Abstract
Objective:
To check the antimicrobial activity of Azadirachta indica (Neem), Ocimum sanctum (Tulsi), Mimusops elelngi (Bakul), Tinospora cardifolia (Giloy) and Chlorhexidine Gluconate (CHX) on common endodontic pathogens like Streptococcus mutans, Enterococcus faecalis and staphylococcus aureus.
Materials and Methods:
The agar diffusion test was used to check the antimicrobial activity of the Methanolic extracts of the medicinal plants along with CHX. Six different concentrations of the tested agents were used for the study. The values of Zone of Inhibition were tabulated according to the concentration of the tested agent and data was statistically analyzed using ANOVA and Bonferroni post- hoc tests. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentrations (MBC) values were also recorded.
Results:
All the plants extracts showed considerable antimicrobial activity against selected endodontic pathogens. At 3mg. concentration, O.sanctum was the most effective against S. mutans, M. elengi showed highest zone of inhibition against E.faecalis, whereas CHX was the most effective agent against S.aureus. CHX was also the most consistent of all the medicaments testes, showing inhibitory effect against all the tree pathogens at all the selected concentrations.
Conclusions:
The Methanolic extract of A.Indica, O.sanctum, M. Elengi, T.cardifolia and Chlorhexidine Gluconate has considerable antimicrobial activity against S. mutans, E. faecalis and S. aureus.
Keywords: Antimicrobial activity, chlorhexidine gluconate, endodontic pathogens, medicinal plant extracts
INTRODUCTION
Microorganisms are primary etiological factor in the development of pulpal and periapical diseases.[1] Endodontic treatment is aimed at complete elimination of microbes form the pulp space. This goal is achieved by thorough chemo mechanical preparation followed by three dimensional obturation of the root canal system. Although mechanical instrumentation can remove a significant number of bacteria from the root canal system,[2] the bacteria remaining in the intricacies of the canal can cause or sustain periradicular tissue inflammation.[3] Therefore, mechanical instrumentation of the pulp space is accompanied by use of different types of irrigation solutions. According to current literature, sodium hypochlorite and 2% chlorhexidine remains to be the most preferred irrigating solutions.[4]
Sodium hypochlorite has an excellent antimicrobial and tissue dissolution properties. However, it can cause soft-tissue inflammation if expressed out of the confines of the root canal. This event is associated with extreme pain and/or widespread swelling.[5] In addition, it has an unpleasant odor and taste. Chlorhexidine gluconate (CHX) has a wide antimicrobial spectrum. However, at 2% concentration, which is most commonly used in endodontics, may have toxic effects on host tissues if expressed beyond the confines of the root canal and may impair healing.[6] It also lacks tissue dissolving capacity.[7]
Natural products are known to play an important role in human life. Various parts of the plants like root, bark, seed and leaves have been an important source of medicine since thousands of years. In recent years a predominant interest has been observed in evaluating different plant extracts for their antimicrobial properties against bacteria causing dental caries and periradicular pathology. A study by Murray et al. evaluated the possible use of Morinda citrifolia juice as an alternative to Sodium hypochlorite as an irrigant.[8] Berberine, a plant alkaloid isolated from many medicinal plants, when combined with CHX was found to be comparable to Sodium hypochlorite in its bactericidal efficacy.[9] India has a rich flora of medicinal plant species that are widely distributed throughout the country.
Mimusops elengi, locally known as Bakul is a small to large tree found all over India. The plant finds an important place in the indigenous system of medicine and its various parts are used in the treatment of various systemic diseases including dental problems. It has shown significant anti-inflammatory, analgesic and antipyretic activity.[10] The bark of M. elengi is acrid, astringent and is used as a gargle for odontopathy, inflammation and bleeding gums.[11] The tender stems are used as tooth brushes.[12]
Azadirachta indica (Neem) is perhaps the most useful traditional medicinal plant. Every part of the tree has been used as traditional medicine for household remedy against various human ailments. The tree is still regarded as “Village dispensary” in India. Most of the parts of the plant such as fruits, seeds, leaves, bark and roots contain compounds with proven antiseptic, antiviral, antipyretic, anti-inflammatory, antiulcer and antifungal properties.
