Abstract
Background
Previous studies have demonstrated that gene expression levels change with age. These changes are hypothesized to influence the aging rate of an individual. We analyzed gene expression changes with age in abdominal skin, subcutaneous adipose tissue and lymphoblastoid cell lines in 856 female twins in the age range of 39-85 years. Additionally, we investigated genotypic variants involved in genotype-by-age interactions to understand how the genomic regulation of gene expression alters with age.
Results
Using a linear mixed model, differential expression with age was identified in 1,672 genes in skin and 188 genes in adipose tissue. Only two genes expressed in lymphoblastoid cell lines showed significant changes with age. Genes significantly regulated by age were compared with expression profiles in 10 brain regions from 100 postmortem brains aged 16 to 83 years. We identified only one age-related gene common to the three tissues. There were 12 genes that showed differential expression with age in both skin and brain tissue and three common to adipose and brain tissues.
Conclusions
Skin showed the most age-related gene expression changes of all the tissues investigated, with many of the genes being previously implicated in fatty acid metabolism, mitochondrial activity, cancer and splicing. A significant proportion of age-related changes in gene expression appear to be tissue-specific with only a few genes sharing an age effect in expression across tissues. More research is needed to improve our understanding of the genetic influences on aging and the relationship with age-related diseases.
Keywords: Aging, gene expression, skin, adipose, brain, microarrays
Background
Aging has been described as a progressive decline in the ability to withstand stress, damage, and disease resulting in degeneration [1,2]. Age is also a major risk factor in the development of many diseases, although the relationship between the aging process and the etiology of age-related diseases is not fully understood. Previous gene expression studies of aging have primarily concentrated on model organisms [3] or have been confined to specific aging-associated disorders such as progeria syndromes[4]. A study of postmortem human brain tissue from 30 individuals aged 26 to 106 years [5] showed that approximately 4% of approximately 11,000 genes analyzed show a significant age-related expression change (1.5-fold or more) in individuals aged >40 years. These genes were reported to play central roles in synaptic plasticity, vesicular transport, and mitochondrial function. Another study [6]examined gene expression changes with age in healthy renal tissue removed at nephrectomy from 74 patients ranging in age from 27 to 92 years old; identifying 985 genes differentially expressed with age. More recently, a meta-analysis of age-related gene expression profiles combined multiple disparate gene expression studies in an attempt to identify common signatures of aging across both tissue and species [7]. However, to date, studies published using human tissuehave all been carried out on a limited number of samples, making them underpowered for the detection of normal age-related expression differences.
The aims of this study were to determine which genes and pathways show differential expression with age in multiple tissues and to understand how the genomic regulation of gene expression alters with age. Age effect on gene expression was explored by examining expression profiles in skin, adipose tissue, and lymphoblastoid cell lines (LCLs) from 856 female twins agedfrom 39 to 85 yearsold (Figure 1)from the Multiple Tissue Human Expression Resource (MuTHER study) [8].Genes significantly affected by age in skin and adipose tissues were followed upin 932 postmortembrain samples (representing from 10 brain regions) from 100 individuals;provided by the UK Brain Expression Consortium[2]. In addition, the influence of genetic variants on gene expression in aging individuals was explored by examining significant eQTL from the MuTHER dataset [9], for a genotype-by-age interaction.
Figure 1.
Histogram showing the age distribution of the individuals in the study.
Results
Age-related gene expression
Expression profiles were determined from 856 individuals aged between 39 and 85 years from skin, adipose tissue,and LCL samples using the Illumina Human HT-12 V3 Bead chip.All the volunteers were female twins(336 MZ and 520 DZ) recruited from the TwinsUK Adult twin registry [10] aged 39 to 85 years old, with an average age for the cohort of 59.3 years (Figure 1 and Additional file 1, TableS 1).A linear mixed model identified 1,672 genes as differentially expressed with chronological age in skin, 188 in adipose tissue, and two in LCLs (Benjamini-Hochberg corrected Pvalue < 0.01)(Figures 2 and 3, and Additional files 2 and 3, Tables S2 and S3). Of those genes, 43 were found in both skin and adipose tissue. The direction of the age effect on gene expression was similar in each tissue for most of the genes; 48.6% of the genes had lower levels of expression with age in skin compared to50.8% in adipose tissue. Six genes exhibited age effects in opposite directions in different tissues. Two genes, the chromosome 5 open reading frame 4 (C5orf4) and the spondin 1 (SPON1) showed lower expression with age in skin but higher in adipose tissue. Conversely, four genes showed higher expression levels with age in skin but lower in adipose tissue. Those genes were carbonic anhydrase XII (CA12), solute carrier family 47, member 1 (SLC47A1), Rho GTPase activating protein 33 (ARHGAP33 or SNX26), and B-cell CLL/lymphoma 6 (BCL6).
Table 1.
Significantly age-affected genes (in grey) from brain transcription profiles in brain regions with significant values.
