Abstract
The aim of this work is to demonstrate how current molecular techniques should be integrated in the diagnostic process and can have a crucial role in the management of oral fungal infections. A case of median rhomboid glossitis Candida-associated and its resolution will be described step by step. At the time of the first observation, the lesion on the surface of the tongue did not respond to the previous administration of topical antifungal agent, such a nystatin. Firstly, in order to identify the causative agent and confirm Candida albicans infection, a brushing of the lesion was performed and polymerase chain reaction analysis was carried out. In addition, deoxyribonucleic acid sequencing method, known as Pyrosequencing®, was used in the detection of point mutations commonly associated with fluconazole resistance and consequently, in the prediction of susceptibility to azole agents. According to molecular findings, the administration of fluconazole has therefore led to resolution of the case in 2 weeks. This case highlights how the use of molecular techniques, now-a-days, can assist the clinicians to quickly obtain the report with highly accurate and precise results and appropriately support them in the diagnosis and therapeutic process.
Keywords: Candida albicans, drug resistance, median rhomboid glossitis, pyrosequencing
INTRODUCTION
Median rhomboid glossitis (MRG) is a condition characterized by a papillary atrophy of the dorsum of the tongue typically located in an area anterior to the circumvallate papillae. It occurs as a well-demarcated area of depapillation, elliptical or rhomboid in shape, on the mid-line of the tongue. Although the surface of the lesion is usually smooth, it occasionally displays a hyperplastic, exophytic or even lobulated appearance.[1,2]
Clinically, MRG is asymptomatic and it can remain unnoticed prior to the examination of the oral mucosa by the clinician. However, sometimes, persistent pain, burning and tingling sensation and dysgeusia can be referred during the anamnesis. The concomitance of erythematous lesion on the palate surface opposing the lingual lesion may be observed and it is commonly recognized as “kissing lesion.”[2]
Nowadays, MRG is considered as variant of the oral lesion associated with Candida infections, even though its etiology is controversial. In fact, several additional predisposing factors has been taken into consideration such as smoking, denture wearing, diabetes mellitus, use of corticosteroid sprays or inhalers and human immunodeficiency virus infection.[2]
According to many reports, topical antifungal therapy promotes the resolution of MRG in most cases. In the recent decades, however, different effective antifungal agents, administered either topic or systemically and has been implied for the management of oral candidiasis.[2,3] In particular, treatment options include the delivery of either polyenes (e.g. nystatin) or the azole-group antimycotics (e.g. fluconazole, miconazole, clotrimazole) [Table 1].
Table 1.
Active substance, trade name and route of administration of the main existing antifungal drugs

Although the availability of such a multiple therapeutic agents, the outbreak of resistance to antifungal drugs has reflections on the decrease of their potency and consequently, requires the adoption of alternative strategies in order to prevent the problem from growing at an alarming rate.[4] Furthermore, cases of antifungal resistance have been reported for almost all classes of antifungal agents but it have been mainly documented for azole antifungals.[4,5,6] For this reason, inappropriate prophylactic and empirical therapies as well as unnecessary antimycotic drug pressure should be avoided. In this context, the development of diagnostic procedures that allow to predict accurately the susceptibility to antifungal drug treatment enhances the efficacy of drug therapy and at the same time, would contribute to control the emergence of resistance.[3,4]
This report aims to investigate the role of accurate and precise molecular method, such as Pyrosequencing®, in the diagnosis as well as in the prediction of success or failure of treatment in order to control the threatening spread of fungal resistance.[7]
CASE REPORT
A 61-year-old Sardinian man was admitted to the Department of Dental Disease Prevention due to the presence of a suspected lesion localized on the dorsum of the tongue. After performing an accurate clinical evaluation, the presence of an erythematous depapillated area restricted to the forward part of the papillae circumvallate was identified [Figure 1].
Figure 1.