Ocimum sanctum, popularly known as Tulsi is a time-tested premier medicinal herb that is used in ayurvedic medicine since ancient times. It has made an important contribution to the modern research due to its large number of medicinal properties. Different parts of the plant have shown antimicrobial, anti-inflammatory, analgesic, antipyretic, antiulcer, antidiabetic, antioxidant and anticancer activity.
Tinospora cardifolia is a large deciduous climbing shrub found throughout India. The ayurvedic name of the plant is Guduchi, Giloy or Amrita. In India, the extract of the plant is used as a remedy for many diseases including diabetes, hepatitis etc., The plant finds a special mention for its use in tribal or folk medicine in different parts of the country. The drug has been subjected to extensive phytochemical, pharmacological and clinical investigations and many interesting findings have been reported.
This study was aimed to compare the antimicrobial efficacy of various concentrations of selected medicinal plants like A. Indica (Neem), O. sanctum (Tulsi), M. elengi (Bakul), T. cardifolia (Giloy) and CHX against common endodontic pathogens such as Enterococcus faecalis, Streptococcus mutans and Staphylococcus aureus.
MATERIALS AND METHODS
Procurement of material
The leaves of Neem and Tulsi, stem of Giloy and Bark of Bakul were collected from the courtyard. All the plant materials were identified by the senior professor at L. M. College of Pharmacy, Ahmedabad. Plant materials were washed with distilled water and dried under the shade for 10-12 days. All the material was ground in an electric grinder to produce a powder.
Preparation of extracts
The powdered material was again dried in an oven at 40°C for 4 h and used for extraction. Accurately weighed 50 g of powdered leaf sample was extracted with 500 ml methanol. This process was repeated until the residual marc got exhaustively extracted and finally extracts were pooled and evaporated in rota-evaporator. The extracts were concentrated under partial vacuum at 80°C to dryness, leaving behind thick semi-solid residue. This extract was dissolved in dimethyl sulfoxide (DMSO) to get six different concentrations to be tested.
Procurement of microorganisms
The microbial strains investigated in the study were obtained from Imtech-Chandigarh, India. The strains are E faecalis (MTCC 439), S. mutans (MTCC 497), and S. aureus (MTCC 737).
Test for antibacterial assay
Agar diffusion test
The test microorganisms were subcultured on specific media procured by Himedia Laboratory Pvt. Ltd., Mumbai, India and incubated aerobically at 37°C for 24 h. A total of six wells were made into a nutrient agar plate using sterile cork borer (6 mm. in diameter) and inoculums containing 1 × 105 CFU/ml of bacteria were spread on the solid plate with the bacterial suspension. 100 μl of the working solution of different medicinal plant extract carrying different concentration of the medicine was filled in the wells with the help of micropipette. The plates were then incubated at 37°C for 24 h in an aerobic environment. After overnight incubation, the plates were observed for the zone of inhibition and the diameters of the inhibition zone in millimeters were measured using a scale. Each extract was tested three times and mean values were recorded.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using the micro broth dilution method.
Statistical analysis
The collected data was analyzed using the following statistical test (SPSS version 17, Inc. Chicago, USA)
Mean value and standard deviation
One-way analysis of variance
Bonferroni post-hoc test to carry out multiple comparisons in bacterial inhibition zones between the groups (P < 0.05).
