Abstract
Objective:
The aim of this study is to evaluate four different pulpotomy medicaments in primary molars.
Materials and Methods:
A total of 147 primary molars with deep caries were treated with four different pulpotomy medicaments (FC: formocresol, FS: ferric sulfate, CH: calcium hydroxide, and MTA: mineral trioxide aggregate) in this study. The criteria for tooth selection for inclusion were no clinical and radiographic evidence of pulp pathology. During 30 months of follow-up at 6-month intervals, clinical and radiographic success and failures were recorded. The differences between the groups were statistically analyzed using the Chi-square test and Kaplan-Meier analysis.
Results:
At 30 months, clinical success rates were 100%, 95.2%, 96.4%, and 85% in the FC, FS, MTA, and CH groups, respectively. In radiographic analysis, the MTA group had the highest (96.4%), and the CH group had the lowest success rate (85%). There were no clinical and radiographic differences between materials (P > 0.05).
Conclusions:
Although there were no differences between materials, only in the CH group did three teeth require extraction due to further clinical symptoms of radiographic failures during the 30-month follow-up period. None of the failed teeth in the other groups required extraction during the 30-month follow-up period.
Keywords: Calcium hydroxide, ferric sulfate, formocresol, MTA, pulpotomy
INTRODUCTION
In pediatric dentistry, pulpotomy is a common therapy performed in a primary molar with extensive caries but without evidence of radicular pathology when caries removal results in a carious or mechanical pulp exposure. The pulpotomy procedure involves covering pulp stumps with a pulp-capping agent to promote healing or an agent to fix the underlying tissue.[1] Various pulpotomy agents, formaldehyde-based materials, electro surgery, lasers, glutaraldehyde, haemostatic medicaments, zinc oxide eugenol, bone morphogenic protein (BMP), collagen and calcium involving, dentin bridge inducing materials, have been recommended from the past to the present. The first pulpotomy agent used for primary teeth, introduced by Sweet, was formocresol (FC), which fixes and mummifies the tissue completely.[2] However, the ideal pulpotomy treatment should leave the radicular pulp vital and healthy and completely enclosed within an odontoblast-lined dentin chamber;[3] calcium hydroxide (CH) was the first medication that induced dentin bridge formation in pulpotomies.[4] Another alternative pulpotomy agent, ferric sulpfte (FS), a haemostatic medicament, has been used because it might minimize the chances for inflammation and thereby prevent internal resorption (IR).[3,5] In 1995, Torabinejad et al. described mineral trioxide aggregate (MTA) a biocompatible, dentin bridge inducing material and was used as a pulpotomy agent.[6]
Evidence is lacking to determine which material is the most appropriate for primary teeth pulpotomies.[7] To make a decision, it is necessary to examine materials long term.
The aim of this study was to evaluate the clinical and radiographic successes of FC, FS, CH, and MTA as primary teeth pulpotomy agents during a 30-month period. This study tested the null hypothesis that there are no differences among pulpotomy materials inducing dentin bridge formation and fixative or haemostatic agents.
MATERIALS AND METHODS
This study was approved by the Ethics Committee (July 27, 2006/47). This study was performed on 147 primary molars on 88 children aged between 5 and 9 years and systemically healthy. The parents of the children received detailed information concerning the procedures, benefits, and possible risks involved in the study and signed informed consent forms. The pulpotomies were performed by one pediatric dentist under local anesthesia.
The criteria for selecting the teeth to be included in the study were as follows:
Symptomless, cariously exposed vital teeth;
No clinical symptoms or evidence of pulp degeneration, such as history of spontaneous pain and tenderness to percussion, history of swelling or sinus tracts, pathologic mobility;
Teeth were restorable;
No radiographic evidence of pulp degeneration such as internal or external resorption, inter-radicular, and/or periapical bone destruction or pulp stones;
No clinical evidence of pulp degeneration such as excessive bleeding from root canals.
After local anesthesia was administered, caries were removed, and cavity access to the pulp was obtained using a high-speed bur (#330) with water spray. Coronal pulp was amputated using a low-speed sterile round bur (No = #6 or No = #8) with water spray, and hemorrhaging was controlled using sterile saline blotted sterile pellets. If the hemorrhage was not controlled within 5 min,[8] the tooth was excluded from the study. If a child had more than one molar needing pulpotomy, the material used for pulpotomy was randomly selected.
