Abstract
Objective:
The purpose of this study was to investigate and compare the crystalline structures of recently released MTA Plus (MTA-P), MTA Angelus (MTA-A), DiaRoot BioAggregate (BA) by X-ray diffraction (XRD) analysis.
Materials and Methods:
Phase analysis was carried out on powder and set forms of tested materials. The powder specimens placed into sample holders that were packed with a glass slide and the set samples prepared according to the manufacturer's instructions were placed into molds. The samples after being set for three days at 37°C and 100% humidity in an incubator were mounted onto the XRD machine and phase identification was accomplished using a search-match software program.
Results:
XRD findings indicated that major constituents of MTA-P were bismuth oxide, portlandite, dicalcium silicate and tricalcium silicate. The crystal structure of MTA-A were similar to those of MTA-P except for the absence of portlandite. Additionally, MTA-A had tricalcium aluminate differing from MTA-P. BA mainly differed from MTA-P and MTA-A by the radiopacifier (tantalum oxide-TO) in its composition.
Conclusions:
The majority of constituents of the tested materials have shown similarity except for the presence of tricalcium aluminate in MTA-A and the inclusion of TO in BA. In addition, set MTA-P showed a strong peak of portlandite.
Keywords: Dental materials, mineral trioxide aggregate, tricalcium silicate, x-ray diffraction
INTRODUCTION
Mineral trioxide aggregate (MTA) was first introduced in 1990s[1] and it has been proven to be a superior material regarding with its excellent sealing ability, biocompatibility and hard-tissue forming capacity.[2,3,4] It also possesses ideal properties such as its antimicrobial effect, dimensional stability, radiopacity and tolerance to moisture.[5,6] Clinically, MTA is currently being used for a variety of endodontic and restorative dental applications such as vital pulp therapy, apexification, repair of root perforations, and root-end filling.[7]
Although MTA is considered to have ideal properties, its usage remained limited due to its high-cost, difficult handling characteristics, long setting time and potential of discoloration.[7] These shortcomings of MTA led to the continuous efforts in developing the modified versions of MTA. First modification was performed on the original gray formula (ProRoot MTA) by the Manufacturer (Dentsply Tulsa Dental, Tulsa, OK, USA) producing a tooth-colored formulation (White MTA) in order to overcome the potential discoloration problem. White MTA mainly differs from the gray MTA in the absence of iron.[5,8,9] In 2001, MTA Angelus (MTA-A) was introduced as an alternative to ProRoot MTA and used in certain regions with its lower price. The chemical difference between ProRoot MTA and MTA-A is that MTA-A lacks calcium sulfate dehydrate as one of its main compounds, resulting in that MTA-A has shorter setting time than ProRoot MTA (165 minutes for ProRoot MTA, while 10 minutes for MTA-A).[10,11] MTA-A is less radiopaque than ProRoot MTA due to the lower content of bismuth oxide in its composition.[12] BioAggregate (BA) was released in 2007 as a bioceramic material, in which most of the constituents are similar to those in white MTA. It differs from MTA by being aluminum -free and containing calcium phosphate monobasic and tantalum pentoxide.[13,14] Aluminum-free content is one of the most significant features of the BA since it is known that aluminum has a toxic effect on the human body.[15] A novel MTA material called MTA-Plus (MTA-P), which is claimed to have a finer particle size than the currently available MTA products that has been introduced on the market.[16] Because MTA-P has been recently available in the market, there are not many studies reported on this material.
X-Ray diffraction (XRD) is a powerful method used for the identification and characterization of the crystalline phase composition of the materials.[17] Although XRD analysis of the relatively new material, MTA-P, has recently been carried out[18] and studies comparing the major constituents of this material with other MTA derivatives are still lacking. The purpose of this study was to investigate and compare the crystalline structures of MTA-P, white MTA-A and Diaroot BA by XRD analysis.
MATERIALS AND METHODS
Materials used in this study included MTA-P (compounded by PrevestDenpro, Jammu, India for Avalon Biomed Inc., Bradenton, FL, USA), white MTA-A (Angelus, Londrina, PR, Brazil) and DiaRoot BA (Innovative BioCeramix, Vancouver, BC, Canada). The MTA-P is provided with either water or a gel for mixing. In the current study, water was used for mixing.
Phase analysis was carried out on both the powder and the set forms of tested materials. For the powder form, each specimen was placed into the sample holder and packed with a glass slide to provide a uniform surface. For the set form, each mixture was prepared according to their manufacturer's instructions was placed into a mold and the upper surface of the specimen was swept with a spatula. The set samples were set for 3 days at 37°C and 100% humidity in the incubator. Both powder and set materials were then mounted for XRD analysis. An X-ray diffractometer (X’Pert Pro MPD, PANalytical, ALMELO, the Netherlands) with Ni filter and CuKa radiation running at 45 kV voltage and 40 mA current was used in the present study. The scan range was set at 2–70°2θ and with a scan speed of 2°2θ per minute.