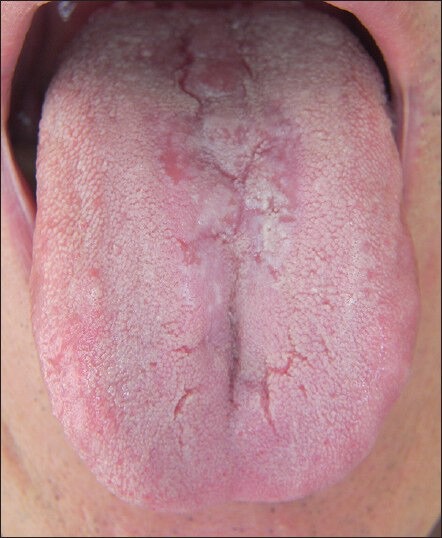
Tongue aspect at the first observation
During the anamnesis, the patient did not report any history of systemic disease nor an immunocompromised status. However, he referred to undergo nystatin (Mycostatin®, 400,000-600,000 IU 4 times/die for 14 days) during the previous 4 weeks that could not be decisive for the resolution of the tongue lesion. Moreover, he did not notice any variation in the aspect of the lesion and kept complaining a light sense of discomfort associated with persistent irritation, pain and dysgeusia in the mentioned area.
In order to confirm Candida albicans infection, a sample was collected by brushing the tongue lesion with a sterile swab and polymerase chain reaction analysis was carried out.[8]
In order to detect mutations commonly associated with antifungal resistance in Candida spp. and then rapidly predict the susceptibility to the treatment, a real-time deoxyribonucleic acid (DNA) sequencing method, known as Pyrosequencing®, was used.[7]
On the basis of molecular findings, fluconazole (Diflucan®, 50 mg 1 time/die for 14 days) was administered systemically and the complete regression of the tongue lesion was observed after 2 weeks [Figure 2]. Lastly, assessment of results performed by classical biochemical technique in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) confirmed, approximately after 1 week, the adequacy of underway therapeutic strategy.
Figure 2.
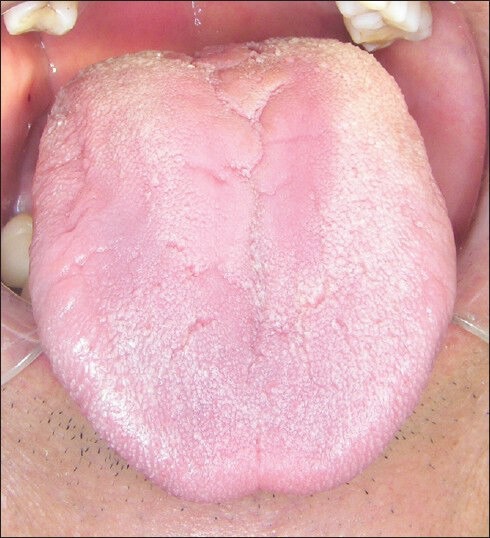
Tongue aspect after 2 weeks of treatment with fluconazole
DISCUSSION
In the last decades, the favorable pharmacokinetic profile of azole agents has encouraged their use in the management of fungal infections. However, the continuous exposure to azole has promoted the emergence of resistant organism, particularly fluconazole-resistant Candida spp. Although clinical resistance can be attributed to the combination of factors related to host, the antifungal agent, or the pathogens, altered gene expression has been identified as playing an essential role and several point mutations have been associated with the azole drug resistance in Candida spp.[5]
Simultaneously, the development of DNA sequencing techniques represents a promising advance in biomedicine as they permit to evaluate accurately, precisely as well as rapidly, the efficacy of therapeutic agents.[7] In fact, although guidelines recommended by CLSI provide a useful method to test the susceptibility of yeast pathogens against existing antifungal agents, clinical response often conflicts with data obtained by in vitro evaluations.[9] According to literature, treatment success rate fluctuates from 34% to 74%, indicating the significant percentage of false positives in vitro.[9] For this reason, clinicians need to count on more accurate and precise diagnostic methods in order to avoid inappropriate use of antimycotic agents [Table 2]. Furthermore, the earlier diagnosis of oral pathologies represents a continuous challenge for clinicians. In this point of view, the application of new molecular technologies may provide a better understanding of pathologic mechanisms as well as enormously facilitate therapeutic decisions as they guarantee to obtain more accurate results in reduced investigation time.[10]
Table 2.
