Abstract
This review will highlight some current areas of difficulty or controversy in diagnosis and treatment of nevoid basal cell carcinoma syndrome (NBCCS). The odontogenic keratocyst (OKC) has significant growth capacity and recurrence potential and is occasionally indicative of the NBCCS. The objective of this study is to clarify the causes of the recurrence of OKC in NBCCS. A literature search was conducted using Medline, accessed via the National Library of Medicine PubMed interface, searching for articles relating to the cause of recurrence of keratocyst in NBCCS written in English. This study has described the previous and the current outcomes of the treatment of OKC (recurrent cause). A protocol was then agreed to search for the possible causes of keratocyst recurrence in NBCCS. The general treatment of other manifestation of NBCCS has excluded from this study. Studies describing cohort, case series and miscellaneous clinical reports were retrieved and evaluated from 2010 to 2012.
Keywords: Basal cell carcinomas, keratocyst, nevoid basal cell carcinoma syndrome
INTRODUCTION
Nevoid basal cell carcinoma syndrome (NBCCS), also known as basal cell nevus syndrome, multiple basal cell carcinomas (BCC) syndrome, Gorlin syndrome and Gorlin-Goltz syndrome, is an inherited medical condition involving defects within multiple body systems such as the skin, nervous system, eyes, endocrine system and bones. People with this syndrome are particularly prone to developing a common and usually non-life-threatening form of non-melanoma skin cancers.[1] The absence of major diagnostic criteria such as BCC or palmar or plantar pits in young patients delay the early diagnosis and the correct screening for medulloblastoma, BCC and cardiac fibromas.[1] The odontogenic keratocyst (OKC) has significant growth capacity and recurrence potential and is occasionally indicative of the NBCCS. The NBCCS is inherited as an autosomal-dominant trait that consists principally of multiple OKC, multiple BCCs, skeletal anomalies and cranial calcifications. Syndrome-associated OKC have the highest recurrence rate and represent approximately 5% of all OKC patients.[2]
The BCCs develop early in life and may number in the tens or hundreds. The most frequently cited skeletal anomaly is bifid rib. Early calcification of falx cerebri is also relatively frequently seen on skull radiograms.[2] This syndrome has been linked to mutations in the PATCHED tumor-suppressor gene that encodes a receptor protein that is a component of the hedgehog (Hh) signaling pathway. Mutations of this gene have been found in syndrome-associated BCCs and OKC.[3,4,5] Gorlin-Goltz syndrome, also known as basal cell nevus syndrome, is an uncommon, autosomal-dominant inherited disorder, which is characterized by numerous BCCs (seen in 50-97% of people with the syndrome), maxillary keratocysts (present in about 75% of patients) and musculoskeletal malformations.
Gorlin and Goltz[6] established the association of multiple basal cell epitheliomas, jaw cysts (which they described as true cysts, having a typical stratified squamous epithelium) and bifid ribs, a combination that is frequently referred to as the “Gorlin-Goltz syndrome, the Gorlin syndrome” or the NBCCS.
Meerkotter and Shear[7] identified the jaw cysts as OKC and numerous clinical and molecular studies have been and continue to be, undertaken on the OKC occurring in patients with the syndrome. The syndrome is inherited as a set of autosomal-dominant characteristics with strong penetrance. It has variable expressivity, including multiple nevoid BCCs, OKC, other congenital skeletal defects, ectopic calcifications, plantar and palmar pits, central nervous system and ocular lesions and fairly typical facial features with frontal bossing and ocular hypertelorism.
It is caused by mutations in the patched tumor suppressor gene (PATCHED gene), a human homologue of the Drosophila gene mapped to chromosome 9q21-23.[8] Chromosomal mapping and genetic studies suggest that the underlying basis for this disease is an abnormality in the Hh signaling pathway. The role of this pathway in embryogenesis is well-known. The PATCHED gene product is part of a receptor for the protein called sonic Hh, which is involved in embryonic development.[9]
Diagnosis of NBCCS can be made when two of the five major criteria or one major and two minor criteria are present.[10,11] Major criteria of this syndrome include the following: (1) Multiple (two or more) BCC or one BCC under 30 years or 10 or more basal cell nevi; (2) OKC of the jaw; (3) multiple (three or more) palmar or plantar pits; (4) ectopic calcification, such as lamellar calcification of the falx cerebri; and (5) family history of Gorlin's syndrome. Minor manifestations include the following: (1) Skeletal anomalies, such as bifid, fused rib or vertebrae; (2) congenital malformation, including cleft lip or palate, macrocephaly, hypertelorism and frontal bossing; (3) medulloblastoma in young children; and (4) cardiac or ovarian fibroma.