RESULTS
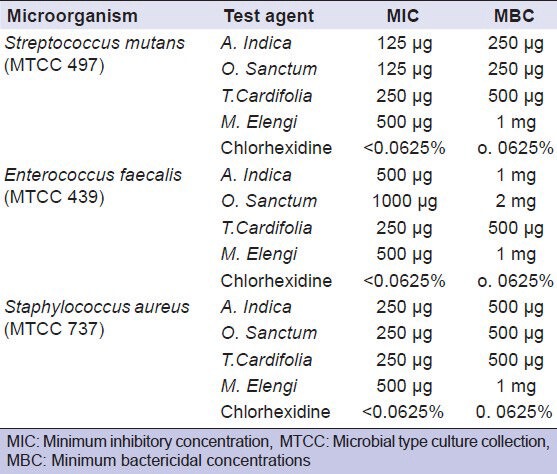
The current study showed that all the plant extracts and 2% CHX exerted antibacterial activity against selected endodontic pathogens. The antimicrobial activities of the test agents were in direct proportion with the concentration used. The effect of different concentrations of Neem, Tulsi, Bakul, Giloy and 2% CHX on selected microorganisms is tabulated in Table 1. The MIC and MBC values are presented in Table 2.
Table 1.
Values of Zone of Inhibition of different concentrations of tested agents
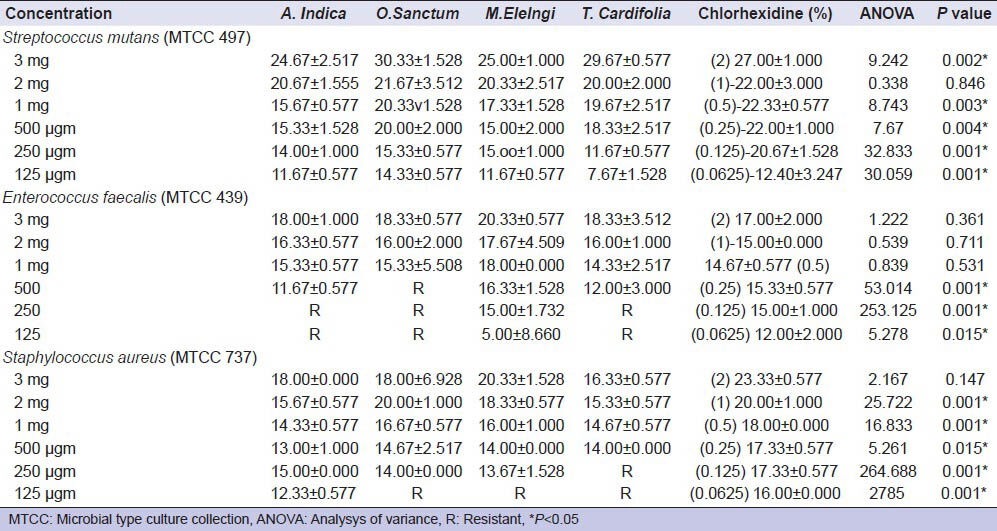
Table 2.
Minimum inhibitory and minimum bactericidal concentrations of the tested agents
It is interesting to note that even at the lowest concentration, all the tested agents showed significant antimicrobial activity. O. sanctum (Tulsi) showed highest zone of inhibition against S. mutans at 3 mg concentration. M. elengi (Bakul) was most effective against E. faecalis at 3 mg concentration, whereas A. indica, O. sanctum and T. cardifolia showed no inhibitory effect at lower concentration. CHX was most effective agent showing highest zone of inhibition at the highest concentration against S. aureus. At the same time it was most consistent of all the medicaments tested, showing inhibitory effect against all the three pathogens at all selected concentrations.
DISCUSSION
In the current study, we explored four medicinal plants such as A. Indica (Neem), O. sanctum (Tulsi), M. elengi (Bakul) and T. cardifolia (Giloy) as well as 2% Chlorhexidine for their antimicrobial effectiveness against target endodontic pathogens like E. faecalis, S. mutans and S. aureus.
