Abstract
Aim:
The aim of this study was to evaluate the surface/mineral changes on enamel before and after the application of acidulated phosphate fluoride (APF) gel, fluoride enhanced hydroxyapatite gel and propolis in conjunction with carbon-dioxide (CO2) laser.
Materials and Methods:
Crowns of 40 human maxillary central incisors were collected and were divided into four groups of 10 each: Topical fluoride application only, topical fluoride application followed by CO2 laser irradiation, CO2 laser irradiation followed by topical fluoride application and CO2 laser irradiation before and after topical fluoride application. The 10 crowns in each group was again sectioned into four equal parts of mesio-incisal, disto-incisal, mesio-cervical and disto-cervical sections rendering 40 samples in each group. Each group was again subdivided into four subgroups: Subgroup C - untreated enamel surface (control). Subgroup A - APF gel application, subgroup R - fluoride enhanced hydroxyapatite gel application and subgroup P - propolis application. The surface morphology of the test samples were analyzed by scanning electron microscopy and mineral changes by energy dispersion X-ray spectrophotometer.
Results:
Total mineral content is maximum in Group 4A (CO2 laser irradiation before and after APF gel application) and calcium/phosphate ratio is highest in Group 4R (CO2 laser irradiation before and after Remin-Pro application). Group 2A (APF gel application followed by CO2 laser irradiation) has the maximum fluoride retention.
Conclusion:
Laser irradiation of enamel through a topically applied APF gel is effective in the prophylaxis and management of dental caries.
Keywords: Acidulated phosphate fluoride gel, carbon-dioxide laser, fluoridated hydroxyapatite gel, propolis, total mineral content
INTRODUCTION
The important strategy in the prevention of dental caries is by increasing the acid resistance capacity of enamel. Fluoride application and carbon-dioxide (CO2) laser have shown a reasonable success to enhance enamel's resistance to acid attack in the prevention of dental caries. The anti-cariogenic effect of professional fluoride application depends on reaction products formed on enamel during the clinical treatment and their retention over time after the application. Professional acidulated phosphate fluoride (APF) application is a well-known method used for dental caries prevention and its efficacy is clearly recognized on an evidence-based perspective.[1] Different types of lasers, such as ruby, CO2, neodymium: Yttrium-aluminum-garnet (YAG) and argon with different operational modes and energy outputs have been used to investigate the possibility of dental caries prevention.[2,3,4,5,6,7,8,9,10,11,12,13] Esteves-Oliveira et al. has shown that CO2 laser was able to decrease the enamel caries progression by causing surface and subsurface thermal changes.[14] Pulsed CO2 laser irradiation of enamel caused marked surface fusion and inhibited the progress of subsurface caries-like lesions by as much as 50%.[5]
Propolis is a resinous wax-like material that is used by the bees as a glue-like matrix in their hives. It has been reported to bear antibacterial activity that may be of benefit in combating dental caries. Studies on propolis applications have increased because of its therapeutic and biological properties.[15] A comparative evaluation of these topical agents in conjunction with CO2 laser was not reported. This study aimed to evaluate the surface and mineral changes on enamel before and after the application of APF gel, fluoride enhanced hydroxyapatite gel and propolis in conjunction with CO2 laser using high resolution-scanning electron microscopy (HR-SEM) and energy dispersion X-ray spectrometry.
MATERIALS AND METHODS
Materials used
APF gel (Pascal company, 2929 N.E. Northrup Way, Bellevue, WA 98004, U.S.A.) used in this study contains 1.23% F (as sodium fluoride and hydrogen fluoride (HF), phosphoric acid and carboxymethyl cellulose as the gelling base. Fluoride enhanced hydroxyapatite gel (Remin-Pro, VOCO GmbH Cuxhaven, Germany) used in this study contains hydroxyapatite, fluoride (1.450 ppm sodium fluoride) and xylitol.
Propolis (Nature's Answer, Hauppauge, NY 11788-3943), which was an alcohol free extract from the herb Apis mellifera was used in this study. The ingredients were propolis-2000 mg, vegetable glycerine and propylene glycol.
Extracted intact 40 human maxillary central incisors were collected and stored in saline. Roots were resected and the crowns were washed in distilled water and stored in saline at the room temperature. The palatal surface of the crowns was flattened using an acrylic trimmer. The 40 crowns were divided into four groups of 10 teeth each.