RECURRENCES OF OKC IN NBCCS
OKC has a particular tendency to recur after surgical treatment. All of studies shown in the literature were case series, case report and miscellaneous studies. Pindborg and Hansen[12] observed no correlation between the size or location of the cyst and its tendency to recur; nor was there any difference in recurrence rate between cases that were treated by extirpation and those treated by fenestration. The increased recurrence of OKC in syndromic patients over non-syndromic patients because of OKC associated with this syndrome has a familial tendency and early family detection and genetic counseling are critical.[13] These cysts arise earlier in patients with NBCCS than in those who are unaffected by the syndrome.[13] OKC associated with NBCCS have occasionally been reported to transform into aggressive neoplasms such as ameloblastomas and squamous cell carcinoma.[14] The cyst lining seen in the NBCSS-related OKC is classically parakeratinized and does not appear to be associated with the orthokeratinized variant of the OKC. The cystic nature of OKC has long been debated, with some investigators classifying the OKC as a benign tumor.[15] In recent years, the WHO has recommended that the term “keratocystic odontogenic tumor” replace the term “OKC”, as it better reflects the neoplastic nature of the lesion.[15]
Hansen[16] reported a recurrence rate of 52% in a series of 52 cases followed for a period of at least 6 months. Browne[17] reported a 25% recurrence rate in 85 cysts followed for 6 months or longer. He found that most recurrences occurred in the first 5 years after surgery, but one of his cases recurred 20 years after operation.
Browne and Miller[18] reported that there was a very similar rate of recurrence following removal of OKCs with satellite cysts (23.7%) and those without satellite cysts (24.4%). There was a higher frequency of recurrence of cysts without epithelial residues (28.1%) than with (8.3%), but the difference was not statistically significant. These observations were confirmed by Vedtofte and Praetorius.[19]
A higher recurrence rate of cysts located in the angle or ascending ramus of the mandible was reported in one study, but the size of the cyst did not appear to have an influence.[20]
A total of 33 patients were followed for at least 6 years in a series of 62 patients with OKC and recurrences were found to be related to the operative procedure employed. The highest frequency of recurrences occurred in patients treated by cystostomy.[21]
A more recent detailed South Korean review of 256 patients showed significantly higher recurrence rates (P = 0.005) for the 14 of 17 patients in the 41-50 year age group; in 30 of 40 patients with cysts in the mandibular molar region (P = 0.001); and in 27 of 37 patients whose cysts had associated daughter cysts (P = 0.03) Myoung et al.[22] Their overall recurrence rate was 58.3% in an average follow-up period of 29 months. Nearly, 99% of the cysts were treated by surgical enucleation, 8.6% of them after marsupialization. A total of 11.7% of patients with recurrences had multiple recurrences.
When 24 patients with orthokeratinized OKC were followed for periods of 6 months to 8 years, only one recurrence was found and it occurred 61/2 years postoperatively.[23] This was the first indication that the orthokeratinized cysts may be less aggressive than the more common parakeratinized type, a contention that has been reinforced in other studies and will be referred to later.
The considerable variation in recurrence rate reported by different workers may be ascribed partly to the variability in the follow-up period. Vedtofte and Praetorius,[19] Forssell[20] and Forssell et al.[24] have reported that the recurrence rate increased with extension of the follow-up period to 5 years or more. It was found that of 75 keratocysts with follow-up times ranging from 5 to 17 years (mean 8.3), 32 (43%) recurred. The cumulative recurrence rate of the 67 annually examined cysts increased from 3% after the 1st year following the operation to 37% after the 3rd year. Thereafter, no new recurrences were noted. They observed that recurrences were more frequent (63%) with cysts in patients with the NBCCS than with cysts in patients without the syndrome (37%). OKC enucleated in one piece recurred significantly less often (P < 0.01) than cysts enucleated in several pieces and the recurrence rate in cases with a clinically observable infection, a fistula or with a perforated bony wall was higher than when these features were not present. The size of the cyst did not seem to influence its prognosis after surgery, but those whose radiographic appearance was multilocular had a higher recurrence rate than those with a unilocular appearance.