S. mutans is believed to be the most common bacteria associated with dental caries. It acts on available carbohydrate in the mouth, breaking it down with the production of lactic acid and subsequent demineralization of tooth structure. S. mutans along with S. aureus is frequently isolated from primary endodontic infection,[13,14,15] as well as in root filled teeth.[16] The presence of these microorganisms is also found to be more strongly associated with pre-operative symptoms and presence of swelling.[17,18]
E. faecalis remains to be the most frequently identified species in canals of root filled teeth with periapical lesions as established by different molecular methods from time to time.[19,20,21,22,23] This may be due to its ability to survive the effects of a wide range of antimicrobial solutions and intracanal medicaments used during endodontic treatment procedures. It also endures prolonged periods of nutritional deprivation. The root canal is hardly a nutrient rich medium, but E. faecalis may survive on serum components from the dentinal fluid. Therefore, even in a well debrided and coronally well-sealed root canal, remaining or surviving cells of E. faecalis may still grow and utilize local sources of energy and nutrients.[24]
The selected plant extracts have shown significant antimicrobial effect against these endodontic pathogens. Tulsi showed highest zone of inhibition against S. mutans at 3 mg concentration. The difference in antimicrobial activity at this concentration was statistically significant when compared with other agents like Neem and Bakul (P < 0.05). It has also shown its antimicrobial property against S. aureus and E. faecalis. However, in lower concentrations, E. faecalis showed resistance against Tulsi with MIC value of 1 mg. The results of our study are in agreement with previous studies where different concentrations of Tulsi have been used against all three tested microorganisms.[25,26,27,28,29] The biological properties of the plant has been attributed to the presence of active compounds like Ursolic acid, flavonoids (epigenin, orientin and vicenin),[30] and phenolic compounds (cirsilineol, circimaritin, isothymusin, eugenol).[31] The leaves of Tulsi contain 0.7% volatile oil comprising about 71% eugenol and 20% methyl eugenol.[32] Eugenol is the most prominent phytoconstituents present in this plant which may be responsible for antimicrobial activity.[33]
Bakul was most effective agent against E. faecalis at 3 mg. concentration. However, the difference with other agents was statistically insignificant (P < 0.05). This plant extract has been tested for its antimicrobial effects against dental pathogens. The extract was found to be effective against Streptococci isolated from tooth tartar of dental patients, thus confirming the traditional claim.[34] In a similar study, a concentration of 450 μg was found to be inhibitory for the growth of most of the tested salivary microflora.[35] Our study has shown that the extract is effective at even lower concentration with MIC value of 250 μg. This may be due to solvent used for the preparation of extract. Methanolic extracts may show greater activity because more phytoconstituents are leached in it when compared to other extracts. Different types of glycosides, alkaloids, phenols, tannins and saponins have been screened in the methanolic extract of this plant.[36]
Neem extract has shown antimicrobial activity against E. faecalis and S. mutans in previous in vitro studies.[37,38,39] Prashant et al., demonstrated that Neem stick extract produced maximum zone of inhibition against S. mutans at 50% concentration. Even at 5% concentration, Neem extract was effective against all four species of microorganisms tested in their study.[39] Bohora et al., concluded that Neem leaf extract has a significant antimicrobial effect against E. faecalis, Candida albicans and mixed culture.