FC pulpotomy
A sterile cotton pellet was moistened with the FC solution and blotted dry on another sterile pellet. The pellet was placed directly over the radicular pulp stumps for 5 min and then pulled away from the cavity. Pulp stumps were covered with zinc oxide eugenol (ZOE) cement (Cavex zinc oxide eugenol cement, Haarlem, Holland). Glass ionomer cement (Voco Argion molar, Cuxhaven, Germany) was placed over the ZOE cement. The restorations were completed with composite resin (Clearfil AP-X, Kuraray Medical Inc, Tokyo, Japan).
FS pulpotomy
A solution of 15.5% FS (Astringedent, Ultradent Products Inc, Salt Lake City, USA) was applied to the pulp stumps for 15 s. The cavity was flushed with water by an air-water syringe and gently dried.
CH pulpotomy
The pulp stumps were gently covered with CH (Sultan Chemists, Englewood, USA) paste with an amalgam condenser and small cotton wool pellets.
MTA pulpotomy
The pulp stumps were covered with an MTA (ProRootMTA, Dentsply, Tulsa, USA) paste obtained by mixing MTA powder with sterile water at a 3:1 powder to water ratio according to manufacturer's instruction.
All the restorations were completed with the same method as in the FC pulpotomy.
The patients were recalled for clinical and radiographic examination after 6, 12, 18, 24, and 30 months, and examinations and failures were determined.
Restorations of pulpotomized primary molar teeth evaluations were carried out at baseline and control sessions under normal clinical conditions with a dental operating light, mouth mirror, and dental explorer. They were examined regarding marginal discoloration, anatomic form, marginal integrity, and recurrent caries. Clinically unacceptable situations were recorded, and teeth with failed restoration were excluded from the study to ensure that the pulpotomy success rate was not affected.
The clinical and radiographic outcomes of the teeth were assessed by two unblinded standardized pediatric dentist. Teeth were scored as clinically successful if they had no pain symptoms, tenderness to percussion, swelling, fistulation with sinus tract, or pathologic mobility.
Periapical radiographs were taken with the parallel technique using a film holder (Rinn XCP Instrument Kit, Dentsply, USA). Teeth were scored as radiographically successful if they showed no evidence of radicular radiolucency, internal or external resorption, or periodontal ligament space widening. Any radiographic evidence of pulp canal obliteration (PCO) was not regarded as a failure or success. On clinical and radiographic examination, if any of these criteria was observed, the treatment was recorded as unsuccessful.
The differences between the four materials were statistically analyzed using the Chi-square test at 6, 12, 18, 24, and 30 months. The groups’ survival time was analyzed with the Kaplan-Meier test. A log-rank test was conducted to compare the four groups’ survival rate.
To test radiographic failures, the Chi-square test was used to analyze the differences between age groups. The difference between first and second molars, and teeth that have no root resorption and teeth that have root resorption of less than one-fourth of the root was analyzed by Fisher's exact test and Chi-square test.
For all of the statistical analyses, the significance level was set at P ≤ 0.05.
RESULTS
This study was performed on 44 boys and 44 girls, a total of 88 children aged between 5-9 years old. A total of 147 pulpotomies were performed using four different pulpotomy materials. At the end of 30 months, only 90 teeth were evaluated because patients did not attend the follow-up visits. Before the clinical and radiographic examinations of the pulpotomies, restorations of pulpotomized primary molar teeth evaluations were carried out at control sessions. Seven pulpotomized molars (4.7%) with failed restorations were excluded from the study according to the criteria described in the methods section.