Each phase (crystalline substance) of a compound has a characteristic diffraction pattern consisting of several X-ray peaks. The peaks at the specified intensity representing the diffraction patterns of the tested materials were matched with the standard data documented in the Powder Diffraction Files (PDF) found in the International Centre for Diffraction Data (ICDD) database.
RESULTS
The XRD results of the samples are shown in Figures 1–3.
Figure 1.
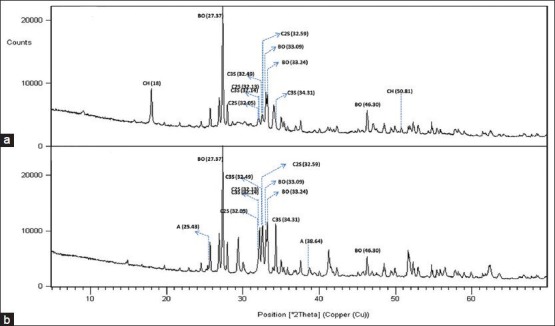
X-Ray diffraction patterns of MTA-Plus in the set (a) and powder (b) forms. (BO, bismuth oxide; C3S, tricalcium silicate, C2S, dicalcium silicate; CH, calcium hydroxide; A, anhydrite)
Figure 3.
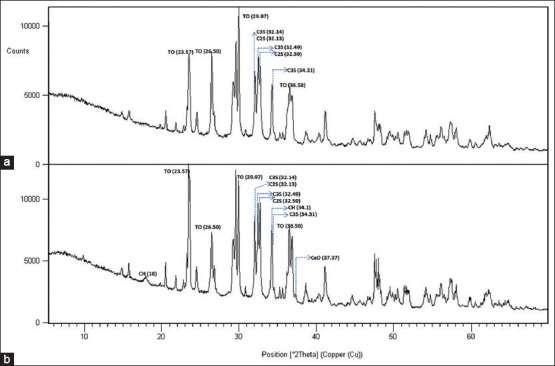
X-Ray diffraction patterns of BioAggregate in the set (a) and powder (b) forms. (TO, tantalum oxide; C3S, tricalcium silicate, C2S, dicalcium silicate; CH, calcium hydroxide)
Figure 2.
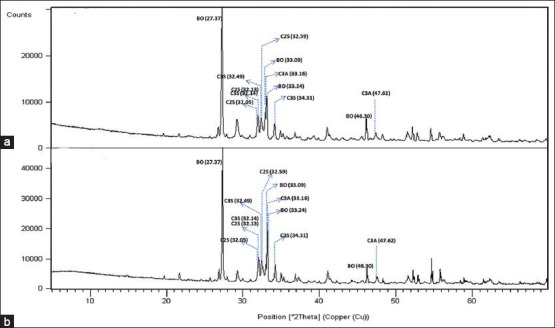
X-Ray diffraction patterns of MTA-Angelus in the set (a) and powder (b) forms. (BO, bismuth oxide; C3S, tricalcium silicate, C2S, dicalcium silicate; C3A, tricalcium aluminate)
Both MTA-P and MTA-A showed strong peaks of bismuth oxide at 27.37, 33.03 and 46.30° 2θ. There were no peaks for bismuth oxide in the BA sample. It showed large peaks at 29.97, 26.50 and 36.58° 2θ representing tantalum oxide. All samples comprised tricalcium silicate indicated by the main peaks at 32.14°, 32.49° and 34.31° and dicalcium silicate indicated by the main peaks at 32.05°, 32.13° and 32.59° 2θ.
MTA-A showed peaks at 33.16, 47.62 and 59.27° 2θ representing tricalcium aluminate. Portlandite (calcium hydroxide) was identified in MTA-P groups and powder form of BA. Powder form of BA showed also peaks for CaO (Lime). Anhydrite was observed in powder MTA-P and ettringite (hexacalcium aluminate trisulphate hydrate) was observed in the set MTA-P.