[37] Our study has shown the leaf extract of Neem is very effective against S. mutans and S. aureus with MIC value of 125 μg. The maximum antimicrobial activity was observed on S. mutans at 3 mg. concentration with zone of inhibition of (24.67 ± 2.517) mm. However, at lower concentrations, it was ineffective on E. faecalis. This is in agreement with the study by Dhanya Kumar et al. where 10% concentration at the highest volume of 75 μl was not effective on E. faecalis. It was effective only at the highest concentration of 50% and at the highest volume of 75 μl.[38] Neem contains different active phytoconstituents such as alkaloids, glycosides, trepenoids, steroids and tannins.[36] Neem has been found to be highly effective in the treatment of periodontal diseases, thus exhibiting its biocompatibility with human PDL fibroblasts. The use of Neem as an endodontic irrigant might be recommended because it is biocompatible antioxidant and thus not likely to cause severe injuries to patients.[40,41] Bitter test associated with this plant can be altered by the addition of sweeteners and flavors to increase the patient compliance and acceptability.[40]
Nearly 2% CHX showed the most consistent antimicrobial activity against all three pathogens with MIC value of <0.0625%. In our study we have used six different concentrations of CHX with two fold reduction (2%, 1%, 0.5%, 0.25%, 0.125% and 0.0625%). The concentration often used in endodontic therapy is 2%. This has been found to be more effective in the least time when compared with other concentrations of CHX ranging from 0.002% to 2%.[42] CHX is active against a wide range of micro-organisms, such as Gram-positive and Gram-negative bacteria, bacterial spores, lipophilic virus, yeast and dermatophytes being bacteriostatic at lower concentrations and bactericidal at high concentration.[43] It shows substantial antimicrobial effect against common endodontic pathogens like S. aureus, Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia, E. faecalis, C. albicans and S. mutans.[42,44,45] The antimicrobial effect is because of reaction of CHX molecule with negatively charged groups on the cell surface, causing an irreversible loss of cytoplasmic constituents, membrane damage and enzyme inhibition. At higher concentrations CHX results in extensive cell damage, coagulation of cytoplasm and precipitation of proteins and nucleic acids.[46]
Giloy also exerted considerable antimicrobial effectiveness against tested pathogens. However, it is ineffective against E. faecalis and S. aureus at lower concentrations with MIC value of 500 μg. This plant has been subjected to chemical investigations extensively and a number of chemical constituents belonging to different groups such as trepenoids, alkaloids, lignans and flavonoids, tannins, cardiac glycosides and steroids have been reported.[47] which may account for the antimicrobial property of this agent. Similar results have been observed in previous studies where this plant extract has shown promising results against the pathogens used in our study.[47,48,49]
CONCLUSION
Under the limitations of this study, it can be concluded that A. Indica, O. sanctum, T. cardifolia, M. elengi and Chlorhexidine have antimicrobial effects against the endodontic pathogens like S. mutans, S. aureus and E. faecalis. However, further preclinical and clinical trials are required to evaluate the cytotoxicity and safety issues of these plant extracts before they can be recommended as an endodontic irrigant or intracanal medicament. Furthermore, looking at the polymicrobial nature of endodontic infections these agents needs to be tested for their antimicrobial effectiveness against a wide range of microorganisms including strict anaerobes.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Ercan E, Ozekinci T, Atakul F, Gül K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: In vivo study. J Endod. 2004;30:84–7. doi: 10.1097/00004770-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Siqueira JF, Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34:1291–301. doi: 10.1016/j.joen.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 5.Kleier DJ, Averbach RE, Mehdipour O. The sodium hypochlorite accident: Experience of diplomates of the American Board of Endodontics. J Endod. 2008;34:1346–50. doi: 10.1016/j.joen.