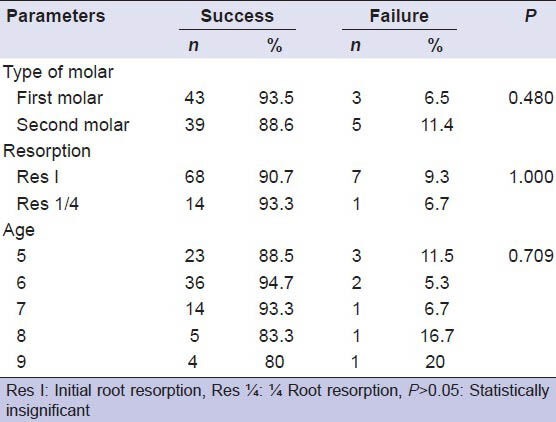
The differences between the success rates of teeth according to age groups, first and second molars, and teeth that have no preoperative physiological root resorption and teeth that have preoperative physiological root resorption of less than one-fourth of the root were analyzed. Statistical analyses revealed that these parameters were not affected by the success of the pulpotomies (P > 0.05) [Table 1].
Table 1.
Distribution of the success and failure rates of teeth according to type of molar, resorption degree, and age
The clinical and radiographic success rates of the pulpotomy agents were compared using a Chi-square test for each follow-up period. There were no statistical differences between the materials’ success rates at each follow-up period in terms of clinical and radiographic successes [Tables 2 and 3].
Table 2.
Clinical success and failure rates of materials
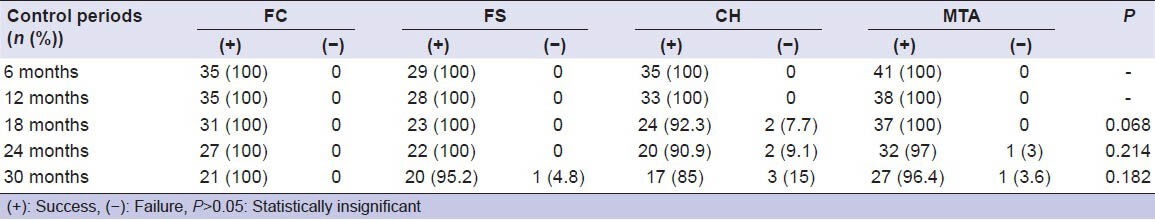
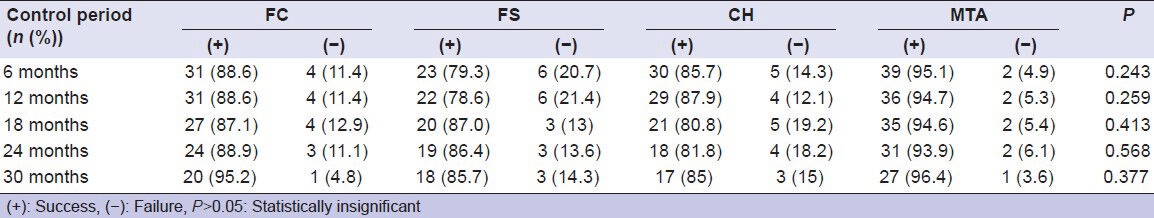
Table 3.
Radiographic success and failure rates of materials
According to the clinical exams, no failure was observed in the FC during the 30-month period. In the FS, 1 tooth was considered failed because of tenderness to percussion. One tooth in the MTA was recorded as unsuccessful due to tenderness to percussion. In the CH, three teeth were observed as unsuccessful. One tooth was diagnosed as having tenderness to percussion after 18 months. Spontaneous pain and tenderness to percussion were diagnosed in a second tooth that had IR in the sixth month, so the tooth was extracted. At the end of the 30 months, tenderness to percussion, spontaneous pain, fistula, and mobility were diagnosed in the third tooth and this tooth was also extracted.
Radiographic failures were observed as IR, furcation and periapical radiolucency. In this study, IR was observed in four teeth (11.47%) in the FC, seven (24.13%) in the FS, five (14.28%) in the CH, and two (4.87%) in the MTA.
In the FC, IR in three teeth and furcation radiolucency in one tooth were observed at 6 months, but furcation radiolucency did not progress in time. Teeth that had IR were not extracted as they had no clinical symptoms. At 18 months, one additional tooth had IR. In the FS, six teeth had IR at 6 months. At 18 months one additional tooth had IR. At 30 months, one additional tooth was recorded as unsuccessful due to furcation radiolucency. In the CH, five teeth showed IR at 6 months. At 18 months, one additional tooth failed due to furcation and periapical radiolucency. In the MTA, two teeth showed IR, but resorption did not progress and perforate the root; moreover, in one tooth, dentin deposition was observed.