Group 1 (n = 10): Topical fluoride application only.
Group 2 (n = 10): Topical fluoride application followed by CO2 laser irradiation. For CO2 laser irradiation, a device fabricated with orthodontic wire was fixed to the laser tip such that a distance of 4 mm from the tip of the hand piece to the specimen was maintained during the irradiation. The specimens were exposed for approximately 15 s by moving the laser tip manually. Necessary precautionary measures were taken by the operator during the laser irradiation procedure. Laser irradiation was carried out by a pulsed CO2 laser (sunny surgical laser, model number-PC015C; Shanghai, China) at 10.6 μm wavelength with the following parameters: 0.5 W, 50 μs pulse duration, 1 Hz repetition rate and a 0.8 mm beam diameter. The CO2 laser, with an emission wavelength of 10.6 μm, which is very close to the phosphate and carbonate absorption bands of dental enamel apatite, is absorbed more efficiently by dental enamel. Furthermore, CO2 laser at 4 W continuous wave for 15 s caused a pulpal temperature raise of 3.5-4.1°C. At this temperature no irreversible thermal damage to the pulp will occur.[16] In this study, only 0.5 W with 50 μs pulse duration was used, which further reduces the observed pulpal temperature rise.
Group 3 (n = 10): CO2 laser irradiation followed by topical fluoride application.
Group 4 (n = 10): CO2 laser irradiation before and after topical fluoride application.
The 10 crowns in each group was again sectioned into four equal parts using a diamond disc, such that it had mesio-incisal, disto-incisal, mesial-cervical and distocervical sections with dimensions of approximately 3 mm × 3 mm × 4 mm, rendering 40 samples in each group. Nail varnish was applied such that only the labial surface was exposed. Each group was again subdivided into four subgroups.
Subgroup C ([n = 10] disto-cervical half)]: Control group (untreated enamel surface).
Subgroup A ([n = 10] mesio-cervical half)]: A single application of APF gel was made on the labial surface of the specimen with a microbrush for 1 min.
Subgroup R ([n = 10] mesio-incisal half)]: A single application with fluoride-enhanced hydroxyapatite gel (Remin-Pro) was done on the labial surface of the specimen with a microbrush for 1 min.
Subgroup P ([n = 10] disto-incisal half)]: Treatment with propolis was done. The application was carried out in the same method as that of fluoride application. All the specimens were then immersed in artificial saliva for 21 h. The surface morphology of the test samples was analyzed by SEM analysis (SEM, JEOL model JSE 5610-LV) and mineral changes by energy dispersion X-ray spectrophotometer (Quanta series ESEM, Quanta 200 Netherland, FEI Company, Philips) where the following parameters were analyzed; total mineral content (TMC), calcium-phosphate ratio and the mean fluoride retention. Two samples from each group were selected randomly for surface evaluation using SEM energy dispersive X-ray analysis was used to determine calcium, phosphate and fluoride content in weight %. The principle of Energy Dispersive X-ray (EDAX) Analysis is based on the energy emitted in the form of X-ray photons where the electrons from external sources hit the atoms in a material, thus generating characteristic X-rays of the element. When the sample is bombarded by the electron beam of the SEM, electrons are ejected from the atoms on the specimen's surface (secondary electrons). A resulting electron vacancy is filled by an electron from higher shell and an X-ray is emitted (characteristic X-rays) to balance the energy difference between the two electrons. The EDAX X-ray detector measures the number of emitted X-rays their energy. The energy of the X-ray is characteristic of the element from, which the X-ray was emitted. A spectrum of the energy versus relative counts of the detected X-rays obtained and evaluated for qualitative and quantitative determinations of the elements present in the specimen using a computer based program. The results were tabulated and statistically analyzed using the statistical package for social sciences (SPSS software version 10.5 Chicago, USA). One-way ANOVA was used to calculate the P value. The mean difference is significant at 0.05 levels. Post hoc multiple comparison Least Significant Difference (LSD) lattice was used to identify the significant groups.
RESULTS
TMC was seen maximum in 4A (127.74 ± 2.76) where laser irradiation was performed followed by the application of APF gel, which was again irradiated, when compared with all the other groups.
Fluoride retention was seen maximum in Group 2A where the application of APF gel was followed by laser irradiation when compared with all other groups.