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Faria G, Celes MR, De Rossi A, Silva LA, Silva JS, Rossi MA. Evaluation of chlorhexidine toxicity injected in the paw of mice and added to cultured l929 fibroblasts. J Endod. 2007;33:715–22. doi: 10.1016/j.joen.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30:785–7. doi: 10.1097/00004770-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Murray PE, Farber RM, Namerow KN, Kuttler S, Garcia-Godoy F. Evaluation of Morinda citrifolia as an endodontic irrigant. J Endod. 2008;34:66–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Xie Q, Johnson BR, Wenckus CS, Fayad MI, Wu CD. Efficacy of berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a mixed-culture biofilm in an in vitro tooth model. J Endod. 2012;38:1114–7. doi: 10.1016/j.joen.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Purnima A, Koti BC, Thippeswamy AH, Jaji MS, Swamy AH, Kurhe YV, et al. Antiinflammatory, analgesic and antipyretic activities of Mimusops elengi Linn. Indian J Pharm Sci. 2010;72:480–5. doi: 10.4103/0250-474X.73908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kritikar KR, Basu BD. 2nd ed. Vol. 2. Allahabad: Published by Lalit Mohan Basu; 1991. Indian Medicinal Plants; pp. 1494–6. [Google Scholar]
- 12.Satyavati GV, Gupta AK. 2nd ed. New Delhi: Indian Council of Medical Research; 1987. Medicinal Plants of India; pp. 257–61. [Google Scholar]
- 13.Rani A, Chopra A. Isolation and identification of root canal bacteria from symptomatic non-vital tooth with periapical pathosis. Endodontology. 2006;18:12–7. [Google Scholar]
- 14.Gomes BP, Pinheiro ET, Gadê-Neto CR, Sousa EL, Ferraz CC, Zaia AA, et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–6. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 15.Siqueira JF, Jr, Rôças IN, Alves FR, Silva MG. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:721–6. doi: 10.1016/j.tripleo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Mindere A, Kundzina R, Nikolajeva V, Eze D, Petrina Z. Microflora of root filled teeth with apical periodontitis in Latvian patients. Stomatologija. 2010;12:116–21. [PubMed] [Google Scholar]
- 17.Fouad AF, Barry J, Caimano M, Clawson M, Zhu Q, Carver R, et al. PCR-based identification of bacteria associated with endodontic infections. J Clin Microbiol. 2002;40:3223–31. doi: 10.1128/JCM.40.9.3223-3231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimarães NL, Otoch HM, Andrade LC, Ferreira CM, Rocha MM, Gomes FD. Microbiological evaluation of infected root canals and their correlation with pain. evista Sul-Brasileira de Odontologia. 2012;9:31–7. [Google Scholar]
- 19.Gomes BP, Pinheiro ET, Jacinto RC, Zaia AA, Ferraz CC, Souza-Filho FJ. Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. J Endod. 2008;34:537–40. doi: 10.1016/j.joen.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Rôças IN, Siqueira JF, Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30:315–20. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Siqueira JF, Jr, Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 22.Siqueira JF, Jr, Rôças IN. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J Clin Microbiol. 2005;43:3314–9. doi: 10.1128/JCM.43.7.3314-3319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endo MS, Ferraz CC, Zaia AA, Jose FA, Gomes BP. Quantitative and qualitative analysis of microorganisms in root filled teeth with persistent infection: Monitoring of the endodontic re-treatment. Eur J Dent. 2013;7:302–9. doi: 10.4103/1305-7456.115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15:308–20. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal P, Nagesh L, Murlikrishnan Evaluation of the antimicrobial activity of various concentrations of Tulsi (Ocimum sanctum) extract against Streptococcus mutans: An in vitro study. Indian J Dent Res. 2010;21:357–9. doi: 10.4103/0970-9290.70800. [DOI] [PubMed] [Google Scholar]
- 26.Mishra P, Mishra S. Study of antibacterial activity of Ocimum Sanctum extract against gram-positive and gram-negative bacteria. Am J Food Technol. 2011;6:336–41. [Google Scholar]