| Affy transcript ID | Affy Probe ID | Illumina Probe | Symbol | CRBL | HIPP | PUTM | TCTX | WHMT | Skin | Adipose |
|---|---|---|---|---|---|---|---|---|---|---|
| t2378077 | NA | ILMN_1811370 | HSD11B1 | 2.89E-08 | 2.80E-01 | 8.90E-01 | 2.07E-01 | 3.58E-01 | 2.33E-09 | 7.40E-01 |
| t2378077 | 205404_at | ILMN_2389501 | HSD11B1 | 2.89E-08 | 2.80E-01 | 8.90E-01 | 2.07E-01 | 3.58E-01 | 3.53E-11 | 4.31E-01 |
| t2378077 | NA | ILMN_2389506 | HSD11B1 | 2.89E-08 | 2.80E-01 | 8.90E-01 | 2.07E-01 | 3.58E-01 | 2.82E-09 | 7.11E-01 |
| t3219885 | 203997_at | ILMN_1717294 | PTPN3 | 1.52E-05 | 6.45E-01 | 8.28E-01 | 2.09E-01 | 8.81E-01 | 5.08E-04 | 1.88E-01 |
| t3883207 | 203650_at | ILMN_1717262 | PROCR | 3.85E-05 | 5.78E-01 | 9.16E-01 | 8.73E-01 | 6.27E-01 | 5.83E-03 | 2.09E-02 |
| t3168066 | 205199_at | ILMN_1725139 | CA9 | 6.79E-03 | 8.11E-01 | 9.75E-01 | 9.70E-01 | 9.92E-01 | 9.75E-03 | 5.86E-01 |
| t3349719 | NA | ILMN_1750496 | ZBTB16 | 3.13E-04 | 3.17E-01 | 8.28E-01 | 9.38E-01 | 5.35E-01 | 1.00E+00 | 7.71E-03 |
| t2753732 | 222738_at | ILMN_2207170 | WWC2 | 3.62E-04 | 9.49E-01 | 9.28E-01 | 7.53E-01 | 7.32E-01 | 1.84E-01 | 1.91E-03 |
| t2316605 | 206080_at | ILMN_2061565 | PLCH2 | 3.05E-01 | 6.94E-01 | 8.85E-01 | 7.00E-03 | 4.43E-01 | 1.27E-05 | 8.98E-01 |
| t3690154 | 218888_s_at | ILMN_1760849 | NETO2 | 7.64E-01 | 1.94E-01 | 8.72E-01 | 9.43E-03 | 5.77E-01 | 3.39E-04 | 9.21E-01 |
| t3374934 | 230550_at | ILMN_1721035 | MS4A6A | 3.05E-01 | 5.89E-05 | 1.38E-04 | 1.39E-01 | 9.35E-01 | 2.56E-05 | 9.33E-01 |
| t3374934 | 219666_at | ILMN_1797731 | MS4A6A | 3.05E-01 | 5.89E-05 | 1.38E-04 | 1.39E-01 | 9.35E-01 | 9.10E-04 | 8.87E-01 |
| t3692999 | NA | ILMN_1715401 | MT1G | 3.49E-01 | 2.10E-01 | 1.83E-03 | 3.09E-01 | 2.16E-01 | 3.57E-04 | 8.42E-01 |
| t3703885 | 201195_s_at | ILMN_1720373 | SLC7A5 | 5.80E-02 | 5.09E-01 | 8.28E-01 | 7.54E-01 | 3.50E-03 | 1.54E-07 | 8.96E-01 |
| t2830465 | 219728_at | ILMN_1656395 | MYOT | 6.65E-01 | 5.50E-02 | 8.28E-01 | 2.77E-01 | 4.04E-03 | 4.08E-03 | 9.30E-01 |
| t2924492 | 219743_at | ILMN_1682034 | HEY2 | 7.26E-01 | 1.56E-01 | 7.31E-01 | 9.63E-02 | 2.31E-03 | 2.99E-04 | 5.77E-01 |
| t2478269 | NA | ILMN_1678403 | TMEM178 | 8.91E-01 | 6.45E-01 | 9.20E-01 | 2.20E-01 | 2.31E-03 | 9.81E-03 | 6.41E-05 |
| t2478269 | 229302_at | ILMN_2104295 | TMEM178 | 8.91E-01 | 6.45E-01 | 9.20E-01 | 2.20E-01 | 2.31E-03 | 2.26E-05 | 7.67E-08 |
Benjamini-Hochberg corrected Pvalues for adipose and skin genes are also shown. Abbreviations:CRBL =Cerebellum, HIPP= Hippocampus, PUMT= Putamen, TCTX= Temporal cortex, WHMT= Intralobular white matter.
Figure 2.
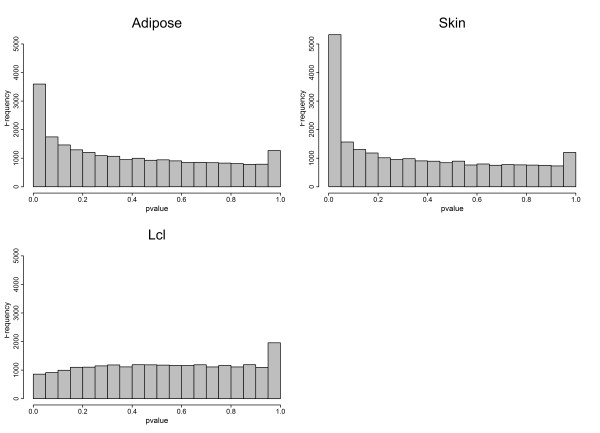
Venn diagram showing the overlap between significant age-affected genes in skin and adipose tissues (Benjamini-Hochberg corrected Pvalue < 0.01).
Figure 3.
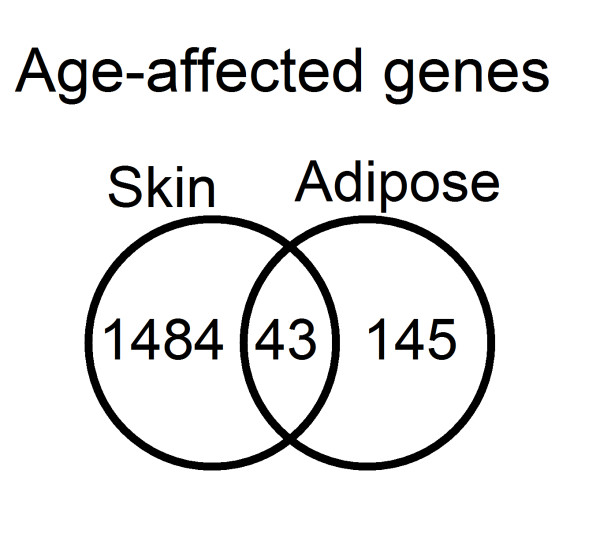
Pvalues distribution of age-affected genes in adipose, skin, and LCL tissues.
GO processes and gene expression enrichment for functional classification were investigated using DAVID [11]. In skin the enrichment included terms related to epidermal development, keratinization, epithelial cell differentiation, extracellular matrix organization, intermediate filament cytoskeleton, collagen fibril organization, and fatty acid metabolic processes(Benjamini-Hochberg corrected Pvalue < 0.05).The enrichment analysis of those genes in skin that significantly increase expression with age identified GO terms relating to alternative splicing and RNA processing as well as cell and organelle structure-related cellular components. Genes that have lower expression with age in skin were enriched for gene ontology terms related to metabolism, biosynthetic processes, and mitochondrial function. In adipose tissue, enrichment analysis only revealed one significant GO term: extracellular structure organization. Separate analysis of genes in fat with either increased or decreased expression did not identify any enriched term. Enrichment analysis in DAVID of the genes in common between tissues (43 genes, Figure 1) did not identify any significant GO terms but were enriched for genes with a tissue-specific expression in the adrenal cortex, as defined by the Genomics Institute of the Novartis Research Foundation [12]. The adrenal cortex forms an active part of the hypothalamic pituitary adrenal (HPA) axis, implicated in the aging process as being the central method by which the body responds to stress, a response which may transcend tissue-specific aging[13].
We also investigated whether known aging-related genes from The Human Ageing Genomic Resources (HAGR)[7,14]were included in the genes found to be differentially expressed in skin and adipose tissues in our dataset. Within HAGR, GenAge is a database of 288 genes potentially associated with human aging, 25 of which were found to be differentially expressed with age in skin and three in adipose tissue in our study(Additional file 4, Table S4).
Many of the age-altered expression genes in skin and adipose tissue belong to cancer-related pathways like p53, Wnt, or Notch, or have been previously implicated in many cancer types. In skin, some of the genes belong to the p53 pathway like p21 (CDKN1A), tripeptidyl peptidase 1 (TPP1), and the tumor protein p53 regulated apoptosis inducing protein 1 (TP53AIP1). Also, differentially expressed genes with age in skin belong to the Wnt and the NOTCH pathways like WNT4, WNT3 or the SMAD family member 3 (SMAD3), the jagged 2 (JAG2) or the nuclear receptor corepressor 2 (NCOR2). In adipose tissue, other genes differentially expressed with age were also related to p53 or cancer and include the vascular endothelial growth factor C (VEGFC), the ret proto-oncogene (RET), the zinc finger and BTB domain containing 16 (ZBTB16) or notch 3 (NOTCH3).