DISCUSSION
The current study investigated the beneficial effects when enamel surfaces were wetted with a topical demineralizing agent before and after they were laser-irradiated. Laser treatment in combination with fluoride application appears to have several advantages over fluoride application alone. Following the CO2 laser irradiation, the F-veneer layer on the surface along with the superficial layer of enamel surface is thermally melted, resulting in recrystallization and rearrangement of a new Fluorapatite (FAP) mineral. This firmly bound fluoride minimizes the mineral loss from the enamel surface thereby making it more resistant to acid attack.[17,18]
The results of our study have been discussed under subheadings of TMC, calcium/phosphate ratio and fluoride retention.
Total mineral content
Table 1 shows the Comparison of mean TMC between different groups. In Group 1, there was no significant difference in TMC between APF (1A) and Remin-Pro (1R). APF gel contains 1.23% fluoride (12,300 ppm, as sodium fluoride and HF), phosphoric acid and carboxymethyl cellulose as the gelling base. The phosphoric acid, which has an acidic pH of 3.5 partly, dissolves the enamel surface. This creates a microporous surface for better penetration of fluoride into the enamel surface to form CaF2. Hence, the application of APF gel retains more fluoride. This might have attributed for the increase in mineral content in Group 1A. The etching pattern of APF was confirmed by the HR-SEM images [Figure 1]. Hydroxyapatite and fluoride (1,450 ppm sodium fluoride) present in Remin-Pro may have contributed to the increase in the TMC when compared with Groups 1P and 1C.
Table 1.
Comparison of mean TMC between different groups
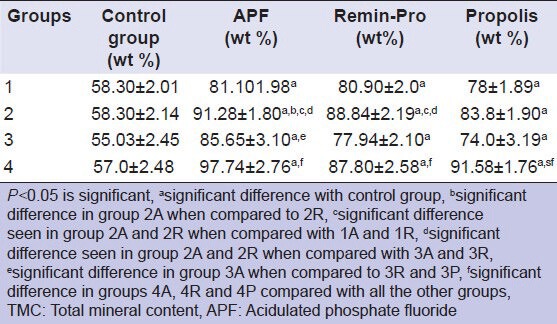
Figure 1.
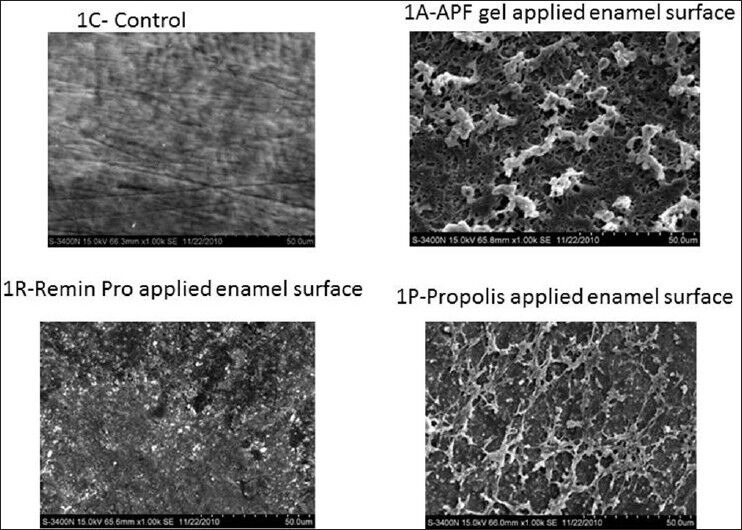
High resolution-scanning electron microscopic pictures of Group 1 at ×1.00 K magnification
In Group 2, there is a significant increase in TMC in Group 2A when compared with 2R. This may be due to the presence of 0.5% phosphoric acid in APF gel, which etches the enamel surface resulting in more penetration of fluoride ions when compared to Remin-Pro. Both Groups 2A and 2R (application followed by CO2 lasing) showed significant increase in TMC as compared with Groups 1A and 1R respectively. This might be due to the increase in fluoride retention by transformation of hydroxyapatite crystals to fluorapatite. Group 2P also showed an increase in TMC compared to 1P though not significant. The above mentioned mechanism may be the reason for this marginal increase in the TMC.
