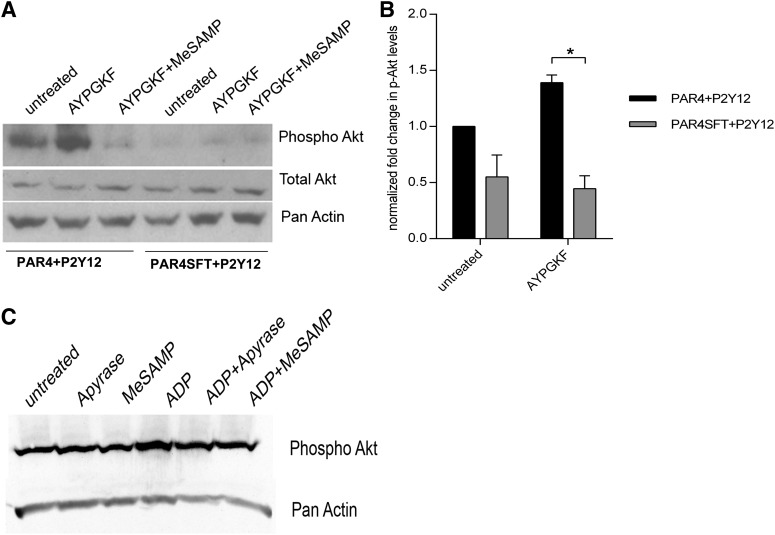
Using the previously described stable cell lines, we next tested whether PAR4-P2Y12 interaction was required for Akt activation. Stimulation with PAR4-AP peptide resulted in substantial Akt activation in PAR4 and P2Y12 coexpressing 293T cells; however, PAR4-AP failed to cause Akt activation in cells coexpressing PAR4SFT and P2Y12, implying that the failure to dimerize with P2Y12 abolishes the ability of PAR4 to associate with arrestin and influences downstream Akt activation (Fig. 6). When expressed alone, however, P2Y12 can activate Akt upon ADP stimulation (Fig. 6C).
Fig. 6.
Akt phosphorylation in response to PAR4 activation in cells coexpressing PAR4 or PAR4SFT and P2Y12. Stable HEK293T cell lines were stimulated with AYPGKF (200 μM) 10 minutes at 37°C with and without MeSAMP (150 μM) 10 minutes at room temperature, lysed, and immunoblotted for phospho Akt using anti–p-Akt473 (1:500; Cell Signaling Technology). Pan-actin and total Akt were used as loading controls (1:1000; Cell Signaling Technology). Shown is a representative blot (A) and an averaged quantification of normalized phospho Akt levels ± S.E.M. for 3 independent experiments (*P < 0.05) (B). (C) P2Y12 stable cells were treated as shown, lysed, and immunoblotted for phospho Akt using anti–p-Akt473 (1:500; Cell Signaling Technology). Pan-actin was used as loading control (1:1000; Cell Signaling Technology).
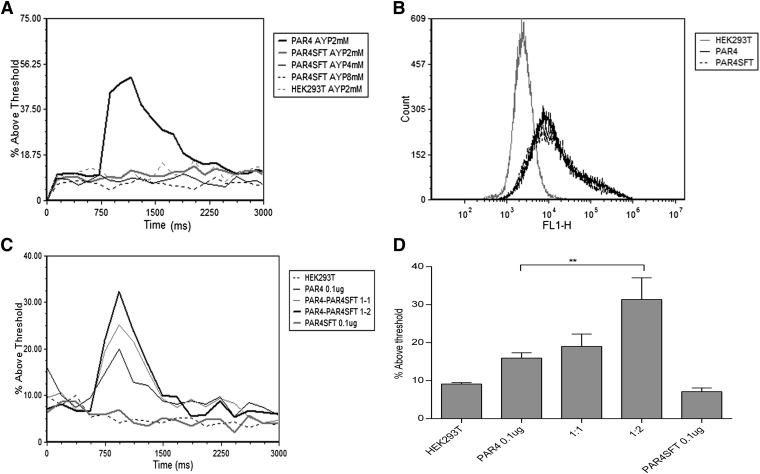
PAR4SFT Fails to Induce Calcium Mobilization Independently but Potentiates PAR4-Mediated Calcium Flux.