PCO and dentin bridge that were not recorded as a failure or success were also observed. PCO were observed in two teeth in the CH (6%), one tooth in the MTA (2%), nine teeth in the FC (26%), and six teeth in the FS group (21%). Dentin barriers were observed in eight teeth in the CH (23%) and 11 teeth in the MTA (27%).
In the 30-month period, 19 teeth were exfoliated within normal physiological exfoliation time and 7 of these had IR.
According to the Kaplan-Meier analysis, there were no differences among the materials’ survival times (P = 0.069).
DISCUSSION
In teeth with deep caries lesions, pulpotomy treatment is common therapy when the microorganisms or their toxins may have reached the pulp. In this study, FC, CH, FS, and MTA, which has been used recently as pulpotomy materials, were evaluated.
In various studies, primary teeth were restored with a stainless steel crown (SSC) after endodontic treatments.[9,10] Guelmann et al. reported in their in vitro study that SSC did not prevent microleakage even if the marginal adaptation was perfect.[11] On the contrary, adhesive resin restorations preserve sound tooth tissue and decrease microleakage as well as providing esthetic demands.[11,12] In addition, when compared with amalgam and SSC, composite resins are reported to be successful in restoring endodontically treated primary teeth.[13,14] In this study, composite resin was used to restore the pulpotomized teeth, and at the end of 30 months, few failures were recorded for the restorations. In the MTA group, gray discolorations which were probably associated with MTA were observed on the crown portion of the teeth, but the colorations did not seem to be affecting the success of the treatment. This result may confirm that composite resins can be an alternative for restoring pulpotomized primary teeth.
According to the Chi-square test, there were no differences among the groups for all follow-up periods. The results of this study support the hypothesis that there are no differences among pulpotomy materials inducing dentin bridge formation and fixatives or haemostatic agents.
High clinical and radiographic success rates were reported for FC.[8,15,16,17,18] In this study, the FC group had 100% clinical and 95.2% radiographic success rate at 30 months, similar to that reported earlier. In contrast to these rates, Sonmez et al. reported a 76.9% success rate for the FC group.[19] Many of the studies reported insignificant differences among FC and MTA or FS,[8,15,17,18,19] while CH reported to be worse than FC, FS, and MTA.[8,15] However, CH was reported to have the similar success rate with FC and FS[19,20] which is consistent with those of given in this study.
Eighty-nine percent to hundred percent clinical[8,18,20] and 86-97% radiographic success rates[8,18,19,20,21] for FS pulpotomies were reported. Differently from these studies, and lower clinical (67%)[22] and radiographic success rates (73%) have also been reported.[19] In this study, a 95.2% clinical and 85.7% radiographic success rate was observed in the FS group.
In the studies about CH pulpotomy, Moretti et al. reported a 71% success rate at 12 months.[15] Markovic et al. reported an 82% clinical success rate at 18 months.[20] Huth et al. reported 87% success rate after 24 months.[8] Studies have reported 80-88% radiographic success rates in CH.[8,20,23,24] In this study, the clinical success rate in the CH group was 85% at 30 months, which is similar to Huth et al. results and 85% radiographic success rate was observed consistent with the studies reported earlier.
In the studies that assessed MTA as a pulpotomy agent, 94-100% clinical success rates were reported.[6,16,17,25] MTA was reported to be successful in some studies, showing 94-100% radiographic success rates.[15,17,25,26] In this study, a 96.4% clinical and radiographic success rate was observed in the MTA, similar to these reports. In contrast to these results, Sönmez et al. reported a 67% success rate.[19] The difference may be the restoration technique. A wet cotton pellet was placed over the MTA for a day, and then the final restoration was performed.[19] In this study, the MTA pulpotomy was performed according to the manufacturer's recommendation.