CAUSES OF RECURRENCES
There are several possible reasons why OKC recur so frequently and require meticulous surgical planning and execution. The first of these is related to their tendency to multiplicity in some patients, including the occurrence of satellite cysts, which may be retained during an enucleation procedure. If enucleation procedures are incomplete, some instances of recurrence may be new cysts arising from retained satellite microcysts or retained mural cell islands. Second, OKC linings are very thin and fragile, particularly when the cysts are large and are, therefore, more difficult to enucleate than cysts with thick walls. Portions of the lining may be left behind Fickling[25] and constitute the origin of a recurrence.
Forssell et al.[20,24,26] showed that recurrences were extremely infrequent if the cyst was enucleated in one piece, but occurred in over half of cases when the cyst was removed in several pieces. An attempt to save vital adjacent teeth or nerves during the operation may lead to incomplete eradication and hence to recurrence. Likewise, enucleation in one piece may be more difficult with cysts that have scalloped margins and this may explain the higher recurrence rates than with those with a smoother contour.
Toller[27] reported that the epithelial linings of OKCs had intrinsic growth potential and suggested that there was some basis for regarding them as benign neoplasms. Ahlfors et al.[28] also proposed that the OKC should be regarded as a benign cystic neoplasm. This was supported by further studies that reported the neoplastic nature of the OKC.[29,30]
Yet another source of the recurrences has been proposed by some authors.[31,32,33,34] They have demonstrated convincingly that OKC may also arise from proliferations of the basal cells of the oral mucosa, often referred to as basal cell hamartias, particularly in the third molar region and ascending ramus of the mandible. They have referred to the frequent observation of perforation of the overlying bone and firm adhesion of the cysts to the overlying mucosa and recommended that when the cysts were surgically removed, the overlying mucosa should be excised with them in an attempt to prevent possible recurrence or the formation of new cysts from residual basal cell proliferations. The two theories of origin are not incompatible as both dental lamina and basal cell hamartias have common parentage, the stomodeal ectoderm and both are influenced by ectomesenchyme or residual ectomesenchymal inductive influences.[35] This being the case, it seems reasonable to speculate that mucosal basal cells could be targeted by the same genetic influences as dental lamina.
Voorsmit et al.[36] reported that a recurrent OKC may develop in three different ways: By incomplete removal of the original cyst lining; by the retention of daughter cysts, from microcysts or epithelial islands in the wall of the original cyst or by the development of new OKC from epithelial off-shoots of the basal layer of the oral epithelium. Myoung et al.[22] reported that OKC had a significantly higher recurrence rate in patients in the fifth decade of life than in patients in the other age groups (P = 0.005). Recurrence rates were significantly dependent on the sites of involvement and OKCs in the mandibular molar region had significantly higher recurrence rates than those in other sites (P = 0.001). The histopathologic presence of one or more daughter cysts was significantly related to recurrence (P = 0.03). Stoelinga et al.[34] reported on 82 OKC diagnosed in 80 patients over a 25 year period. The clinical and radiographic data were correlated, which resulted in an accurate picture of the clinical presentation, relationship with teeth and incidence of lingual perforations in mandibular OKC. In 40% of the cysts, no suspicion had arisen before surgery; in 60%, the diagnosis was secured before surgery. Some was treated with the exception of the maxillary OKC, which entailed excision of the attached, overlying mucosa and enucleation of the cyst, after which the defect was treated with Carnoy's solution. The other patients underwent just enucleation of the cysts. For the first 5 years, the patients were seen every year, thereafter, every 2 years if possible. Recurrences (9/82) were mainly found in patients in whom the cyst had just been enucleated. Only three cysts recurred in the group treated according to the above-mentioned protocol. Most recurrences presented within 5 years, but late recurrences did occur even after 25 years.
Emerson et al.[37] Partridge and Towers[38] reported that the behavior of OKC so aggressive that they have penetrated cortical bone and involved surrounding soft-tissues. Another reported OKC extended from the maxilla and eventually involved the base of the skull, “behaving rather like a low-grade squamous cell carcinoma” as studied by Jackson et al.,[39] whereas others extended from the maxilla into orbit and infratemporal fossa as reported by Chuong et al.[40] or into the infratemporal fossa as per Worrall.[41] DeGould and Goldberg[42] described the recurrence of an OKC in a bone graft after partial mandibulectomy and the source of this may well have been the mucosa. Many studies have been carried out on patients with multiple OKC and in patients with the NBCCS in an attempt to find explanations for the recurrences. Payne[43] compared the histological features of recurrent OKC with non-recurrent specimens and those from patients with the syndrome. The presence of inflammation and the type of keratin produced did not seem to be significant. He found bud-like proliferations of the basal cell layer in 5 of 11 recurrent cysts (45%) and four of nine cysts from patients with the syndrome (44%). By comparison, only six of 72 non-recurrent OKC (8%) showed this feature. Satellite microcysts were observed in the cyst walls of 78% of cysts from patients with the syndrome, 18% of the recurrent cysts and 4% of the non-recurrent cysts.