Finally, Gq-coupled calcium mobilization responses of PAR4 and PAR4SFT were assessed by evaluating the relative fluorescence of PAR4-stimulated, Fluo4-AM–loaded HEK293T cells transiently expressing PAR4 or PAR4SFT. When expressed individually, PAR4SFT failed to induce a calcium flux upon stimulation with increasing AYPGKF concentration (from 1 to 8 mM), although PAR4 and PAR4SFT were expressed with equal efficiency (Fig. 7, A and B), implying that it is unable to activate Gq by itself. PAR4 homodimerization has been reported to be essential for calcium mobilization (de la Fuente et al., 2012); hence, we reasoned that the defect in calcium mobilization seen with PAR4SFT could be due to the inability of the mutant to form homodimers. Importantly, however, we showed that PAR4SFT can dimerize with wild-type PAR4 (Fig. 3B). Therefore, we hypothesized that dimeric PAR4 is required for functional Gq coupling. Hence we tested whether increased expression of PAR4SFT with (wild-type) PAR4 might alter the minimal amount of PAR4 required to generate a calcium response. When minimal amounts of PAR4 (0.1 μg) (enough to see minimal detectable calcium flux) were cotransfected with increasing amounts of PAR4SFT (PAR4:PAR4SFT 1:1 and 1:2), a PAR4SFT receptor dose-dependent increase in calcium response was detected (Fig. 7, C and D), suggesting that PAR4SFT can dimerize with PAR4 to generate a functional dimer. This, together with our BRET data (Fig. 3B), shows that PAR4SFT can dimerize with PAR4 but not with itself to induce Gq-mediated responses.
Fig. 7.
Mutating TM4 residues LGL (194–196) to SFT disrupts the ability of PAR4 to induce calcium mobilization in response to PAR4-AP. HEK293T cells transiently transfected with V5-PAR4-GFP or HA-PAR4SFT-GFP. Forty-eight hours post-transfections, cells were collected and loaded with Fluo4-AM for 20 minutes at 37°C. Calcium mobilization was measured as Fluo4-AM intensity. Baseline cytosolic calcium levels were recorded for 1 minute followed by AYPGKF 2 mM stimulation on AccuriC6 Flow cytometer. (A) Representative overlay of the calcium mobilization in response to AYPGKF. (B) Transfection efficiency of PAR4 and PAR4SFT was tested by the GFP signal in unloaded HEK293T cells. (C and D) Coexpression of PAR4SFT with PAR4 potentiates calcium mobilization above minimal detectable PAR4 response to AYPGKF. HEK293T cells were transiently transfected with 0.1 μg of PAR4 alone and or cotransfected with PAR4 (0.1 μg):PAR4SFT (0.1 and 0.2 μg) at 1:1 and 1:2 ratio. Calcium mobilization was assessed as described in A. Shown is the % increase in Fluo4-AM intensity above threshold ±S.E.M. of 4 independent experiments (**P < 0.01).
PAR4-Induced Calcium Mobilization Influences PAR4-P2Y12 Dimerization.
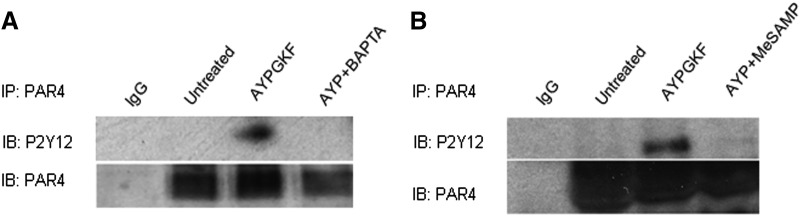
Given that PAR4SFT fails to elicit normal calcium responses when expressed in isolation, we reasoned that the inability of PAR4SFT to dimerize with P2Y12 could be secondary to its defect in eliciting Gq-coupled calcium responses. Hence, we evaluated whether calcium mobilization is required for association of PAR4 with P2Y12 in human platelets and HEK293T cells. Washed human platelets or HEK293T cells coexpressing PAR4 and P2Y12 were treated with calcium chelator BAPTA-AM 10 μM and then stimulated with AYPGKF 150 μM followed by coimmunoprecipitation of PAR4 and P2Y12. Figure 8A and Supplemental Fig. 2 show that in human platelets and HEK293T cells, respectively, PAR4 stimulation promotes PAR4-P2Y12 association and can be blocked by BAPTA-AM. Thus, calcium mobilization is important in promoting receptor-receptor interaction, suggesting at least one mechanism by which receptor activation is required for PAR4-P2Y12 association. Consistent with our previous report, we also demonstrate in human platelets that PAR4-stimulated PAR4-P2Y12 association is prevented by inhibition of P2Y12 (Fig. 8B).