In Group 3, Group 3A (irradiation followed by APF application) showed a significant increase in TMC when compared to Groups 3R, 3P and 3C. The probable reason may be the higher fluoride content (12,300 ppm) in APF as compared with 1,450 ppm fluoride in Remin-Pro. Similarly, 3A and 3R showed a significant increase in TMC as compared to 3P and 3C. Group 3A and 3R showed a significant reduction in TMC when compared with Groups 2A and 2R respectively. This might be due to the lack of retention of topical agents after CO2 laser irradiation. Superficially located minerals may be lost by back exchange, back diffusion and migration from the enamel surface to the surrounding tissue fluid/saliva.[17]
An increase in mineral content was seen when CO2 laser irradiation was done before and after the application of the topical agents. Groups 4A, 4R and 4P showed the highest mineral content compared to all the other groups, which can be attributed to the multiple thermal effects caused by the laser. Márquez et al. in 1993 explained that 30% of the carbonate is lost between 400 and 600°C and it is completely removed only after repeated irradiation beyond the melting temperature of the enamel itself (more than 800°C). The complete removal of the carbonate derives both from the absorption depth of the surface and from pulse intensity and duration.[19] So, a multiple irradiation before and after the application of topical gels might have led to the complete removal of carbonate, thereby incorporating fluoride into the enamel.
Calcium/phosphate ratio
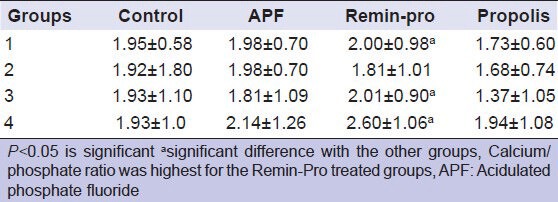
Table 2 shows the comparison of mean calcium/phosphate ratio between different groups. Calcium/phosphate ratio was highest for the Remin-Pro treated groups when compared with all other groups. This may be due to the presence of hydroxyapatite crystals in the topical agent. The least calcium/phosphate ratio for Group 1P can be explained by the absence of minerals present in propolis. Lee et al. in 2004 reported that there is no significant difference between non-irradiated and irradiated enamel surface in calcium/phosphate ratio when erbium YAG laser was used.[20] However, in the present study, the increase in calcium/phosphate ratio for Groups 1R, 3R and 4R might be due to the presence of hydroxyapatite crystals in Remin-Pro.
Table 2.
Comparison of mean calcium/phosphate ratio between different groups
Fluoride retention
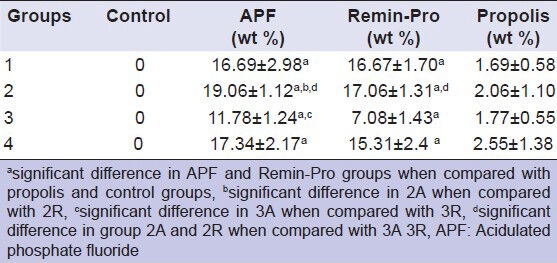
Table 3 shows the comparison of mean fluoride retention between different groups. EDX revealed very negligible or absence of fluoride in all the control groups. The amount of fluoride in all the propolis groups was very negligible. This suggests the absence of fluoride in propolis. Though, the fluoride content of APF is much higher than Remin-Pro there was no significant difference between Group 1A and 1R. This may be due to the loss of fluoride by back exchange, back diffusion and migration when stored in artificial saliva. Previous studies have shown high uptake of fluoride ions from APF gel compared with a neutral gel. Depth of penetration for fluoride ion was found to be 54 μm with APF as compared to 36.6 μm with neutral gel.[21] When this enamel is irradiated by laser it enhances fluoride uptake into the crystalline structure of the enamel in the form of firmly bound fluoride. Hence, the mineral loss from the enamel surface is minimized resulting in significant increase in fluoride retention with Group 2A when compared with Group 2R.
Table 3.
Comparison of mean fluoride retention between different groups
As the solubility of the crystals decreases with their increasing size, CaF2 particles occurring as a result of APF application will dissolve slower than those of sodium fluoride application. Topical fluoride application increases the amount of CaF2 deposited on the enamel surface at low pH. This accounts for the increase of fluoride in Group 3A when compared to Group 3R.[22]
The loss of ions by back exchange, back diffusion and migration when immersed in artificial saliva might be the reason for decreased fluoride intake in Group 3A and 3R when compared with Group 2A and 2R respectively.