DISCUSSION
The identification of the major constituents or compounds present in a material is important as it will contribute to understanding of the material's physical, chemical and mechanical properties. The use of XRD permits the identification of the major constituents or compounds present in a material.[19] The key principle of the technique is based on identifying the diffraction pattern of each crystalline phase characterized by a unique set of peaks (known as Bragg's peaks), with a specific diffracted intensity (y-axis) and diffracted angle at a specified position. Phase identification is accomplished by comparing the data of the tested specimens by using peaks and relative intensities with a very large set of “standard” data provided by the ICDD.[17,20]
MTA contains largely crystalline phases, with the calcium silicate hydrate being the only amorphous phase.[21] All the tested materials in this study had two common constituents, tricalcium silicate and dicalcium silicate. Tricalcium silicate is the major component in the formation of calcium silicate hydrate which gives early strength to the cement. Dicalcium silicate hydrates more slowly than tricalcium silicate and is responsible for the latter strength.[22,23] MTA-A has also included the tricalcium aluminate phase as one of its main phases. Tricalcium aluminate is the most reactive constituent and reacts rapidly with water, however, its contribution to the strength is very little.[23]
The crystal structure of BA has been recently investigated by XRD analysis and all the crystalline phases were identified.[13,24] The XRD results obtained from BA samples in this study were largely similar to those of Park et al.[13] Tantalum oxide, tricalcium silicate, dicalcium silicate demonstrated strong peaks in BA samples. BA also includes hydroxyapatite and amorphous silicon oxide together with the main cementitious and radiopacifier phases. The hydroxyapatite and amorphous silicon oxide are added by the manufacturer to reduce the levels of the calcium hydroxide, which is a weak hydration product of calcium silicates. Calcium hydroxide is leached in solution and is not cementitious. Thus, it does not contribute to the strength of the material.[14,24] The Portlandite peak on XRD analysis indicates the formation of calcium hydroxide. Portlandite was identified in the powder form of BA and this is in contrast to the study by Park et al., which demonstrated portlandite only in set samples.[13] Hydroxyapatite peaks were not observed in this study differing from the study by Park et al. The absence of aluminum specified by the manufacturer was also verified in the present study.
MTA-P is a new version of mineral trioxide aggregate, which is claimed to be similar in composition to the other MTA products. It has been released by the manufacturer highlighting the features of finer particle size and lower cost. MTA-P is sold with either distilled water or anti-washout gel intending to improve its anti-washout resistance.[16] Camilleri et al. determined that the crystalline particles in MTA-P were smaller than those present in ProRoot MTA although the chemical compositions were found to be similar.[18] Smaller particle size is important for the physical properties as it will increase the surface available for hydration and cause greater early strength as well as ease of handling.[6]
There are not many studies published comparing the new MTA-P and the other MTA products, which have been extensively investigated. Formosa et al. were first to investigate the chemical and physical properties of the novel MTA-P.[21] They used the XRD method to identify the crystalline phases of MTA-P and made a comparison between its two set forms, one mixed with the anti-washout gel and the other mixed with the distilled water. The phases identified in their study were similar for each mixture and included tricalcium silicate, dicalcium silicate, bismuth oxide and portlandite, which also corroborated with the findings of this study. They also observed weak peaks of ettringite, which were also identified in the set sample of MTA-P of the present study. It is known that the ettringite peaks are formed as a result of reaction between tricalcium aluminate and water in the presence of calcium sulfate.[18,25] Our results showed that MTA-P powder consisted of anhydrite as the calcium sulfate phase and it is likely that small amounts of tricalcium aluminate are present considering the production of ettringite in set samples. The aluminate phase results from the addition of alumina in the mixture and alumina helps reducing the burning temperatures required to make the cement.[17] The quantity of anhydrite is important for controlling the “flash set” phenomenon attributed to the high reactivity of the aluminate phase with water. Aluminate reacts quickly with water leading to an undesirable shortening of the setting time. It can be controlled by adding a sulfate-involving agent such as gypsum or anhydrite, which delays the setting time.[17,26] Although MTA-A had an aluminate phase, it did not contain calcium sulfate and this results in a shorter setting time (~10 min) of the material.