Fig. 8.
Chelating cytosolic calcium post PAR4 activation blocks PAR4 P2Y12 dimerization. Human platelets (4 × 108/lane) were treated with AYPGKF (150 μM) 5 minutes at 37°C with/without BAPTA-AM (20 μM) for 10 minutes at 37°C (A) or MeSAMP (100 μM) 5 minutes at room temperature (B), then immunoprecipitated with either IgG control or antibody to PAR4 (2 μg/ml). Precipitates were immunoblotted (IB) with anti-P2Y12 antibody (1:1000).
Discussion
An increasing number of studies have shown that GPCRs can exist as homo- or heterodimers or higher order oligomers (Milligan et al., 2004; Minneman, 2007; Milligan, 2008; Smith and Milligan, 2010). Although the structural determinants governing these interactions and their functional relevance are defined for some receptor pairs (Wang et al., 2005; Lagane et al., 2008; de la Fuente et al., 2012; Frey et al., 2013; Ibrahim et al., 2013), new receptor homo- and heterodimerizations continue to be identified in diverse cells. Some receptor pairs have been shown to support differential signaling responses from those attributed to either receptor alone (Rozenfeld and Devi, 2010, 2011). We report here for the first time that PAR4 and P2Y12, two GPCRs expressed in platelets, undergo activation-dependent direct interaction when expressed in HEK293T cells. We identify a three amino acid site in TM4 of PAR4 that, when replaced with aligned residues from its PAR1 counterpart, disrupts the interaction of PAR4 with P2Y12 but not its ability to homodimerize with wild-type PAR4. Furthermore, this mutation also disrupts its ability to recruit arrestin-2 to the PAR4-P2Y12 complex, with commensurate disruption of Akt phosphorylation. Interestingly, expression of PAR4SFT alone fails to support Gq-coupled calcium responses but potentiates PAR4-mediated calcium responses, suggesting that PAR4 homodimer formation is required for Gq coupling. This study suggests a mechanism by which PAR4 and P2Y12 synergistically may contribute to platelet activation because of the activation-dependent formation of PAR4-P2Y12 heterodimers, which permits recruitment of arrestin-2 to initiate a unique signaling cascade that can mediate the PI3K-dependent Akt activation (Li et al., 2011) to influence platelet activation.
In this study, we demonstrate using saturation BRET in HEK293T cells that the thrombin receptor PAR4 interacts directly with P2Y12 upon agonist activation. Although some interaction is detected in the absence of agonist, this is likely caused by overexpression in the heterologous expression system that can elicit increased constitutive activity (Daeffler and Landry, 2000). An increased net BRET output after PAR4 stimulation and a reduction in BRET in the presence of P2Y12 antagonist (Fig. 1) suggest that exposure of platelets in vivo to the physiologic release of thrombin during thrombus formation could promote PAR4-P2Y12 heterodimerization and that P2Y12 or PAR4 inhibitors would block this event. Many GPCRs expressed in platelets have been reported to form homodimers or -oligomers independent of ligand activation, including PAR4, thromboxane A2 receptor, and P2Y12. Agonist-dependent regulation of PAR4-P2Y12 association in platelets suggests that these heterophilic associations might occur in a regulated, reversible fashion to promote sustained signaling events supporting longer-term thrombus stability.