LCLs expression showed little significant age effect
LCLs are derived from lymphoblastoid cell lines infected and immortalized by Epstein-Barr virus (EBV). Analysis of age influence on gene expression in 777 LCLs samples identified only two genes differentially expressed with age (Benjamini-Hochbergcorrected Pvalue < 0.01), a gene coding for aspartoacylase (ASPA) and the OUT domain containing 7A (OTUD7A) (Additional file 5, Table S5). Previous studies analyzing age influence in LCLs expression profiles found also very little influence of chronological age [15]. Although, Joehanes et al. [15] attributed their results to a small sample size, the larger sample size here analyzed added further weight to the hypothesis that there is a lack of detectable chronological age effect on gene expression in transformed lymphocytes. To further investigate the lack of age-related expression differences, expression profiles from 92 fresh lymphocytes obtained from a subset of the individuals were analyzed. No gene showed a significant age-related effect (Benjamini-Hochbergcorrected Pvalue < 0.01)(Additional file 6, Table S6). However, based on the Pvalue distribution and the effect sizes detected in the smaller number of fresh lymphocytes compared to transformed lymphocytes (LCLs), a small age effect of fresh lymphocytes cannot be ruled out (Additional files 7, 8, 9, Figures S1-S3).
Age-affected genes in brain
To explore common expression signatures of aging in other tissues, expression data from the Edinburgh brain bank were interrogated [16]. The brain expression dataset used 932brain samples obtained from 10 different brain regions following sudden death in 100 individuals aged 16 to 83 years. This dataset was examined for transcripts that were differentially expressed with age. Of 1,860 skin or adipose tissue transcripts with a significant age effect described above, 14 brain genes in five of the 10 available brain regions were found to have a significant age-altered expression (Benjamini-Hochberg corrected Pvalue <0.01, Additional file 10, Table S7). Of those 14 genes (Table 1), one was common to all the three tissues (TMRM178);two more genes were in common between adipose and brain only, and 12 more genes between skin and brain. The two genes common between adipose tissue and brain tissue (cerebellum) were a zinc finger transcription factor coding gene (ZBTB16) and the WW and C2 domain containing two genes (WWC2). From the 12genes common to skin and brain, four were differentially expressed with age in cerebellum (PROCR, HSD11B1, PTPN3,and CA9), two in temporal cortex (PLCH2 and NETO2),one in putamen (MT1G),four in intralobular white matter (SLC7A5, MYOT,HEY2 and TMEM178),and one in hippocampus and putamen (MS4A6A).
Many of the age-altered expression genes in brain have been also associated with cancer and other aging-related diseases. ZBTB16 (PLZF) has previously been implicated in acute myeloid leukemia development via protein fusion with the retinoic acid receptor alpha gene product and interaction with p53 [17]. The protein C receptor (PROCR) belongs to the blood coagulation pathway and has been linked both to aging-associated diseases (like cardiovascular diseases) and cancer [18]. The carbonic anhydrase IX (CA9) is a transmembrane enzyme implicated in development that has also been associated with cancer in multiple organs including the brain [19]. Additionally, CA9 mediated changes in SIRT1, a sirtuin involved in cellular differentiation and stress response whose activity reduces p53 mediated apoptosis and FOXO-induced apoptosis [20-22]. SLC7A5is an amino acid transporter light chain, also known as LAT1, that has been established as a biomarker for the development of cancer [23].Another age-related disease gene in brain wasMS4A6, a membrane-spanning four-domains gene which has been associated with Alzheimer's disease [24], a known aging-related disease. Finally TMEM178, the only gene found to be affected by age in skin, adipose tissue, and brain (intralobular white matter) is a transmembrane protein whose function is unknown but has been previously linked to age-related changes in gene expression of murine lung tissue [25].
Genotype-by-age interactions (eQTL analysis)
A common approach to identify factors controlling differential gene expression is to incorporate genomic data to derive a set of expression QTL (eQTL)[26]. Specific gene allele differences may cause variation in gene expression with age and may be detectable by eQTL analysis. Since eQTL identify SNPs that influence the expression of a gene, GxA interactions identify a difference in the SNPs influence on gene expression due to an interaction with age. It suggests that the effect of the SNP on gene expression is not constant throughout the life span of the individual and that age plays a relevant role in the effect that the genetic variant has on gene expression.Therefore, we included an age term in an eQTL analysis to investigate possible genotype-by-age (GxA) interactions that explain differences in gene expression with age among individuals. By determining which eQTL show an age interaction, we have the potential to explain some of the genetic control in age-related gene expression changes and hence differences in aging rates between individuals. We limited our analysis to 3,529 SNPs in adipose tissue and 2,796 SNPs in skin that had been identified as significant eQTL by the MuTHER study[27]. A full linear mixed model with a GxA interaction term was compared with a model without an interaction term and evaluated using the Akaike Information Criterion (AIC). Interaction effects were identified with a better model fit for 610 eQTL in adipose tissue and for 488 eQTL in skin. Of these, 70 eQTL were common to both tissues (Figure 4). These were explored by the UK Brain Expression Consortium dataset but no significant interactions (Pvalue<1e-5) were identified.
Figure 4.
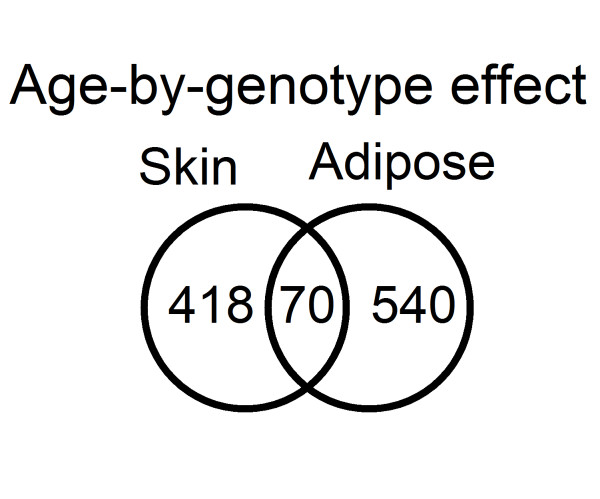
Venn diagrams showing the overlap in skin and adipose tissue for GxA interacting genes.
Genes with an GxA interaction in both skin and adipose tissue included a member of the B-cell lymphoma family (BCL7C)as well as the BRCA1 associated RING domain 1 gene (BARD1) and the YEATS domain containing 4 (YEATS4, also known as GAS41), both of which are p53 related genes previously associated with cancer. Among the genes in skin with a significant GxA interaction we found 31 genes associated with cancer, including the Telomerase reverse transcriptase (TERT), which is also involved in aging via its effect on telomere length [28]. Others include the fibroblast growth factor receptor 4 (FGFR4), the interferon gamma receptor 2 (IFNFR2), and the heat shock 70kDa protein 1A (HSPA1A) (Additional files 11 and 12, Tables S8 and S9). In adipose tissue, we found not only a number of known cancer-related genes, like SOD2 or AMACR, but also diabetes- and obesity-related genes, like MTHFR or ADIPOR2.
Genetic markers of diseases identified by GWAS have been often associated with gene expression changes via eQTL studies. Integration of cis-eQTL data from the MuTHER study and published disease loci has previously identified eQTL associated with diseases [27]. In skin, three SNPs with a GxA interaction were associated with smoking behavior, electrocardiographic traits, and tooth development. Twenty-five SNPs with a GxA interaction identified in adipose tissue (Table 2) were associated with age-related traits and diseases like Parkinson's (rs10516849, rs708726) or cancer (rs402710, rs6715570, rs12912744). Interestingly, one of the disease-associated variants (rs402710) underlying a GxA cis-eQTL in the cleft lip and palate associated transmembrane protein 1 gene (CLPTM1L) has been previously associated to lung cancer susceptibility and is located in a LD region that includes TERT[29].