Diagnostic and therapeutic procedures followed for this case report
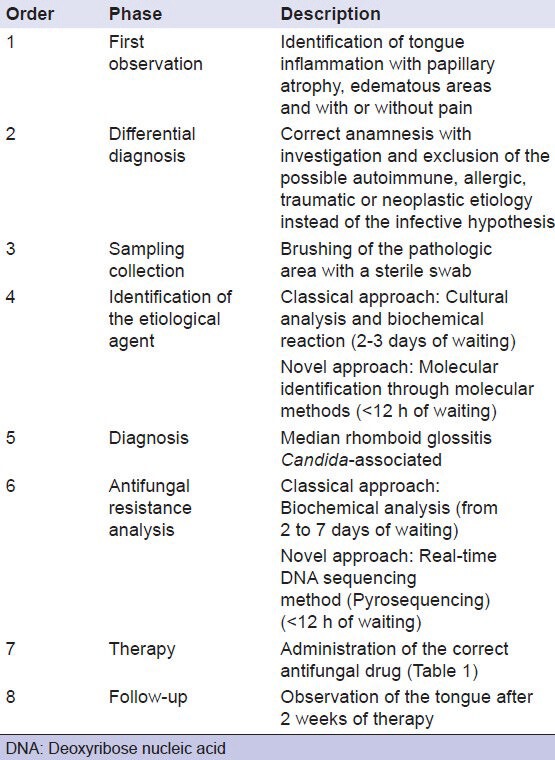
This report would provide an alternative strategy to oppose the emergence of resistance and contemporaneously, to promote an adequate use of azole agents in the perspective of reducing antifungal drug pressure. As fluconazole drug resistance is mainly documented, we focus our attention on genes responsible for it but, in the future, this molecular method could have promising reflections on early diagnosis of pathologies Candida-associated and consequently, it could be considered as an ordinary practice in clinical evaluations.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Goregen M, Miloglu O, Buyukkurt MC, Caglayan F, Aktas AE. Median rhomboid glossitis: A clinical and microbiological study. Eur J Dent. 2011;5:367–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Mahto KK, Prasad R. Lipidomics and in vitro azole resistance in Candida albicans. OMICS. 2013;17:84–93. doi: 10.1089/omi.2012.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masiá Canuto M, Gutiérrez Rodero F. Antifungal drug resistance to azoles and polyenes. Lancet Infect Dis. 2002;2:550–63. doi: 10.1016/s1473-3099(02)00371-7. [DOI] [PubMed] [Google Scholar]
- 5.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–13. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanafani ZA, Perfect JR. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin Infect Dis. 2008;46:120–8. doi: 10.1086/524071. [DOI] [PubMed] [Google Scholar]
- 7.Montero CI, Shea YR, Jones PA, Harrington SM, Tooke NE, Witebsky FG, et al. Evaluation of pyrosequencing technology for the identification of clinically relevant non-dematiaceous yeasts and related species. Eur J Clin Microbiol Infect Dis. 2008;27:821–30. doi: 10.1007/s10096-008-0510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erriu M, Boscarelli F, Peluffo C, Orrù G, Nucaro A, Zorco S, et al. A rapid sample method for HLA haplotype typization. A preliminary study on celiac patients. Minerva Stomatol. 2010;59:477–87. [PubMed] [Google Scholar]
- 9.Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002;35:982–9. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 10.Germano F, Bramanti E, Arcuri C, Cecchetti F, Cicciù M. Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study results. Eur J Dent. 2013;7:152–8. doi: 10.4103/1305-7456.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]