IR after pulpotomy is the most common finding and the most speculated- about. As in many articles,[10,15,16] IR was regarded as a failure in this study. The etiology of IR is thought to be the result of chronic pulpitis.[27] It was thought that the risk of IR may increase since FC and FS do not have the ability to induce new dentin deposition.[18] However, none of the FS and only one of the FC in Sönmez et al. study exhibited failure caused by IR, while IR was observed in the CH and MTA.[19]
IR occurred after CH pulpotomy thought to be a result of direct contact of CH with pulp tissue.[28] In this study and other pulpotomy studies,[6,8,19] IR was observed in all the treatment groups, indicating that it cannot be associated only with CH. It was advocated that CH could stimulate the healing process only in healthy pulp.[29] These failures may be due to the presence of infection that could not be diagnosed.
In Holan et al. study, IR was detected in the MTA and was found to be arrested and calcifications were observed.[6] In this study, two teeth showed IR in the MTA that ceased without any root perforation; moreover, calcifications were observed as in Holan et al. study. The satisfactory results for MTA may be due to hard tissue formation properties and good sealing ability.
In the pulpotomized teeth, dentin bridge formation is not always regarded as a success. Caicedo et al. observed dentin bridge in teeth have infection and thought that infection may have occurred after dentin bridge formation.[30] In contrast, it was reported that dentin bridge may occur as a response by infected pulp.[27,31] In this study, as in others,[10,26] dentin bridges were not considered successful and were observed in the CH (23%) and MTA (27%) groups. In the CH group, a tooth showed a dentin bridge in distal root while IR was observed in the mesial root. It was thought that after the healing process was failed, resorption may have occurred.[27]
According to the Chi-square test and Kaplan-Meier analysis, the groups’ success rates were statistically similar, but the MTA showed a higher radiographic success rate than the CH. MTA and CH stimulate dentin bridge formation probably because of their biocompatibility and alkalinity. However, the most important distinctive feature of MTA is its good sealing ability, and it shows no signs of solubility.[32] The solubility of CH[33] may cause bacterial microleakage from the furcation or coronal restoration.[27] Dentin bridges in MTA pulpotomies are reported be more homogeneous and continuous than in CH,[26,34] thus, leading clinical and radiographic failures of CH related to this matter. The success of FC is thought to be related to its fixative characteristic.[6] However, evidence of purulent exudates in FC as in CH pulpotomy was observed by Waterhouse et al.[27] While FS was the most failed group in terms of IR, only one tooth showed clinical symptoms. However, three of five teeth were unsuccessful in the CH during radiographic observation, and showed clinical symptoms at later periods. Although the radiographic failure rates of these two groups were similar, further clinical failure of radiographic pathologic findings was more common in the CH.
In a histological study, pulp responses of a formaldehyde-based Pulpotec cement and a haemostatic agent, collagen particles were reported to be similar.[35] Another haemostatic agent, Ankaferd, was reported to have 85.7% success rates and have similar success rate with FC.[36] Materials which contain similar chemical elements with MTA are also evaluated in some studies.[37,38,39] Calcium-enriched mixture was evaluated as successful as MTA but superior than CH.[37] Portland cement also showed similar results with MTA.[34,39] Insignificant difference was reported in an histological assessment between calcium phosphate cement and formocresol.[38] Many of the pulpotomy studies show that there were insignificant differences among formaldehyde-based materials, hemostatic agents and dentin bridge inducing materials such as MTA and CH. High clinical success rates for FC, MTA, CH, and FS were observed in this study, which means there was no evidence that any of these materials is better than the others.
The most common failure was IR that was seen in all groups. Follow-up of pulp therapies is essential as IR, which rarely causes clinical symptoms, and is generally evaluated in radiographic examination. Chronically inflamed pulp may be initially symptom free, but acute exacerbation may occur. The only group that had clinical failed teeth requiring extraction was CH. None of the failed teeth required extraction during 30 months in the other groups.