Table 2.
List of SNPs with an age-by-genotype interaction effect associated to GWAS hits.
| eQTL | Gene | Trait |
|---|---|---|
| Adipose | ||
| rs10516849 | MMRN1 | Parkinson's disease |
| rs10870077 | <NA> | Ulcerative colitis |
| rs12006032 | PRKACG | Chronic kidney disease |
| rs12523750 | MRS2 | Radiation response |
| rs12912744 | SERF2 | Lung cancer |
| rs17253722 | SHRM | Serum magnesium levels |
| rs17253722 | SHRM | Chronic kidney disease;renal function and chronic kidney disease |
| rs17253722 | SHRM | Eosinophilic esophagitis (pediatric) |
| rs17671591 | C5orf37 | LDL cholesterol |
| rs17671591 | C5orf37 | Quantitative traits |
| rs402710 | CLPTM1L | Lung cancer |
| rs402710 | CLPTM1L | Bladder cancer;pancreatic cancer |
| rs531676 | CRTAC1 | Metabolic syndrome |
| rs592229 | AGPAT1 | Menopause (age at onset) |
| rs592229 | AGPAT1 | Height |
| rs6143035 | CABLES2 | Colorectal cancer |
| rs6715570 | BARD1 | Neuroblastoma (high-risk) |
| rs708726 | RAB7L1 | Parkinson's disease |
| rs8049897 | DBNDD1 | Blond vs. brown hair color;freckles;redvs. non-red hair color;skin sensitivity to sun |
| rs8049897 | DBNDD1 | Black vs. red hair color;melanoma |
| rs9263871 | HCG27 | Protein quantitative trait loci |
| rs9263871 | HCG27 | Vitiligo |
| rs9263871 | HCG27 | CD4:CD8 lymphocyte ratio |
| rs9926577 | CNOT1 | QT interval |
| Skin | ||
| rs2305797 | RAB4B | Smoking behavior |
| rs1049337 | CAV1 | Electrocardiographic traits;PR interval |
| rs1042815 | HOXB2 | Primary tooth development (number of teeth) |
Discussion
Aging studies from model organisms such as yeast, worms, and flies have repeatedly shown that changes in the expression of certain genes have an effect upon longevity. Although similar aging processes are likely to operate across multiple species [30], it has been much more difficult to identify longevity candidate genes in human studies [30]. A key question in human aging is to what extent a signature of aging may be detectable across tissues. Until now there has been a lack of large transcriptional profiles from the same human individuals in multiple tissues. The MuTHER study provides insight into the human aging process by interrogating the largest multiple human tissue gene expression resource to identify genes in which expression was affected by chronological age. The analysis of the skin and adipose tissues samples identified several hundred genes responsive to changes in chronological age. However, the 43 shared genes in skin and adipose tissue showed a single common identifiable pathway related to the stress response. From over 1,800 transcripts that have altered expression with age in skin and adipose tissues, 14 also had age-related differential expression in brain. The limited overlap in these two experiments may partly reflect the smaller sample size of the brain expression dataset, the differences in age range between the studies (16 to 83 years for brain samples; 39 to 85 years for MUTHER samples), or the inclusion of males in the brain samples. But it may also imply, as other studies have suggested, that the effects of age on gene transcription are tissue specific[6,31,32]. This hypothesis was supported by the comparison with known related aging genes from the GenAge database, which identified an overlap for a small number of aging-related genes with our data. The GenAge database was the result of a meta-analysis using age-related expression profiles from human brain, kidney, and skeletal muscle, and several expression profiles from mouse and rat; no adipose tissue or skin samples were included (Additional file, Table 1 in [7]). The limited overlap between these datasets supports the idea that molecular signatures of aging reflect predominantly a tissue-specific transcriptional response.
The lack of age-related genes in transformed LCLs, suggest that the transformation to immortalize a cell line may mask or even remove the age-related signatures in gene expression. The transformation of primary B lymphocytes into LCLs requires infection by the Epstein-Barr virus which has the effect of disrupting the p53 signaling pathway in order to induce growth and survival [33]. Joehanes et al.[15] identified only five genes with age-associated expression in LCLs, including p53 itself (TP53). Although the authors attribute the lack of age-affected genes to their small sample size (n=50) and narrow age range, our analysis with a much larger sample size found even fewer age-related changes, suggesting a lack of detectable aging signature in LCLs. The analysis in the subset of fresh lymphocytes suggested an age influence in fresh lymphocytes may potentially be detectable with a larger sample size.
Similarly to age-affected genes, genes with a significant GxA interaction are likely to be relevant for the general aging process. More than 300 GxA interactions have previously been identified in the model organism C. elegans[34,35]and more than 600 have been documented in human blood and kidney [36,37]. By focusing on SNPs with a statistically significant effect on expression (eQTL) from the MuTHER study, we were able to identify more than 1,000 genes for which a GxA interaction better explained the SNP effect in expression in skin and adipose tissue. Fewer than 10% of GxA interactions were observed in both tissues, suggesting that these changes were also tissue-specific. Model organism studies have observed that gene expression can influence the aging rate of an individual but also the aging rate of different tissues[38]. A feedback process may be involved here, where the consequences of the aging process may affect the expression of multiple genes like the expression and regulation of gene expression may affect the aging process. However, it is impossible to determine whether the gene expression changes observed were result or causative of the aging process. The GxA interactions identified the interface between tissue-specific gene expression regulation and tissue-specific aging. But resolving the potential feedback mechanism would require deeper knowledge of the regulatory process of gene expression and its relationship with the aging process. Our results, however, provide a starting point for future work in this direction.
Among the 70 genes associated with GxA common to both tissues, we identified cancer-related genes, such as BARD1 and BCL7C.The BARD1/BRCA1 interaction is disrupted by tumorigenic amino acid substitutions in BRCA1, implying that the formation of a stable complex between these proteins may be an essential aspect of BRCA1 tumor suppression. BARD1 may also be the target of oncogenic mutations in breast or ovarian cancer and is also important for DNA repair. CLPTM1L gene, which has been implicated in susceptibility to lung cancer, had a GxA effect in both skin and fat. A recent study has shown that the TERT-CLPTM1L locus is also associated with melanoma risk [39]. This suggests that the associations of genetic variants to certain diseases, in particular the onset of cancer, are modified by chronological age-related effects. Other studies have found associations between genes expression changes with age and the development of cancer [40,41] and while age remains the strongest risk factor for developing the vast majority of cancers, the actual relationship between the aging process and the development of cancer is complex and far from fully understood[42,43]. We provide a description of genes affected by age in multiple tissues. Using the limited knowledge on gene functionality available on the databases, many of those genes have been associated to cancer, in some way. However, we do not talk in terms of enrichment for 'cancer genes' as we are not able to define them accurately enough to generate a set of genes to test in a statistical analysis. Are oncogenes considered the only cancer genes? What about metastasis and tumour suppressor genes? What about other genes? After all, genes involved in authophagy, cell migration, and metabolism in general are also involved in cancer, with immune-system-related genes also being relevant for this process. The distinction is still unclear making the definition of a list of 'cancer' genes difficult. Since epidemiological studies identify age as a strong and consistent risk factor for cancer, it is well accepted that both processes are related, but the actual mechanism behind is still unknown. Our study supports the idea that gene expression changes with age might be linked to the association between aging-related diseases and senescence or lack of it, as both processes share many genes. Recently this relationship has been exploited when using pro-senescence therapies for cancer treatment [44]. Although secondary effects of these therapies are still under investigation, the positive results obtained in animal models suggest that genes which expression changes with age may be relevant to understand the link between cancer and aging.