A multiple irradiation before and after the application of topical gels probably led to the complete removal of carbonate, thereby incorporating the fluoride from APF into apatite lattice, transforming hydroxyapatite into fluorapatite. The increased fluoride retention in Group 4A when compared with Group 4R can be due to the depth of penetration of fluoride in APF gel as explained before. The melted and fused enamel surface could have decreased the penetration of APF gel owing to the decreased retention in Group 3A when compared to Group 4A.
All the HR-SEM images of the control group showed normal surface morphology of enamel. In Figure 1a and p, etching of enamel is seen with probable deposits of CaF2 on surface. Figure 1R presents a relatively smooth surface when compared with 1a and 1p with surface deposits of CaF2. In Figure 2A, irregular surface is seen due to the formation of craters with deposits of CaF2. However/, in Figure 2R, fewer craters are evident with a smoother surface differentiating them. Surface deposits of CaF2 are also seen along with the craters. Figure 3A presents few craters with deposits of CaF2. In Figure 3R, flower shaped craters are seen along with CaF2 deposits. Craters are differentiated from each other by smooth surface morphology. In Figure 3P, there is no evidence of crater formation. Surface morphology is smooth with fewer surface deposits. Large and closely packed craters with no surface deposits of CaF2 are seen in Figure 4A. Closely packed craters resembling an island surrounded by a sea of smooth surface is noticed in Figure 4R. However, in 4p, less number of craters is noticed with surface deposits present on the irregular surface. The entire laser irradiated groups revealed melting and fusion with the formation of craters on the enamel surface. The etched enamel prisms were filled with CaF2 particles where CO2 laser was combined with the topical agent.
Figure 2.
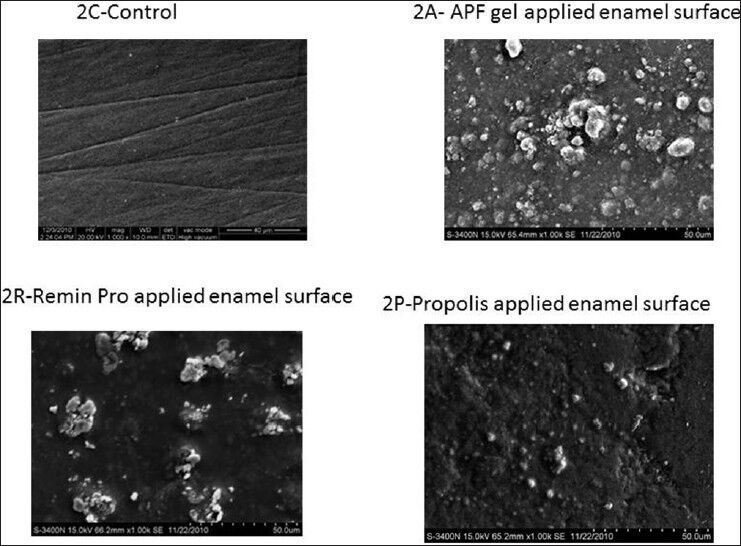
High resolution-scanning electron microscopic pictures of Group 2 at ×1.00 K magnification
Figure 3.
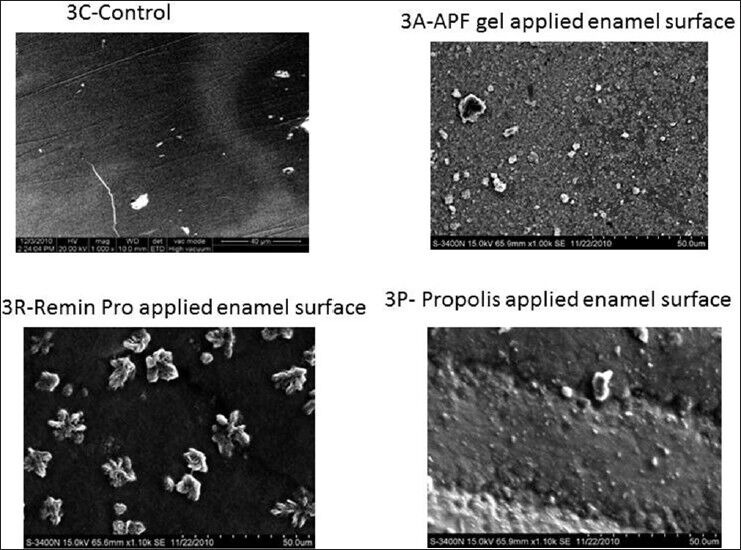
High resolution-scanning electron microscopic pictures of Group 3 at ×1.00 K magnification
Figure 4.