Donatsky and Hjørting-Hansen[44] reported that the jaw cysts removed from patients with NBCCS were clinically, radiologically and histopathologically typical keratocysts with a high occurrence of epithelial islands and/or microcyts in the connective tissue of the capsule. The significantly higher occurrence of proliferative epithelial remnants in the connective tissue of the cyst wall may be an explanation of the high recurrence rate seen in patients with NBCCS and of the recurrence of some solitary keratocysts in patients without any signs or symptoms of NBCCS. In a follow-up period of 2 years, 85% of patients with NBCCS showed recurrence. One-third of these patients did not show any recurrence at the 1 year follow-up. Therefore, a follow-up period of 2 years or more is mandatory in the care of patients with the NBCCS.
Woolgar et al.[45] reported that there are histologic differences between OKC occurring in the BCCs syndrome NBCCS and as single lesions in otherwise healthy persons. This study identifies certain differences in age, gender and site between the two groups. The age at removal of the first keratocyst is significantly lower in the syndrome group. On more thorough examination, patients with multiple keratocysts (excluding recurrences) are found to have other features of NBCCS. The term multiple cysts refers to the lifetime history of the patient and does not necessarily imply that more than one cyst is present at any 1 time.
In another study, Woolgar et al.[46] have studied the clinical features of 44 recurrent OKC were compared with those of 228 single non-recurrent keratocysts, which had been followed for five or more years. Histological comparisons were made with 44 non-recurrent cysts matched for age, sex and site. There were no significant differences in the age, sex and site between patients with recurrent and non-recurrent cysts. There were no significant histological differences except for a greater amount of inflammation in the non-recurrent cysts. It is suggested that operative factors have a major influence on the likelihood of recurrence.
Woolgar et al.[47] have indicated that 164 OKC from 60 patients with the basal cell nevus syndrome were compared with a similar number of single keratocysts matched for age and site. Significant differences between the two groups were found in the numbers of satellite cysts, solid islands of epithelial proliferation and odontogenic rests within the capsule and in the numbers of mitotic figures in the epithelium lining the main cavity. An index of activity derived from these parameters suggests a greater growth potential in syndrome cysts; in addition, the patterns of association of the features support the theory that the odontogenic rests give rise to satellite cysts.
They found no association to support the theory that satellite cysts arose by basal budding of the epithelium lining the parent cyst from histological point view. Their results did, however, support the view that satellite cysts are formed when islands of proliferating epithelial cells derived from small epithelial rests reach a size where cystic breakdown occurs. They found no evidence that the ameloblastomatoid proliferations develop into true ameloblastomas. They suggested that there was some inherent genetic potential for proliferation of odontogenic epithelium in the syndrome patients.
Dominguez and Keszler[48] reported that keratocysts of the solitary type were histologically and histometrically compared with those associated with the NBCCS. It was observed that parakeratinization, intramural epithelial remnants and satellite cysts were a more frequent finding among NBCCS keratocysts than among solitary keratocysts. Conversely, it was also found that the total nuclear density was greater in non-associated cysts and that the total number of nuclei, the number of basal nuclei and the epithelial height values were also higher in solitary keratocysts. Nevoid BCC-keratocysts and solitary keratocysts are considered to be two morphologically distinct populations of the same entity.
Some authors have referred to the occurrence of multiple OKC in patients without obvious signs of other features of the syndrome or of a familial trend. Brannon[49] reported a frequency of 3% with multiple cysts in his sample, Kinard et al.[50] 4%, Ahlfors et al.[28] 6%, Voorsmit et al.[36] 2% and Stoelinga and Bronkhorst[51] 4.5%. As oral surgeons have become increasingly aware of the need to treat OKC more aggressively than other jaw cysts or by the use of special protocols, it is likely that future studies will show a declining frequency of recurrences.