Regarding cancer and aging, Serrano and Blasco (2007) suggested that an equilibrium between mechanisms diminishing cellular damage and mechanisms preventing excessive cellular proliferation is required between both processes[43]. The authors argue that the p53 pathway may be seen as an anti-aging mechanism as it is a key defense mechanism against cellular damage protecting from both aging and cancer. One effect of aging at the cellular level is reduced telomerase activity and progressive shorter telomeres in somatic cells [45]. Shortened telomeres are highly recombinogenic, leading to a genome-susceptible cancer development[46,47]. Genomic instability driven by dysfunctional telomeres is also associated with the transition from benign to malignant tumors[48]. Conversely, telomere dysfunction also acts to induce the p53 gene to suppress tumor development by initiating cell-cycle arrest, cellular senescence or, apoptosis. Our analysis has identified several genes involved in the regulation and activity of the p53 pathway as being affected by age. In skin, the telomerase reverse transcriptase (TERT) showed an age-related expression in association with a genetic variant (rs10866530). In addition p21, a gene directly regulated by p53 and also involved in telomere-driven aging, was shown to be differentially expressed with age [49]. In brain, theZBTB16, CA9,and HEY2, genes associated to the p53 pathway directly or via SIRT1, all showed age-related expression. The activity of p53 has been shown to enhance the transcription of inhibitors of the insulin receptor pathway, preventing cell growth and division after stress signaling[50,51] and many genes from the insulin signaling pathway have been extensively associated with longevity in multiple studies and organisms. Our results suggest that the link between aging and cancer is evident in multiple tissues through differential expression of genes with age.
Conclusion
This study examines for the first time changes in gene expression with chronological age in multiple normal human tissues from the same individuals. While a significant proportion of age-related changes in gene expression appear to be tissue-specific, a few common genes were affected by age in a diverse range of tissues. Many of these genes are important in cell division regulation, senescence and apoptosis, processes prone to dysregulation, and potential oncogenesis with advancing age. Tissues comparison suggested that a significant proportion of age-related changes in gene expression are tissue-specific. This raises the question of to what extent age-related changes in gene expression are due to tissue aging rate differences or to tissue-specific gene expression regulation. Further interrogation of this aging-related expression and eQTL resource has the potential to unravel the complex interactions betwe enaging and gene expression regulation, as well as the link with age-related diseases. However, this may reveal that the two processes are so inextricably linked that it is difficult to consider them independently.
Materials and methods
Sample collection
The study included 856 Caucasian female individuals (336 MZ and 520 DZ twins, respectively) recruited from the TwinsUK Adult twin registry [39]. The age at inclusion ranged from 39 to 85 years with a mean age of 59 years. Punch biopsies (8mm) were taken from relatively photo-protected infra-umbilical skin. Subcutaneous adipose tissue was carefully dissected from the biopsy site using forceps and scalpel, weighed, and immediately stored in liquid nitrogen. Similarly, the remaining skin tissue was weighed and stored in liquid nitrogen. Peripheral blood samples were collected, and LCLs were generated through EBV-mediated transformation of the B-lymphocyte component by the European Collection of Cell Cultures agency. The data presented here are part of the MuTHER project (Multiple Tissue Human Expression Resource)[52], Nica et al.[9],and Grundberget al. [8].The project was approved by the local ethics committee of all institutions involved. All the samples were collected after obtaining written and signed informed consent, in accordance with the Helsinki Declaration.
RNA extraction
RNA was extracted from homogenized tissue samples (adipose and skin) and lysed cells (LCL) using TRIzol Reagent (Invitrogen) according to protocol provided by the manufacturer. RNA quality was assessed with the Agilent 2100 BioAnalyzer (Agilent technologies) and the concentrations were determined using NanoDrop ND-1000 (NanoDrop Technologies) and samples were stored in -80°C until ready to use. cDNA derived from the RNA sample was hybridized with the Illumina Human Sentrix 12 chip.
Expression profiling
Expression profiling of skin, adipose tissue, LCLs, and fresh lymphocites, each with either two or three technical replicates, were performed using the Illumina Human HT-12 V3 BeadChips (IlluminaInc) including 48,804 probes where 200ng of total RNA was processed according to the protocol supplied by Illumina. All samples were randomized prior to array hybridization and the technical replicates were always hybridized on different beadchips. Raw data were imported to the IlluminaBeadstudio software and probes with fewer than three beads present were excluded. Log2-transformed expression signals were then normalized separately per tissue with quantile normalization of the replicates of each individual followed by quantile normalization across all individuals as previously described [9]. Post-QC expression profiles were subsequently obtained for 825 (adipose tissue and LCL), 705 (skin), and 92 (fresh lympholytes) individuals, respectively. The Illumina probe annotations were cross-checked by mapping the probe sequence to the NCBI Build 36 genome with MAQ. Only uniquely mapping probes with no mismatches and either an Ensembl or RefSeq ID were kept for analysis. Probes mapping to genes of uncertain function (LOC symbols) and those encompassing a common SNP (1000G release June 2010) were further excluded leaving 23,596 probes used in the analysis. Microarray data can be download from the ArrayExpress archive, accession no. E-TABM-1140.
Analysis of gene expression with age
A linear mixed model was used to examine gene expression variability by age and confounding factors including as fixed effect batch and RNA concentration (only in skin samples), and as random effects family relationship and zygosity. We fitted the mixed-effects model in R[53] with the lmer function in the lme4 package [54]. The Pvalues to assess significance for age effect were calculated from the Chi-square distribution with 1 degree of freedom using likelihood ratio as the test statistic. We computed Pvalues adjusted for multiple testing by controlling the false discovery rate (FDR) with the Benjamini-Hochberg procedure [55]in R and using a threshold of 0.01. Enrichment analysis was carried out using the DAVID Bioinformatics Resource server with a significant level threshold of 0.05 in Benjamini-Hochberg corrected Pvalues [11].
Genotyping and imputation
Genotyping of the TwinsUK dataset (n= approximately 6,000) was done with a combination of Illumina arrays (HumanHap300, HumanHap610Q, 1M-Duo, and 1.2MDuo 1M).Intensity data for each of the three arrays were pooled separately (with 1M-Duo and 1.2MDuo 1M pooled together) and genotypes called with the Illuminus calling algorithm, thresholding on a maximum posterior probability of 0.95.Similar exclusion criteria were applied to each of the three datasets separately. Exclusion criteria for samples were: (i) sample call rate < 98%, (ii) heterozygosity across all SNPs ≥2 s.d. from the sample mean; (iii) evidence of non-European ancestry as assessed by PCA comparison with HapMap3 populations; and (iv) observed pairwise IBD probabilities suggestive of sample identity errors. Exclusion criteria for SNPs were: (i) Hardy-Weinberg Pvalue<10-6, assessed in a set of unrelated samples; (ii) MAF<1%, assessed in a set of unrelated samples; and (iii) SNP call rate <97% (SNPs with MAF≥5%) or <99% (for 1% ≤MAF <5%).