- 27.Geeta, Vasudevan DM, Kedlaya R, Deepa S, Ballal M. Activity of Ocimum sanctum (the traditional Indian medicinal plant) against the enteric pathogens. Indian J Med Sci. 2001;55:434–8. 472. [PubMed] [Google Scholar]
- 28.Sharma A, Chandraker S, Patel VK, Ramteke P. Antibacterial activity of medicinal plants against pathogens causing complicated urinary tract infections. Indian J Pharm Sci. 2009;71:136–9. doi: 10.4103/0250-474X.54279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi B, Sah GB, Basnet BB, Bhatt M, Sharma D, Subedi K, et al. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum Sanctum (Tulsi), Eugenia caryophyllata (clove), Achytanthes Bidentata (Datiwan) and Azadirachta Indica (Neem) J Microbiol Antimicrob. 2011;3:1–7. [Google Scholar]
- 30.Gupta SK, Prakash J, Srivastava S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol. 2002;40:765–73. [PubMed] [Google Scholar]
- 31.Mumbai, India: IDMA; 2002. Indian Herbal Pharmacopoeia; p. 272. [Google Scholar]
- 32.Shah CS, Qadry JS. A Textbook of Pharmacognosy. 1998:216. [Google Scholar]
- 33.Bhatt MK, Shankar MB, Saluja AK, Dholwani KK, Captain AD. Evaluation of antimicrobial activity of Ocimum Sanctum Methanolic extract. J Pharm Sci Innov. 2012;1:39–41. [Google Scholar]
- 34.Murudkar A, Mundhada SS, Tatke PA. Antibacterial activity of Mimusops Elengi Linn. Bark against dental pathogens. Indian J Pharm Educ Res. 2007;41:114–20. [Google Scholar]
- 35.Deshpande RR, Ruikar A, Panvalkar PS, Ankur K. Comparative evaluation of different concentration of Mimusops Elengi (L) extract as an antimicrobial against salivary microflora. J Biomed Sci Res. 2010;2:151–4. [Google Scholar]
- 36.Prabhat, Ajaybhan, Navneet, Chauhan A. Evaluation of antimicrobial activity of six medicinal plants against dental pathogens. Rep Opin. 2010;2:37–42. [Google Scholar]
- 37.Bohora A, Hegde V, Kokate S. Comparison of antibacterial efficiency of neem leaf extract and 2% sodium hypochlorite against E. faecalis, C. Albicans and mixed culture. Endodontology. 2010;22:10–3. [Google Scholar]
- 38.Dhanya Kumar NM, Sidhu P. The antimicrobial activity of Azadirachta Indica, Glycyrrhiza glabrat, Cinnamum zeylanicum, Syzygium aromaticum, Acacia nilotica on Streptococcus mutans and Enterococcus faecalis: An in vitro study. Endodontology. 2011;23:18–25. [Google Scholar]
- 39.Prashant GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: An in vitro study. Indian J Dent Res. 2007;18:148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 40.Botelho M, Santos AD, Martin J, Carvalho C, Paz M, Azenha C, et al. Efficacy of a mouthrinse based on leaves of neem in the treatment of patient s with chronic gingivitis. J Med Plant Res. 2008;2:341–6. [Google Scholar]
- 41.Behl M, Sidhu O, Kumar V, Singh D, Saimbi C. Efficacy of neem active metabolites for prevention of dental plaque and gingivitis. Neem Foundation. 2002 [Google Scholar]
- 42.Lessa FC, Nogueira I, Vargas Fda S, Spolidorio DM, Hebling J, García-Godoy F, et al. Direct and transdentinal antibacterial activity of chlorhexidine. Am J Dent. 2010;23:255–9. [PubMed] [Google Scholar]
- 43.Denton GW. Chlorhexidine. In: Block SS, editor. Disinfection, Sterilization and Preservation. 4th ed. Philadelphia: Lea and Febiger; 1991. pp. 274–89. [Google Scholar]
- 44.Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 45.Dammaschke T, Nina J, Inga H, Schafer E. The effect of different root canal medicaments on the elimination of Enterococcus faecalis-ex vivo. Eur J Dent. 2013;7:442–8. doi: 10.4103/1305-7456.120683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 47.Bansal D, Bhasin P, Anita P, Sehrawat AR. Evaluation of antimicrobial activity and phytochemical screening of extracts of Tinospora cardifolia against some pathogenic microbes. J Pharm Res. 2012;5:127–9. [Google Scholar]
- 48.Duraipandiyan V, Ignacimuthu S, Balakrishna K. Antimicrobial activity of Tinospora cardifolia: an ethnomedicinal plant. Asian J Tradit Med. 2012;7:59–65. [Google Scholar]
- 49.Upadhyay R, Tripathi R, Ahmad S. Antimicrobial activity of two Indian medicinal plants-Tinospora Cardifolia and Cassia Fistula against human pathogenic bacteria. J Pharm Res. 2011;4:167–70. [Google Scholar]