It is essential to understand that after clinical and radiographic evaluations are performed carefully to achieve correct diagnosis, all the evaluated materials can be used successfully.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Camp JH, Fuks AB. Pediatric endodontics: Endodontic treatment fort the primary and young permanent dentition. In: Cohen S, Hargreaves KM, editors. Pathways of the Pulp. 9th ed. St. Louis: Mosby Elseiver; 2006. pp. 822–53. [Google Scholar]
- 2.Sweet CA. Procedure for treatment of exposed and pulpless deciduous teeth. J Am Dent Assoc. 1930;17:1150–3. [Google Scholar]
- 3.Ranly DM. Pulpotomy therapy in primary teeth: New modalities for old rationales. Pediatr Dent. 1994;16:403–9. [PubMed] [Google Scholar]
- 4.Zander HA. Reaction of the pulp to calcium hydroxide. J Dent Res. 1939;18:373–9. [Google Scholar]
- 5.Fei AL, Udin RD, Johnson R. A clinical study of ferric sulfate as a pulpotomy agent in primary teeth. Pediatr Dent. 1991;13:327–32. [PubMed] [Google Scholar]
- 6.Holan G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent. 2005;27:129–36. [PubMed] [Google Scholar]
- 7.Nadin G, Goel BR, Yeung CA, Glenny AM. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev. 2003:CD0033220. doi: 10.1002/14651858.CD003220. [DOI] [PubMed] [Google Scholar]
- 8.Huth KC, Paschos E, Hajek-Al-Khatar N, Hollweck R, Crispin A, Hickel R, et al. Effectiveness of 4 pulpotomy techniques--randomized controlled trial. J Dent Res. 2005;84:1144–8. doi: 10.1177/154405910508401210. [DOI] [PubMed] [Google Scholar]
- 9.Maroto M, Barbería E, Planells P, García Godoy F. Dentin bridge formation after mineral trioxide aggregate (MTA) pulpotomies in primary teeth. Am J Dent. 2005;18:151–4. [PubMed] [Google Scholar]
- 10.Sönmez D, Durutürk L. Ca (OH) 2 pulpotomy in primary teeth. Part I: Internal resorption as a complication following pulpotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e94–8. doi: 10.1016/j.tripleo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Guelmann M, Bookmyer KL, Villalta P, García-Godoy F. Microleakage of restorative techniques for pulpotomized primary molars. J Dent Child (Chic) 2004;71:209–11. [PubMed] [Google Scholar]
- 12.el-Kalla IH, García-Godoy F. Fracture strength of adhesively restored pulpotomized primary molars. ASDC J Dent Child. 1999;66:238–42. 228. [PubMed] [Google Scholar]
- 13.Zulfikaroglu BT, Atac AS, Cehreli ZC. Clinical performance of Class II adhesive restorations in pulpectomized primary molars: 12-month results. J Dent Child (Chic) 2008;75:33–43. [PubMed] [Google Scholar]
- 14.Hutcheson C, Seale NS, McWhorter A, Kerins C, Wright J. Multi-surface composite vs stainless steel crown restorations after mineral trioxide aggregate pulpotomy: A randomized controlled trial. Pediatr Dent. 2012;34:460–7. [PubMed] [Google Scholar]
- 15.Moretti AB, Sakai VT, Oliveira TM, Fornetti AP, Santos CF, Machado MA, et al. The effectiveness of mineral trioxide aggregate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int Endod J. 2008;41:547–55. doi: 10.1111/j.1365-2591.2008.01377.x. [DOI] [PubMed] [Google Scholar]
- 16.Farsi N, Alamoudi N, Balto K, Mushayt A. Success of mineral trioxide aggregate in pulpotomized primary molars. J Clin Pediatr Dent. 2005;29:307–11. doi: 10.17796/jcpd.29.4.n80t77w625118k73. [DOI] [PubMed] [Google Scholar]
- 17.Jabbarifar SE, Khademi AA, Ghasemi D. Success rate of formocresol pulpotomy versus mineral trioxide aggregate in human primary molar tooth. J Res Med Sci. 2004;6:304–7. [Google Scholar]
- 18.Ibricevic H, al-Jame Q. Ferric sulfate as pulpotomy agent in primary teeth: Twenty month clinical follow-up. J Clin Pediatr Dent. 2000;24:269–72. doi: 10.17796/jcpd.24.4.d7u6405nw1132705. [DOI] [PubMed] [Google Scholar]
- 19.Sonmez D, Sari S, Cetinbaş T. A comparison of four pulpotomy techniques in primary molars: A long-term follow-up. J Endod. 2008;34:950–5. doi: 10.1016/j.joen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Markovic D, Zivojinovic V, Vucetic M. Evaluation of three pulpotomy medicaments in primary teeth. Eur J Paediatr Dent. 2005;6:133–8. [PubMed] [Google Scholar]