Prior to merging the three datasets, we performed pairwise comparison among the three datasets and further excluded SNPs and samples as follows: (i) concordance at duplicate samples <1%; (ii) concordance at duplicate SNPs <1%; (iii) visual inspection of QQ plots for logistic regression applied to all pairwise dataset comparisons; (iv) Hardy-Weinberg Pvalue <10-6, assessed in a set of unrelated samples; and (v) observed pairwise IBD probabilities suggestive of sample identity errors.
Imputation was performed using the IMPUTE software package (v2)26 using two reference panels, P0 (HapMap2, rel 22, combined CEU+YRI+ASN panels) and P1 (610k+, including the combined TwinsUK HumanHap610k and 1M array).Post-imputation, SNPs were filtered at a MAF >5% and IMPUTE info value of >0.8.
The UK Brain Expression Consortium dataset
The samples from the UK Human Brain Expression Consortium [16] used in this study were provided by the MRC Sudden Death Brain and Tissue Bank in Edinburgh [56] and originated from 100 individuals (78 men and 22 women) of European descent. For each individual, up to 10 anatomical brain regions were sampled: cerebellar cortex (CRBL), frontal cortex (FCTX), hippocampus (HIPP), medulla (more specifically the inferior olivary nucleus, MEDU), occipital cortex (OCTX), putamen (PUTM), substantianigra (SNIG), temporal cortex (TCTX), thalamus (THAL), and intralobular white matter (WHMT). A detailed description of the samples used in the study, tissue processing, and dissection is provided in Trabzuniet al. (2011). All samples had fully informed consent for retrieval and were authorized for ethically approved scientific investigation (Research Ethics Committee number 10/H0716/3).
The tissues were profiled using the Affymetrix Human Exon 1.0 ST array (n=932 arrays) and subsequently preprocessed using RMA using a high confidence list of probesets (unique hybridization, gene annotation, and at least three valid probes after removal of probes containing SNPs). Exon-level expression data was corrected for sex and batch effects and summarized into transcript-level expression values using 10% trimmed mean.
Each individual was genotyped on two chips: the IlluminaInfinium Omni1-Quad BeadChip and the ImmunoChip, a custom genotyping array designed for the fine-mapping of auto-immune disorders [57,58]. Individuals suspected of being of non-European ancestry were identified using principal components projection and excluded from analysis. After standard quality controls, both genotype datasets were combined and imputed using MaCH[59,60]and minimac [61]using the 1000Genomes (March 2012). We used the resulting approximately 5.8 million SNPs with good post-imputation quality (r2 >0.50) and minor allele frequency of at least 5% in subsequent analyses.
The Illumina IDs were converted to Affymetrix transcript ID using available annotation files from the two company websites and matched to the best sequence similarity. This resulted in a match for 63.6% of the genes of interest. For the remaining genes, we matched by gene symbol. Overall, approximately 98% of the replication list was matched in the UK Brain Expression Consortium. The log-likelihood comparing a model that regressed gene expression against age with a model with the intercept only was calculated and tested under an F-distribution with 1 degree of freedom and the corresponding Pvalues reported here and corrected with the Benjamini-Hochberg procedure for FDR correction.
Genotype-by-age (GxA) interaction effect on gene expression
The genetic effect of transcripts to imputed genotypes by genome wide cis-eQTL mapping on more than 23,500 expression traits was previously mapped in three tissues [7]. In total, 776 adipose, 777 LCL, and 667 skin samples had expression profiles and imputed genotypes and were included in the analysis. Across all transcripts significant eQTL were 3,529 in adipose, 4,625 in LCL, and 2,796 in skin. Here we investigate the genotype-by-age (GxA) interaction contribution to gene expression variance observed in those genes. For that we question each gene and the most significant SNP (pick of the eQTL) association with a linear mixed model that included GxA interaction as contributing factor to the gene expression variance. The linear mixed model test was done using the lmer() function in the lme4 package [45] and adjusted for age, experimental batch effect, and sample processing effect in skin (fixed effects) and for family relationship and zygosity (random effects). The Akaike Information Criterion (AIC) was used as a selection method to identify the best fit explaining the origin of the variation per gene expression. Genes for which the model with a genotype-by-age interaction factor fit better the data were used in the functional analysis.
Accession codes
Microarray data of skin, adipose tissue, LCLs can be download from the ArrayExpress archive, accession no. E-TABM-1140. Details about the MuTHER Resource can be found in the bibliography [8,52].
Microarray data of brain can be downloaded from GEO, accession no. GSE46706. Details about the UK Brain Expression Consortium and expression resource can be found in the bibliography[2,62]
Ethics Statement
This project was approved by the ethics committee at St Thomas' Hospital London, where all the biopsies were carried out. Volunteers gave informed consent and signed an approved consent form prior to the biopsy procedure. Volunteers were supplied with an appropriate detailed information sheet regarding the research project and biopsy procedure by post prior to attending for the biopsy.
Authors' contributions
DG, AV, MIM, PD, ETD, MW, VB, and TDS conceived and designed the experiments. AV, MND, AR, LP, DK, and AAB analyzed the data. DG, AKH, KSS, AB, EG, ACN, RD, PD, and FN contributed reagents, materials and analysis tools. DG, AV, MND, AR, MW, VB, and TD wrote the manuscript.
Supplementary Material
Table S1:Age for individuals with microarray expression in skin, adipose tissue, and LCLs.
Table S2: Pvalues for ageeffect in skin tissue.
Table S3: Pvalues for ageeffect in adipose tissue.
Table S4:List of genes with age effect in skin and adipose tissue included in the GenAge database [6].
Table S5: Pvalues for ageeffect in LCLs.
Table S6: Pvalues for ageeffect in fresh lymphocytes.
Figure S1:Pvalues distribution for age effect analysis in 777 individuals with lymphocytes cell lines (LCLs) and 92 individuals with fresh lymphocytes expression arrays.
Figure S2: Pvalues distribution for age effect analysis in 40 individuals with both lymphocytes cell lines (LCLs) and fresh lymphocytes expression profiles.
Figure S3: Beta values from lymphocytes cell lines (LCLs) and fresh lymphocytes expression association with age in 40 common samples (left) and in whole dataset (777 LCL and 92 fresh lymphocytes) (right).
Table S7: Pvalues for ageeffect in brain.
Table S8: Pvalues for genotype-by-age interactions in skin tissue.
Table S9: Pvalues for genotype-by-age interactions in adipose tissue.
TableS10: List of contributors to the MuTHER and the UK Brain Expression Consortiums.
Contributor Information
Daniel Glass, Email: dan.glass@kcl.ac.uk.
Ana Viñuela, Email: ana.vinuela@kcl.ac.uk.
Matthew N Davies, Email: matthew.n.davies@kcl.ac.uk.
Adaikalavan Ramasamy, Email: adaikalavan.ramasamy@kcl.ac.uk.
Leopold Parts, Email: leopold.parts@gmail.com.
David Knowles, Email: knowles84@gmail.com.
Andrew A Brown, Email: ab25@sanger.ac.uk.