Association of P2Y12 with PAR4 is specific, because P2Y12 does not interact with PAR1 when assessed by saturation or competition BRET (Fig. 2). Structural motifs governing GPCR interaction have been mapped to the TMs in many receptors (complement factor A receptor, chemokine receptors, dopamine, and β-adrenergic receptors) (Floyd et al., 2003; Klco et al., 2003). Using the sequence divergence of PAR1 from PAR4 in the TM helices as a basis for site-directed mutagenesis, we identified a site at the base of the TM4 helix of PAR4 that disrupts the association between PAR4 and P2Y12, as well as Gq-coupled calcium mobilization. The impairment in PAR4-P2Y12 association could be a consequence of the defect in Gq signaling, implying that PAR4-dependent calcium mobilization is required to promote PAR4 and P2Y12 dimerization. This further supports the idea that PAR4 activation is necessary for PAR4-P2Y12 dimerization. Interestingly, the leucine zipper of PAR4 TM4 has been reported to be required for PAR4 homodimerization and intracellular calcium mobilization (de la Fuente et al., 2012). Of note, the association of PAR4SFT with wild-type PAR4 is maintained despite the mutation (Fig. 3B). This region is near the cytoplasmic interface, but C terminal to the residues that have previously been shown to be required for PAR4 homodimerization, suggesting that discrete regions of TM4 govern association of PAR4 with another PAR4 protomer versus with P2Y12.
Given the reported existence of constitutive PAR4-PAR1 heterodimers (Leger et al., 2006) in platelets, the inability of PAR1 to compete with the interaction of PAR4 and P2Y12 raises at least two possible interpretations. 1) PAR4 interacts with P2Y12 at a site different from its interaction with PAR1. This possibility is likely, given that the two discrete mutations in TM4 show differential effect on the interaction of PAR4 with itself versus P2Y12. 2) The affinity of PAR4 for P2Y12 in this HEK293T system is greater than that of PAR4 for PAR1. This may be the case in platelets only when PAR4 and P2Y12 are agonist activated, because the PAR1-PAR4 interaction is constitutive in platelets but the PAR4-P2Y12 is not. Although each of these complexes has been detected in platelets by coimmunoprecipitation, the relative amounts of PAR4 in each of these GPCR complexes remains to be quantified, as does whether these different complexes of PAR4 exist in different membrane domains, such as lipid rafts.
Given that receptor activation drives PAR4-P2Y12 association and these receptors have been shown to play a role in thrombus stability in vivo, we wondered whether PAR4-P2Y12 association could drive unique signaling events. Multiple studies have observed that signaling consequences of GPCR heterodimers can be different from those detected due to expression of either receptor alone; for example, heterodimers of δ-κ-opioid receptors, δ-μ-opioid receptors, and α2a- and α2c-adrenergic receptors yield arrestin-dependent signaling responses as heterodimers that are not detected upon expression of the monomers alone (Small et al., 2006; Rozenfeld and Devi, 2007, 2010). Arrestins serve as scaffolding molecules to signal through complexes that include clathrin, adaptin, mdm2, and, of particular relevance to our study, src family kinases, and PI3K (Gurevich and Gurevich, 2003; Lodeiro et al., 2009; Yang et al., 2009). We previously demonstrated that in mouse and human platelets, PAR4-AP induces a signaling complex of arrestin-2, the p85 subunit of PI3K and the src family kinases, Lyn, which contributes to Akt activation in a P2Y12-dependent manner (Li et al., 2011). Here, we show that PAR4 supports arrestin recruitment and arrestin-dependent Akt phosphorylation only in the presence of P2Y12 in HEK293T cells (Figs. 5 and 6). In contrast, when PAR4SFT and P2Y12 are coexpressed, arrestin recruitment to AYPGKF-activated PAR4SFT is abolished, and Akt phosphorylation is inhibited. Although many studies have reported that PAR4 signaling to Akt is P2Y12 dependent (Kim et al., 2006; Li et al., 2008), a recent study demonstrated that PAR4 can independently activate Akt in P2Y12 knockout mice and in COS-7 cells at high agonist concentration (AYPGKF 500–1000 μM; Xiang et al., 2010). In the current study, coexpression of P2Y12 and PAR4 was required to detect PAR4-dependent Akt phosphorylation. Also our previous study demonstrated that arrestin-2 deletion reduced but did not ablate PAR4-dependent Akt phosphorylation. Taken together, these studies support a model whereby arrestin scaffolding of PI3K complexes permits the potentiation of Akt phosphorylation by low concentrations of PAR4-AP (150–200 μM). Additionally P2Y12 has been reported to bind arrestin-2 and undergo rapid internalization, suggesting a model whereby the direct interaction of P2Y12 with arrestin allows PAR4 to recruit arrestin together with P2Y12 upon PAR4-AP activation (Mundell et al., 2006).