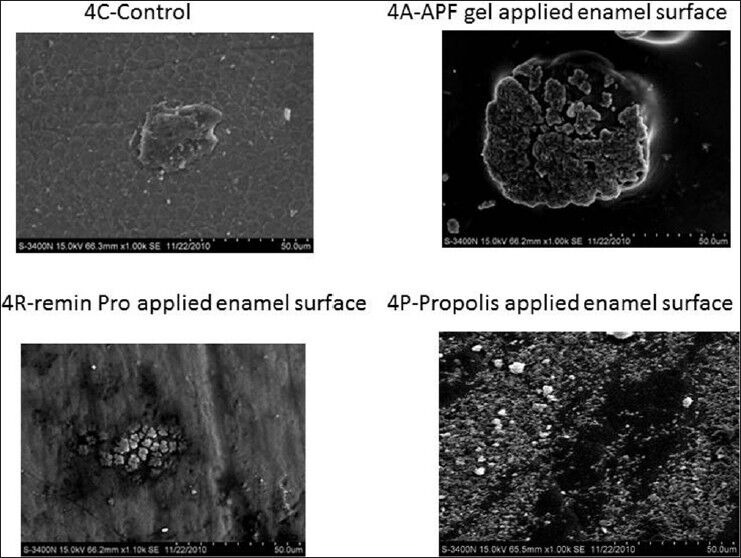
High resolution-scanning electron microscopic pictures of Group 4 at 1.00 K magnification
CONCLUSION
Within the limitations of this in-vitro study, it can be concluded that:
TMC is increased in the specimen where the enamel was irradiated before and after APF gel application
There was an increase in calcium/phosphate ratio for specimens treated with Remin-Pro irrespective of the radiation protocol
Maximum fluoride retention was seen in specimens where the application of APF gel was followed by laser irradiation when compared with all other groups
CO2 laser irradiation, at 10.6 μm, in combination with a single application of APF can increase the retention of fluoride by transforming hydroxyapatite crystals into fluorapatite
The SEM images presented with craters on all the irradiated enamel surfaces. However, there was no evidence for an additional effect when the enamel was treated with APF alone.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Stern RH, Vahl J, Sognnaes RF. Lased enamel: Ultrastructural observations of pulsed carbon dioxide laser effects. J Dent Res. 1972;51:455–60. doi: 10.1177/00220345720510023501. [DOI] [PubMed] [Google Scholar]
- 2.Stern RH, Sognnaes RF, Goodman F. Laser effect on in vitro enamel permeability and solubility. J Am Dent Assoc. 1966;73:838–43. doi: 10.14219/jada.archive.1966.0319. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Ooya K. Potential of yttrium-aluminum-garnet laser in caries prevention. J Oral Pathol. 1974;3:7–15. doi: 10.1111/j.1600-0714.1974.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Sato K. Prevention of dental caries by acousto-optically Q-switched Nd: YAG laser irradiation. J Dent Res. 1980;59:137. doi: 10.1177/00220345800590020801. [DOI] [PubMed] [Google Scholar]
- 5.Nelson DG, Shariati M, Glena R, Shields CP, Featherstone JD. Effect of pulsed low energy infrared laser irradiation on artificial caries-like lesion formation. Caries Res. 1986;20:289–99. doi: 10.1159/000260948. [DOI] [PubMed] [Google Scholar]
- 6.Nelson DG, Wefel JS, Jongebloed WL, Featherstone JD. Morphology, histology and crystallography of human dental enamel treated with pulsed low-energy infrared laser radiation. Caries Res. 1987;21:411–26. doi: 10.1159/000261047. [DOI] [PubMed] [Google Scholar]
- 7.Tagomori S, Morioka T. Combined effects of laser and fluoride on acid resistance of human dental enamel. Caries Res. 1989;23:225–31. doi: 10.1159/000261182. [DOI] [PubMed] [Google Scholar]
- 8.Oho T, Morioka T. A possible mechanism of acquired acid resistance of human dental enamel by laser irradiation. Caries Res. 1990;24:86–92. doi: 10.1159/000261245. [DOI] [PubMed] [Google Scholar]
- 9.Fox JL, Yu D, Otsuka M, Higuchi WI, Wong J, Powell G. Combined effects of laser irradiation and chemical inhibitors on the dissolution of dental enamel. Caries Res. 1992;26:333–9. doi: 10.1159/000261464. [DOI] [PubMed] [Google Scholar]