Åsa K Hedman, Email: asa.hedman@well.ox.ac.uk.
Kerrin S Small, Email: kerrin.small@kcl.ac.uk.
Alfonso Buil, Email: alfonso.buil@unige.ch.
Elin Grundberg, Email: elin.grundberg@mcgill.ca.
Alexandra C Nica, Email: Alexandra.Nica@unige.ch.
Paola Di Meglio, Email: pdimegl@nimr.mrc.ac.uk.
Frank O Nestle, Email: frank.nestle@kcl.ac.uk.
Mina Ryten, Email: mina.ryten@ucl.ac.uk.
the UK Brain Expression consortium, Email: tim.spector@kcl.ac.uk.
the MuTHER consortium, Email: muther@kcl.ac.uk.
Richard Durbin, Email: rd@sanger.ac.uk.
Mark I McCarthy, Email: mark.mccarthy@drl.ox.ac.uk.
Panagiotis Deloukas, Email: panos@sanger.ac.uk.
Emmanouil T Dermitzakis, Email: emmanouil.dermitzakis@unige.ch.
Michael E Weale, Email: michael.weale@kcl.ac.uk.
Veronique Bataille, Email: bataille@doctors.org.uk.
Tim D Spector, Email: tim.spector@kcl.ac.uk.
Acknowledgments
A complete list of the members of the UK Human Brain Expression and MuTHER (Multiple Tissue Human Expression Resource) Consortiums is available in Additional file 13, TableS10.
The MuTHER study was funded by the Wellcome Trust Program grant # 081917.Genotyping of TwinsUK samples was provided by the Wellcome Trust Sanger Institute and the National Eye Institute via a US National Institutes of Health (NIH)/Center for Inherited Disease Research (CIDR) genotyping project. This research was supported in part by Wellcome Trust grant WT098051 to the Wellcome Trust Sanger Institute.
TwinsUK also receives support from the ENGAGE project grant agreement HEALTH-F4-2007-201413 and from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's ƒ St. Thomas' National Health Service Foundation Trust in partnership with King's College London. TDS is an NIHR senior investigator and European Research Council (ERC) senior investigator. MIM is supported by the Oxford NIHR Biomedical Research Centre. Additional support was provided by the Louis-Jeantet Foundation to ETD and ACN and via NIH-NIMH grant R01 MH090941 to ETD and MIM. DG was supported by an MRC Research Training Fellowship. DG, AV, MND, AAB, and AB are supported by the EU FP7 grant EuroBATS (No. 259749).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Reznick DN. The genetic basis of aging: an evolutionary biologist's perspective. Sci Aging Knowl Environ. 2005;14:pe7. doi: 10.1126/sageke.2005.11.pe7. [DOI] [PubMed] [Google Scholar]
- Trabzuni D, Wray S, Vandrovcova J, Ramasamy A, Walker R, Smith C, Luk C, Gibbs JR, Dillman A, Hernandez DG, Arepalli S, Singleton AB, Cookson MR, Pittman AM, de Silva R, Weale ME, Hardy J, Ryten M. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012;14:4094–4103. doi: 10.1093/hmg/dds238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Kayo T, Lee C-K, Prolla TA. Gene expression profiling of aging using DNA microarrays. Mech Ageing Dev. 2002;14:177–193. doi: 10.1016/S0047-6374(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;14:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao S-Y, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;14:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Rodwell GEJ, Sonu R, Zahn JM, Lund J, Wilhelmy J, Wang L, Xiao W, Mindrinos M, Crane E, Segal E, Myers BD, Brooks JD, Davis RW, Higgins J, Owen AB, Kim SK. A transcriptional profile of aging in the human kidney. PLoS Biol. 2004;14:e427. doi: 10.1371/journal.pbio.0020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;14:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, Bell JT, Yang TP, Meduri E, Barrett A, Nisbett J, Sekowska M, Wilk A, Shin SY, Glass D, Travers M, Min JL, Ring S, Ho K, Thorleifsson G, Kong A, Thorsteindottir U, Ainali C, Dimas AS, Hassanali N, Ingle C, Knowles D, Krestyaninova M, Lowe CE, Di Meglio P. et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet. 2012;14:1084–1089. doi: 10.1038/ng.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nica AC, Parts L, Glass D, Nisbet J, Barrett A, Sekowska M, Travers M, Potter S, Grundberg E, Small K, Hedman ÅK, Bataille V, Tzenova Bell J, Surdulescu G, Dimas AS, Ingle C, Nestle FO, di Meglio P, Min JL, Wilk A, Hammond CJ, Hassanali N, Yang T-P, Montgomery SB, O'Rahilly S, Lindgren CM, Zondervan KT, Soranzo N, Barroso I, Durbin R. et al. The architecture of gene regulatory variation across multiple human tissues: The MuTHER Study. PLoS Genet. 2011;14:e1002003. doi: 10.1371/journal.pgen.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort Profile: TwinsUK and healthy ageing twin study. Int J Epidemiol. 2013;14:76–85. doi: 10.1093/ije/dyr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols. 2008;14:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med GenetB Neuropsychiatr Genet. 2006;14:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- Aguilera G. HPA axis responsiveness to stress: Implications for healthy aging. Exp Gerontol. 2011;14:90–95. doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhães JP. Human Ageing Genomic Resources: Integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2013;14:D1027–D1033. doi: 10.1093/nar/gks1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R, Johnson AD, Barb JJ, Raghavachari N, Liu P, Woodhouse KA, O'Donnell CJ, Munson PJ, Levy D. Gene expression analysis of whole blood, peripheral blood mononuclear cells, and lymphoblastoid cell lines from the Framingham Heart Study. Physiol Genomics. 2012;14:59–75. doi: 10.1152/physiolgenomics.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabzuni D, Ryten M, Walker R, Smith C, Imran S, Ramasamy A, Weale ME, Hardy J. Quality control parameters on a large dataset of regionally dissected human control brains for whole genome expression studies. J Neurochem. 2011;14:275–282. doi: 10.1111/j.1471-4159.2011.07432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinga A, Monestiroli S, Ronzoni S, Carbone R, Pearson M, Pruneri G, Viale G, Appella E, Pelicci P, Minucci S. Impairment of p53 acetylation, stability and function by an oncogenic transcription factor. Embo J. 2004;14:1144–1154. doi: 10.1038/sj.emboj.7600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menschikowski M, Hagelgans A, Tiebel O, Klinsmann L, Eisenhofer G, Siegert G. Expression and shedding of endothelial protein C receptor in prostate cancer cells. Cancer Cell Int. 2011;14:4. doi: 10.1186/1475-2867-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proescholdt MA, Mayer C, Kubitza M, Schubert T, Liao S-Y, Stanbridge EJ, Ivanov S, Oldfield EH, Brawanski A, Merrill MJ. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. NeuroOncol. 2005;14:465–475. doi: 10.1215/S1152851705000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Miura M. Caspase-mediated changes in Sir2α during apoptosis. FEBS Letters. 2006;14:5875–5879. doi: 10.1016/j.febslet.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S-i, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 2001;14:137–148. doi: 10.1016/S0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng H-L, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;14:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;14:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;14:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra V, Lee H, Singh A, Huang K, Thimmulappa RK, Mitzner W, Biswal S, Tankersley CG. Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol Genomics. 2007;14:429–440. doi: 10.1152/physiolgenomics.00060.2007. [DOI] [PubMed] [Google Scholar]