It is difficult to ignore the possibility that the variability in reported recurrence rates may at least partly be attributable to differences in the surgical techniques used and in the experience of the surgeons. Voorsmit et al.[36] reported the results of a follow-up study of two groups of patients treated for OKC. In the first group of 52 cases, the cysts were treated conservatively by careful enucleation of the entire wall. In the second group of 40 cases, the cysts were removed by enucleation along with excision of the mucosa overlying a perforation of the cortical bone, which was determined at operation. Before removal, all cysts in this group were treated with Carnoy's solution. The recurrence rate in their first group was 13.5% in a 1-21 year follow-up while the recurrence rate in their second group was 2.5% in a 1-10 year follow-up.
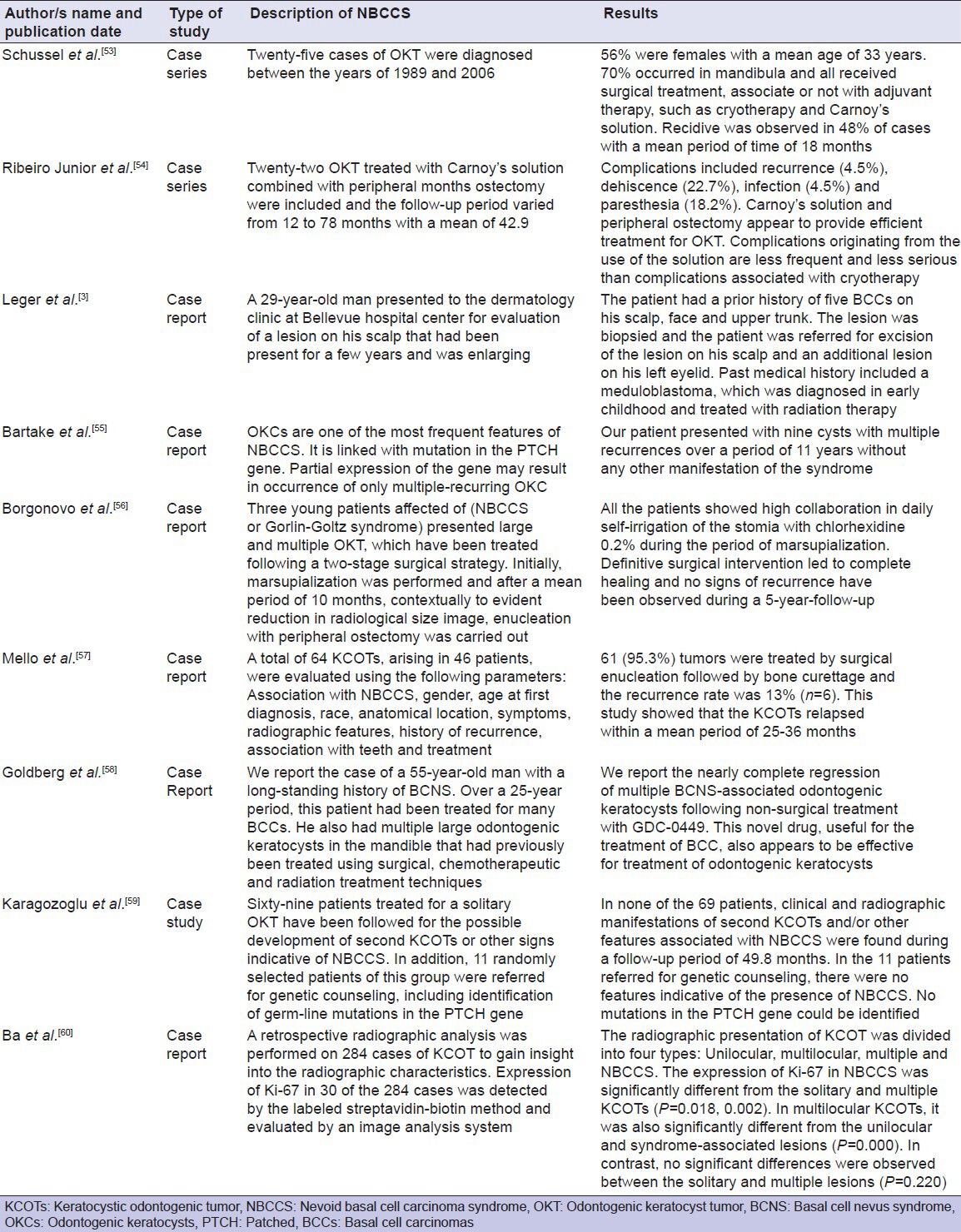
Furthermore, the current studies show similar results [Table 1] regarding the frequent recurrence of the keratocyst in NBCCS. Furthermore, all of those studies describing cohort, case series and miscellaneous clinical reports. No randomized controlled trials for the treatment of keratocyst in NBCCS were located in the literature.