Curiously, mutation of TM4 LGL to residues SFT also disrupted PAR4-dependent calcium responses. This was a surprise, considering that PAR1, like PAR4, also couples to Gq. Nevertheless, it raises the question of whether this site is required both for PAR4-P2Y12 interaction and G protein association independently or whether mutation of PAR4 affects G protein interaction, which, in turn, influences PAR4-P2Y12 interaction. Interestingly, expressing increasing concentrations of PAR4SFT with coexpressed PAR4 potentiated calcium responses compared with expression of PAR4 alone. This implies that one protomer of PAR4 containing an intact TM4 is sufficient to interact with a PAR4SFT protomer. It also is consistent with a model in which PAR4 exists in a stoichiometric ratio of 2 GPCRs:1 G protein, which has been the model suggested by previous reports of rhodopsin and leukotriene receptors (Liang et al., 2003; Maurice et al., 2011; Jastrzebska et al., 2013).
In summary, we have shown that agonist-activated PAR4 recruits P2Y12-associated arrestin to PAR4 through direct association with P2Y12. We previously showed that arrestin-dependent pathways contribute to Akt activation in platelets, which, in turn, is important for the growth and stability of platelet-rich thrombi in vivo (Li et al., 2011; Woulfe, 2010). Taken together, these studies suggest that the agonist-regulated association of PAR4 with P2Y12 has functional consequences in promoting the stability of platelet thrombi. Although GPCR heterodimerization has been demonstrated in a variety of cell systems, including platelets, their functional relevance in platelets is just beginning to be elucidated. This is the first demonstration of an agonist-regulated formation of a GPCR heterodimer of receptors expressed in platelets that results in a specific signaling consequence. Because we previously showed that arrestin contributes to Akt activation, which in turn stabilizes platelet thrombi, we propose that agonist-regulated PAR4-P2Y12 association is one mechanism by which PAR4 and P2Y12 synergize to stabilize platelet-rich thrombi. We will test this hypothesis in future studies.
Supplementary Material
Acknowledgments
The authors thank Dr. Marvin Nieman (Case Western Reserve University, Cleveland, OH) and Dr. Ji-Fang Zhang (Thomas Jefferson University, Philadelphia, PA) for providing PAR4 and vector constructs, respectively. Flow Cytometry was performed on AccuriC6 for calcium mobilization assays at the Nemours Cell Science Core. The authors are grateful to Dr. Sonali Barwe and Sona Lakshme Balasubramanium (A. I. Dupont Children’s hospital, Centre for Childhood Cancer research, Wilmington, DE) for assistance with calcium mobilization assays.
Abbreviations
- AYPGKF
Ala-Tyr-Pro-Gly-Lys-Phe
- BAPTA-AM
1,2-bis(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid
- BRET
bioluminescence resonance energy transfer
- DMEM
Dulbecco’s modified Eagle’s medium
- EYFP
enhanced yellow fluorescent protein
- FBS
fetal bovine serum
- FOB
fold over basal
- GFP
green fluorescent protein
- GPCR
G protein–coupled receptor
- HA
hemagglutinin
- HEK
human embryonic kidney
- IP
immunoprecipitation
- mBU
milli-BRET units
- MeSAMP
2-methylthioadenosine 5′-monophosphate triethylammonium salt
- PAR
protease activated receptor
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PMSF
phenylmethanesulfonyl fluoride
- TM
transmembrane helix
Authorship Contributions
Participated in research design: Khan, Li, Ibrahim, Smyth, Woulfe.