MTA-P and MTA-A contained the same constituent, bismuth oxide, as a radiopacifier while BA included tantalum oxide instead. All these phases were also verified by XRD analysis in this study. Considering that tantalum oxide is the major difference between MTA and BA, it may be important to examine the differences between bismuth oxide and tantalum oxide in terms of toxicity and biocompatibility. The effects of containing tantalum oxide instead of bismuth oxide are not known well.[13] Bismuth oxide, which is one of the main compounds of MTA, may not contribute to its excellent biocompatibility. Steinemann reported a strong inhibition zone when osteoblasts were grown, whereas fibroblasts proliferated well on the tantalum disc.[27] Camilleri et al. reported no cell growth over bismuth oxide. However, they could not infer whether those results were due to the surface roughness or the chemical nature of the material.[28]
The set MTA-P exhibited strong peak of calcium hydroxide, which is in accordance with the results of a previous study by Camilleri et al.[18] The calcium hydroxide peak in this study was found to be much more pronounced in MTA-P than in the other MTA products tested. The high portlandite peak, which is indicative of a greater rate of reaction, is reported to be related to the fine grinding of MTA-P cement.[18]
CONCLUSION
All the tested materials were similarly composed of tricalcium silicate and dicalcium silicate crystalline structures. MTA-P showed anhydrite in a powder sample and ettringite in a set sample differing from MTA-A and BA. In addition, set MTA-P exhibited a strong peak of portlandite, which is attributable to its fine grinding property. MTA-A differed from MTA-P and BA by the presence of tricalcium aluminate phase. BA mainly differed from MTA-A and MTA-P by the radiopacifier added. MTA-P and MTA-A contained the same constituent, bismuth oxide, as a radiopacifier while BA included tantalum oxide instead.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19:541–4. doi: 10.1016/S0099-2399(06)81282-3. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Parirokh M. Mineral trioxide aggregate: A comprehensive literature review-part II: Leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Yáñez Sánchez A, Leco-Berrocal MI, Martínez-González JM. Metaanalysis of filler materials in periapical surgery. Med Oral Patol Oral Cir Bucal. 2008;13:E180–5. [PubMed] [Google Scholar]
- 4.Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37:432–9. doi: 10.1002/(sici)1097-4636(19971205)37:3<432::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:809–15. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31:101–3. doi: 10.1097/01.don.0000133156.85164.b2. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira MG, Xavier CB, Demarco FF, Pinheiro AL, Costa AT, Pozza DH. Comparative chemical study of MTA and Portland cements. Braz Dent J. 2007;18:3–7. doi: 10.1590/s0103-64402007000100002. [DOI] [PubMed] [Google Scholar]
- 11.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri J, Gandolfi MG. Evaluation of the radiopacity of calcium silicate cements containing different radiopacifiers. Int EndodJ. 2010;43:21–30. doi: 10.1111/j.1365-2591.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 13.Park JW, Hong SH, Kim JH, Lee SJ, Shin SJ. X-Ray diffraction analysis of white ProRoot MTA and Diadent BioAggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:155–8. doi: 10.1016/j.tripleo.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Diadent DiaRoot® BioAggregate material safety data sheet. [Last accessed on 2013 Feb 10]. Available from: http://www.henryschein.ca/MSDS/105Y951.pdf .
- 15.Forbes WF, Gentleman JF. Risk factors, causality, and policy initiatives: The case of aluminum and mental impairment. Exp Gerontol. 1998;33:141–54. doi: 10.1016/s0531-5565(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 16.MTA Plus Directions for use. [Last accessed on 2013 Feb 15]. Available from: http://avalonbiomed.com/wp-content/uploads/2012/07/DFUs-MTAPLUS-6-30-12EN.pdf .
- 17.Belío-Reyes IA, Bucio L, Cruz-Chavez E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder diffraction. J Endod. 2009;35:875–8. doi: 10.1016/j.joen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri J, Formosa L, Damidot D. The setting characteristics of MTA Plus in different environmental conditions. Int Endod J. 2013;46:831–40. doi: 10.1111/iej.12068. [DOI] [PubMed] [Google Scholar]
- 19.Islam I, Chng HK, Yap AU. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J. 2006;39:220–5. doi: 10.1111/j.1365-2591.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 20.Pecharsky VK, Zavalij PY. 2nd ed. New York: Springer; 2009. Fundamentals of Powder Diffraction and Structural Characterization of Materials; pp. 377–399. [Google Scholar]
- 21.Formosa LM, Mallia B, Camilleri J. Mineral trioxide aggregate with anti-washout gel-properties and microstructure. Dent Mater. 2013;29:294–306. doi: 10.1016/j.dental.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SA, Chang TN. The hydration of tricalcium silicate. J Phys Chem. 1965;69:553–61. [Google Scholar]
- 23.Wang WH, Wang CY, Shyu YC, Liu CM, Lin FH, Lin CP. Compositional characteristics and hydration behavior of mineral trioxide aggregates. J Dent Sci. 2010;5:53–9. [Google Scholar]
- 24.Grech L, Mallia B, Camilleri J. Characterization of set ýntermediate restorative material, biodentine, bioaggregate and a prototype calcium silicate cement for use as root-end filling materials. Int Endod J. 2013;46:632–41. doi: 10.1111/iej.12039. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008;11:141–3. doi: 10.4103/0972-0707.48834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondal P, Jeffery JW. The crystal structure of tricalcium aluminate, Ca3Al2O6. Acta Cryst. 1975;B31:689–97. [Google Scholar]
- 27.Steinemann SG. Titanium-the material of choice? Periodontol 2000. 1998;17:7–21. doi: 10.1111/j.1600-0757.1998.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J. 2004;37:699–704. doi: 10.1111/j.1365-2591.2004.00859.x. [DOI] [PubMed] [Google Scholar]