- 10.Kato IT, Kohara EK, Sarkis JE, Wetter NU. Effects of 960-nm diode laser irradiation on calcium solubility of dental enamel: An in vitro study. Photomed Laser Surg. 2006;24:689–93. doi: 10.1089/pho.2006.24.689. [DOI] [PubMed] [Google Scholar]
- 11.Hicks MJ, Flaitz CM, Westerman GH, Berg JH, Blankenau RL, Powell GL. Caries-like lesion initiation and progression in sound enamel following argon laser irradiation: An in vitro study. ASDC J Dent Child. 1993;60:201–6. [PubMed] [Google Scholar]
- 12.Westerman GH, Hicks MJ, Flaitz CM, Blankenau RJ, Powell GL, Berg JH. Argon laser irradiation in root surface caries: In vitro study examines laser's effects. J Am Dent Assoc. 1994;125:401–7. doi: 10.14219/jada.archive.1994.0060. [DOI] [PubMed] [Google Scholar]
- 13.Westerman GH, Hicks MJ, Flaitz CM, Powell GL, Blankenau RJ. Surface morphology of sound enamel after argon laser irradiation: An in vitro scanning electron microscopic study. J Clin Pediatr Dent. 1996;21:55–9. [PubMed] [Google Scholar]
- 14.Esteves-Oliveira M, Pasaporti C, Heussen N, Eduardo CP, Lampert F, Apel C. Prevention of toothbrushing abrasion of acid-softened enamel by CO (2) laser irradiation. J Dent. 2011;39:604–11. doi: 10.1016/j.jdent.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Giamalia I, Steinberg D, Grobler S, Gedalia I. The effect of propolis exposure on microhardness of human enamel in vitro. J Oral Rehabil. 1999;26:941–3. doi: 10.1046/j.1365-2842.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 16.Anić I, Vidović D, Luić M, Tudja M. Laser induced molar tooth pulp chamber temperature changes. Caries Res. 1992;26:165–9. doi: 10.1159/000261437. [DOI] [PubMed] [Google Scholar]
- 17.Tepper SA, Zehnder M, Pajarola GF, Schmidlin PR. Increased fluoride uptake and acid resistance by CO2 laser-irradiation through topically applied fluoride on human enamel in vitro. J Dent. 2004;32:635–41. doi: 10.1016/j.jdent.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Wu CC, Roan RT, Chen JH. Sintering mechanism of the CaF2 on hydroxyapatite by a 10.6-l microm CO 2 laser. Lasers Surg Med. 2002;31:333–8. doi: 10.1002/lsm.10124. [DOI] [PubMed] [Google Scholar]
- 19.Márquez F, Quintana E, Roca I, Salgado J. Physical-mechanical effects of Nd: YAG laser on the surface of sound dental enamel. Biomaterials. 1993;14:313–6. doi: 10.1016/0142-9612(93)90124-k. [DOI] [PubMed] [Google Scholar]
- 20.Lee BS, Lin CP, Hung YL, Lan WH. Structural changes of Er: YAG laser-irradiated human dentin. Photomed Laser Surg. 2004;22:330–4. doi: 10.1089/pho.2004.22.330. [DOI] [PubMed] [Google Scholar]
- 21.Pai N, McIntyre J, Tadic N, Laparidis C. Comparative uptake of fluoride ion into enamel from various topical fluorides in vitro. Aust Dent J. 2007;52:41–6. doi: 10.1111/j.1834-7819.2007.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 22.Featherstone JD, Barrett-Vespone NA, Fried D, Kantorowitz Z, Seka W. CO2 laser inhibitor of artificial caries-like lesion progression in dental enamel. J Dent Res. 1998;77:1397–403. doi: 10.1177/00220345980770060401. [DOI] [PubMed] [Google Scholar]