Conducted experiments: Khan, Li, Ibrahim.
Contributed new reagents or analytic tools: Ibrahim, Smyth.
Performed data analysis: Khan, Li, Ibrahim, Woulfe.
Wrote or contributed to the writing of the manuscript: Khan, Woulfe.
Footnotes
This work was funded, in part, by an American Heart Association Grant-in-Aid GRNT12040216; by a pilot project awarded from the National Center for Research Resources [Grant 5P30RR031160-03]; and the National Institutes of Health National Institute of General Medical Sciences [Grant P30-GM103519-03]. Nemours Cell Science Core is supported, in part, by grants from the National Institutes of Health National Institute of General Medical Sciences [Grants 5P20-GM103464-09 and 5P20-GM103446-13].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Angers S, Salahpour A, Bouvier M. (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42:409–435 [DOI] [PubMed] [Google Scholar]
- Covic L, Gresser AL, Kuliopulos A. (2000) Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry 39:5458–5467 [DOI] [PubMed] [Google Scholar]
- Daeffler L, Landry Y. (2000) Inverse agonism at heptahelical receptors: concept, experimental approach and therapeutic potential. Fundam Clin Pharmacol 14:73–87 [DOI] [PubMed] [Google Scholar]
- de la Fuente M, Noble DN, Verma S, Nieman MT. (2012) Mapping human protease-activated receptor 4 (PAR4) homodimer interface to transmembrane helix 4. J Biol Chem 287:10414–10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filizola M, Weinstein H. (2005) The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J 272:2926–2938 [DOI] [PubMed] [Google Scholar]
- Floyd DH, Geva A, Bruinsma SP, Overton MC, Blumer KJ, Baranski TJ. (2003) C5a receptor oligomerization. II. Fluorescence resonance energy transfer studies of a human G protein-coupled receptor expressed in yeast. J Biol Chem 278:35354–35361 [DOI] [PubMed] [Google Scholar]
- Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, et al. (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107:1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AJ, Ibrahim S, Gleim S, Hwa J, Smyth EM. (2013) Biased suppression of TP homodimerization and signaling through disruption of a TM GxxxGxxxL helical interaction motif. J Lipid Res 54:1678–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía J, Lluís C, Ferré S, Franco R, Ciruela F. (2008) Light resonance energy transfer-based methods in the study of G protein-coupled receptor oligomerization. Bioessays 30:82–89 [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. (2003) The new face of active receptor bound arrestin attracts new partners. Structure 11:1037–1042 [DOI] [PubMed] [Google Scholar]
- Holinstat M, Voss B, Bilodeau ML, McLaughlin JN, Cleator J, Hamm HE. (2006) PAR4, but not PAR1, signals human platelet aggregation via Ca2+ mobilization and synergistic P2Y12 receptor activation. J Biol Chem 281:26665–26674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie JA, Ahuja M, Belmonte E, Pizarro S, Parton R, Brass LF. (1993) Internalization and recycling of activated thrombin receptors. J Biol Chem 268:13756–13763 [PubMed] [Google Scholar]
- Ibrahim S, McCartney A, Markosyan N, Smyth EM. (2013) Heterodimerization with the prostacyclin receptor triggers thromboxane receptor relocation to lipid rafts. Arterioscler Thromb Vasc Biol 33:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska B, Orban T, Golczak M, Engel A, Palczewski K. (2013) Asymmetry of the rhodopsin dimer in complex with transducin. FASEB J 27:1572–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. (1999) Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 103:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jin JG, Kunapuli SP. (2006) Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood 107:947–954 [DOI] [PubMed] [Google Scholar]
- Klco JM, Lassere TB, Baranski TJ. (2003) C5a receptor oligomerization. I. Disulfide trapping reveals oligomers and potential contact surfaces in a G protein-coupled receptor. J Biol Chem 278:35345–35353 [DOI] [PubMed] [Google Scholar]
- Lagane B, Chow KYC, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. (2008) CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood 112:34–44 [DOI] [PubMed] [Google Scholar]
- Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. (2006) Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation 113:1244–1254 [DOI] [PubMed] [Google Scholar]