- 21.Vij R, Coll JA, Shelton P, Farooq NS. Caries control and other variables associated with success of primary molar vital pulp therapy. Pediatr Dent. 2004;26:214–20. [PubMed] [Google Scholar]
- 22.Casas MJ, Kenny DJ, Johnston DH, Judd PL. Long-term outcomes of primary molar ferric sulfate pulpotomy and root canal therapy. Pediatr Dent. 2004;26:44–8. [PubMed] [Google Scholar]
- 23.Gruythuysen RJ, Weerheijm KL. Calcium hydroxide pulpotomy with a light-cured cavity-sealing material after two years. ASDC J Dent Child. 1997;64:251–3. [PubMed] [Google Scholar]
- 24.Waterhouse PJ, Nunn JH, Whitworth JM. An investigation of the relative efficacy of Buckley's formocresol and calcium hydroxide in primary molar vital pulp therapy. Br Dent J. 2000;188:32–6. doi: 10.1038/sj.bdj.4800380. [DOI] [PubMed] [Google Scholar]
- 25.Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: A preliminary report. Pediatr Dent. 2001;23:15–8. [PubMed] [Google Scholar]
- 26.Agamy HA, Bakry NS, Mounir MM, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent. 2004;26:302–9. [PubMed] [Google Scholar]
- 27.Waterhouse PJ, Nunn JH, Whitworth JM, Soames JV. Primary molar pulp therapy--histological evaluation of failure. Int J Paediatr Dent. 2000;10:313–21. doi: 10.1046/j.1365-263x.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 28.Schröder U. Effect of an extra-pulpal blood clot on healing following experimental pulpotomy and capping with calcium hydroxide. Odontol Revy. 1973;24:257–68. [PubMed] [Google Scholar]
- 29.Mathewson RJ, Primosch RE. Fundamentals of Pediatric Dentistry. 3rd ed. Chicago: Quintessence Publishing Co; 1995. Pulp treatment; pp. 257–84. [Google Scholar]
- 30.Caicedo R, Abbott PV, Alongi DJ, Alarcon MY. Clinical, radiographic and histological analysis of the effects of mineral trioxide aggregate used in direct pulp capping and pulpotomies of primary teeth. Aust Dent J. 2006;51:297–305. doi: 10.1111/j.1834-7819.2006.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003;29:324–33. doi: 10.1097/00004770-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 33.Miller LB, Witt JC. Solubility of calcium hydroxide. J Phys Chem. 1929;33:285–9. [Google Scholar]
- 34.Menezes R, Bramante CM, Letra A, Carvalho VG, Garcia RB. Histologic evaluation of pulpotomies in dog using two types of mineral trioxide aggregate and regular and white Portland cements as wound dressings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:376–9. doi: 10.1016/S107921040400215X. [DOI] [PubMed] [Google Scholar]
- 35.Kakarla P, Avula JS, Mellela GM, Bandi S, Anche S. Dental pulp response to collagen and pulpotec cement as pulpotomy agents in primary dentition: A histological study. J Conserv Dent. 2013;16:434–8. doi: 10.4103/0972-0707.117525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaman E, Görken F, Pinar Erdem A, Sepet E, Aytepe Z. Effects of folk medicinal plant extract Ankaferd Blood Stopper(® ) in vital primary molar pulpotomy. Eur Arch Paediatr Dent. 2012;13:197–202. doi: 10.1007/BF03262870. [DOI] [PubMed] [Google Scholar]
- 37.Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010;43:565–71. doi: 10.1111/j.1365-2591.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 38.Jose B, Ratnakumari N, Mohanty M, Varma HK, Komath M. Calcium phosphate cement as an alternative for formocresol in primary teeth pulpotomies. Indian J Dent Res. 2013;24:522. doi: 10.4103/0970-9290.118370. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira TM, Moretti AB, Sakai VT, Lourenço Neto N, Santos CF, Machado MA, et al. Clinical, radiographic and histologic analysis of the effects of pulp capping materials used in pulpotomies of human primary teeth. Eur Arch Paediatr Dent. 2013;14:65–71. doi: 10.1007/s40368-013-0015-x. [DOI] [PubMed] [Google Scholar]