- Grundberg E, Kwan T, Ge B, Lam KCL, Koka V, Kindmark A, Mallmin H, Dias J, Verlaan DJ, Ouimet M, Sinnett D, Rivadeneira F, Estrada K, Hofman A, van Meurs JM, Uitterlinden A, Beaulieu P, Graziani A, Harmsen E, Ljunggren Ö, Ohlsson C, Mellström D, Karlsson MK, Nilsson O, Pastinen T. Population genomics in a disease targeted primary cell model. Genome Res. 2009;14:1942–1952. doi: 10.1101/gr.095224.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, Bell JT, Yang T-P, Meduri E, Barrett A, Nisbett J, Sekowska M, Wilk A, Shin S-Y, Glass D, Travers M, Min JL, Ring S, Ho K, Thorleifsson G, Kong A, Thorsteindottir U, Ainali C, Dimas AS, Hassanali N, Ingle C, Knowles D, Krestyaninova M, Lowe CE, Di Meglio P. et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet. 2012;14:1084–1089. doi: 10.1038/ng.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njajou OT, Blackburn EH, Pawlikowska L, Mangino M, Damcott CM, Kwok P-Y, Spector TD, Newman AB, Harris TB, Cummings SR, Cawthon RM, Shuldiner AR, Valdes AM, Hsueh W-C. A common variant in the telomerase RNA component is associated with short telomere length. PLoS ONE. 2010;14:e13048. doi: 10.1371/journal.pone.0013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, McLaughlin J, Shepherd F, Montpetit A, Narod S, Krokan HE, Skorpen F, Elvestad MB, Vatten L, Njolstad I, Axelsson T, Chen C, Goodman G, Barnett M, Loomis MM. et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;14:1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;14:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Wheeler HE, Kim SK. Genetics and genomics of human ageing. Philos Trans R SocLond B Biol Sci. 2011;14:43–50. doi: 10.1098/rstb.2010.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006;14:e115. doi: 10.1371/journal.pgen.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E, Luftig MA. MDM2-dependent inhibition of p53 is required for Epstein-Barr virus B-cell growth transformation and infected-cell survival. J Virol. 2009;14:2491–2499. doi: 10.1128/JVI.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela A, Snoek LB, Riksen JAG, Kammenga JE. Genome-wide gene expression regulation as a function of genotype and age in C. elegans. Genome Res. 2010;14:929–937. doi: 10.1101/gr.102160.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela A, Snoek LB, Riksen JAG, Kammenga JE. Aging uncouples heritability and expression-QTL in Caenorhabditis elegans. G3 (Bethesda) 2012;14:597–605. doi: 10.1534/g3.112.002212. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler HE, Metter EJ, Tanaka T, Absher D, Higgins J, Zahn JM, Wilhelmy J, Davis RW, Singleton A, Myers RM, Ferrucci L, Kim SK. Sequential use of transcriptional profiling, expression quantitative trait mapping, and gene association implicates MMP20 in human kidney aging. PLoS Genet. 2009;14:e1000685. doi: 10.1371/journal.pgen.1000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JW Jr, Göring HHH, Charlesworth JC, Drigalenko E, Diego VP, Curran JE, Johnson MP, Dyer TD, Cole SA, Jowett JBM, Mahaney MC, Comuzzie AG, Almasy L, Moses EK, Blangero J, Williams-Blangero S. Genotype × age interaction in human transcriptional ageing. Mech Ageing Dev. 2012;14:581–590. doi: 10.1016/j.mad.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;14:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Law MH, Montgomery GW, Brown KM, Martin NG, Mann GJ, Hayward NK, MacGregor S. Meta-analysis combining new and existing data sets confirms that the TERT-CLPTM1L locus influences melanoma risk. J Invest Dermatol. 2012;14:485–487. doi: 10.1038/jid.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;14:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;14:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LCW, Mooi WJ, Peeper DS. BRAFE600 in benign and malignant human tumours. Oncogene. 2007;14:877–895. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;14:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. Trends Cell Biol. 2012;14:211–219. doi: 10.1016/j.tcb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;14:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Keeping telomerase in its place. Nat Med. 2002;14:934–936. doi: 10.1038/nm0902-934. [DOI] [PubMed] [Google Scholar]
- O'Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, DePinho RA. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell. 2002;14:149–155. doi: 10.1016/S1535-6108(02)00094-6. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Gabrielson E, Strauss WM, De Marzo AM, Argani P. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. AmJPathol. 2004;14:925–935. doi: 10.1016/S0002-9440(10)63180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, Lee H-W, von Zglinicki T, Ganser A, Schirmacher P, Nakauchi H, Rudolph KL. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;14:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- Feng Z, Levine AJ. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;14:427–434. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK β1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;14:3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- The MuTHER project (Multiple Tissue Human Expression Resource). http://www.muther.ac.uk
- The R Project for Statistical Computing. http://www.r-project.org/
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. Rpackage version 0.999375-41. 2011.
- Dobbin K, Shih JH, Simon R. Statistical design of reverse dye microarrays. Bioinformatics. 2003;14:803–810. doi: 10.1093/bioinformatics/btg076. [DOI] [PubMed] [Google Scholar]
- Millar T, Walker R, Arango JC, Ironside JW, Harrison DJ, MacIntyre DJ, Blackwood D, Smith C, Bell JE. Tissue and organ donation for research in forensic pathology: the MRC Sudden Death Brain and Tissue Bank. J Pathol. 2007;14:369–375. doi: 10.1002/path.2247. [DOI] [PubMed] [Google Scholar]
- International Parkinson's Disease Genomics C; Wellcome Trust Case Control C. A two-stage meta-analysis identifies several new loci for Parkinson's disease. PLoS Genet. 2011;14:e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics Consortium; Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;14:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;14:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;14:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minimac. http://genome.sph.umich.edu/wiki/Minimac
- The UK Brain Expression Consortium and expression resource.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1:Age for individuals with microarray expression in skin, adipose tissue, and LCLs.
Table S2: Pvalues for ageeffect in skin tissue.
Table S3: Pvalues for ageeffect in adipose tissue.
Table S4:List of genes with age effect in skin and adipose tissue included in the GenAge database [6].
Table S5: Pvalues for ageeffect in LCLs.
Table S6: Pvalues for ageeffect in fresh lymphocytes.
Figure S1:Pvalues distribution for age effect analysis in 777 individuals with lymphocytes cell lines (LCLs) and 92 individuals with fresh lymphocytes expression arrays.
Figure S2: Pvalues distribution for age effect analysis in 40 individuals with both lymphocytes cell lines (LCLs) and fresh lymphocytes expression profiles.
Figure S3: Beta values from lymphocytes cell lines (LCLs) and fresh lymphocytes expression association with age in 40 common samples (left) and in whole dataset (777 LCL and 92 fresh lymphocytes) (right).
Table S7: Pvalues for ageeffect in brain.
Table S8: Pvalues for genotype-by-age interactions in skin tissue.
Table S9: Pvalues for genotype-by-age interactions in adipose tissue.
TableS10: List of contributors to the MuTHER and the UK Brain Expression Consortiums.