- Li D, August S, Woulfe DS. (2008) GSK3beta is a negative regulator of platelet function and thrombosis. Blood 111:3522–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DJ, D’Angelo L, Chavez M, Woulfe DS. (2011) Arrestin-2 differentially regulates PAR4 and ADP receptor signaling in platelets. J Biol Chem 286:3805–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. (2003) Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem 278:21655–21662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Trejo J. (2013) Transactivation of the PAR1-PAR2 heterodimer by thrombin elicits β-arrestin-mediated endosomal signaling. J Biol Chem 288:11203–11215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodeiro M, Theodoropoulou M, Pardo M, Casanueva FF, Camiña JP. (2009) c-Src regulates Akt signaling in response to ghrelin via beta-arrestin signaling-independent and -dependent mechanisms. PLoS ONE 4:e4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P, Kamal M, Jockers R. (2011) Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol Sci 32:514–520 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2008) A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol 153 (Suppl 1):S216–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Bond RA, Lee M. (1995) Inverse agonism: pharmacological curiosity or potential therapeutic strategy? Trends Pharmacol Sci 16:10–13 [DOI] [PubMed] [Google Scholar]
- Milligan G, Pediani J, Fidock M, López-Giménez JF. (2004) Dimerization of alpha1-adrenoceptors. Biochem Soc Trans 32:847–850 [DOI] [PubMed] [Google Scholar]
- Minneman KP. (2007) Heterodimerization and surface localization of G protein coupled receptors. Biochem Pharmacol 73:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. (2006) Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic 7:1420–1431 [DOI] [PubMed] [Google Scholar]
- Niitsu Y, Jakubowski JA, Sugidachi A, Asai F. (2005) Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost 31:184–194 [DOI] [PubMed] [Google Scholar]
- Novi F, Stanasila L, Giorgi F, Corsini GU, Cotecchia S, Maggio R. (2005) Paired activation of two components within muscarinic M3 receptor dimers is required for recruitment of beta-arrestin-1 to the plasma membrane. J Biol Chem 280:19768–19776 [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2007) Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 21:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2010) Receptor heteromerization and drug discovery. Trends Pharmacol Sci 31:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. (2011) Exploring a role for heteromerization in GPCR signalling specificity. Biochem J 433:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Hervé C, Uzabiaga MF, Pereillo JM, Culouscou JM, Bono F, Ferrara P, et al. (2006) The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA 103:11069–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. (2000) Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J Biol Chem 275:25216–25221 [DOI] [PubMed] [Google Scholar]
- Small KM, Schwarb MR, Glinka C, Theiss CT, Brown KM, Seman CA, Liggett SB. (2006) Alpha2A- and alpha2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry 45:4760–4767 [DOI] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev 62:701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S, Nakazawa T, Kamae T, Kiyomizu K, Kashiwagi H, Honda S, Kanakura Y, Tomiyama Y. (2011) A potential role for α-actinin in inside-out αIIbβ3 signaling. Blood 117:250–258 [DOI] [PubMed] [Google Scholar]
- Tiberi M, Caron MG. (1994) High agonist-independent activity is a distinguishing feature of the dopamine D1B receptor subtype. J Biol Chem 269:27925–27931 [PubMed] [Google Scholar]
- Trejo J, Coughlin SR. (1999) The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J Biol Chem 274:2216–2224 [DOI] [PubMed] [Google Scholar]
- Wang DX, Sun XC, Bohn LM, Sadée W. (2005) Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol 67:2173–2184 [DOI] [PubMed] [Google Scholar]
- Woulfe DS. (2010) Akt signaling in platelets and thrombosis. Expert Rev Hematol 3:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang B, Zhang G, Liu J, Morris AJ, Smyth SS, Gartner TK, Li Z. (2010) A G(i) -independent mechanism mediating Akt phosphorylation in platelets. J Thromb Haemost 8:2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, He RL, Benovic JL, Ye RD. (2009) beta-Arrestin1 interacts with the G-protein subunits beta1gamma2 and promotes beta1gamma2-dependent Akt signalling for NF-kappaB activation. Biochem J 417:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.