Table 1.
A description of odontogenic keratocyst in nevoid basal cell carcinoma syndrome from clinical view (2010-2012)
DISCUSSION
The OKC represent from 65% to 75% of the cases of the NBCCS.[52] These cysts represent a particular entity that has been of interest, mainly due to biological aggressiveness and to the great amount of recurrence.[1,7]
Recently and based on the intrinsic growth potential of its epithelial coating, they have been re-classified and called OKC tumors and they have been included in the odontogenic neoplasias.[7,11,26] The keratocysts have a well-defined scale-like parakeratinized stratified epithelium with an average thickness of 5-8 cells, with a basal layer in which cells present themselves fenced up in a corrugated surface and a connective wall rich in mucopolisacarids, without inflammatory infiltration and with a variable number of microcysts and epithelial islets.[9,50] Its high potential of recurrence is justified by the high mitotic epithelial activity, the frequency of satellite cysts, pieces of epithelium and prolific dental sheet and by the existence of a epithelial coating thicker than in other jaw cysts.[7,10,11]
The treatment modalities for the keratocysts vary from simple enucleation with curettage, to the enucleation with peripheral osteotomy or to osseous resection in block. This last technique is the most aggressive and it logically follows that the recurrence rate decreases.[61] There are also more conservative options such as the local parietal therapy with Carnoy solution, with cryotherapy or marsupialization of the cysts, or decompression followed by a secondary enucleation.[8] Nevertheless, those methods are not efficient in the long-term and their use is considered to be controversial. It is believed that the nature of the treatment of keratocyst is depending on the following factors: Lesion size, lesion extension, location, possible cortical and soft parts damage, the age and whether it is a primary or recurrent lesion.[8] It is also important to detect if it is an isolated keratocyst or if it is associated with the syndrome, since in the last case, the rate of recurrence is higher as Forssell[20] have suggested the recurrence rate is of 63% in keratocysts associated to the syndrome and of 37% in the isolated ones.[20] Keratocyst is different from other odontogenic cyst. Matsumoto and Ribeiro[62] indicated that immunoreactivity of inducible nitric oxide expression was expressed in several cellular types present in periapical cyst, being positively correlated with the level of inflammation. Therefore, inducible nitric oxide expression plays an important role in the pathogenesis of periapical cysts. Pre-operative diagnosis is important to achieve the optimal treatment planning of cystic lesions of the maxillofacial region.
Matsumoto and Ribeiro[62] reported that the value of fine-needle aspiration biopsy in cystic lesions of the maxillofacial region is found as successful as in the solid lesions.
This study has shown that there is a lack of published evidence regarding the cause of frequent recurrent of OKC that presented in NBCCS. The findings of the study revealed differences in opinion regarding the treatment modalities.
This literature review revealed many papers, which reported the possible causes of the recurrence of keratocyst in nevoid BCC. None of the publications included randomized clinical trials and formal meta-analysis study was not possible. The evidence base was, therefore, determined by comparing case series. Only literature relating to treatment of keratocyst in NBCCS was included.
The limitations of this kind of this study are well-recognized and bias can arise from many sources.[63] In general, literature review offers a number of advantages and disadvantages for data collection, though their limitations must be recognized. The reviews often aim to provide background, identifying relationships between ideas and practice, establishing the context of the topic or problem, rationalizing the significance of the problem, identifying methodologies and techniques that have been used[64] and are designed to collect information from online database (PubMed) as accurately and precisely as possible. Another important limitation is that a review of this kind cannot address the issue of individual case variation. A number of factors determine any treatment selected including lesion size, lesion extension, location, possible cortical and soft parts damage, the age and whether it is a primary or recurrent lesion. This is not surprising as only a limited number of clinical guidelines can be found relating to the management of keratocyst in NBCCS. It would be necessary to identify an expert panel, which was inclusive of other surgical specialties, pathologists and systematic reviewers experienced in formulating guidelines. Additional data would have to be gathered.
As mentioned above, this study has shown that there is a lack of published evidence relating to the cause of recurrence of keratocyst in NBCCS.
Randomized controlled clinical trials could, in theory, be used to address these issues, but are difficult to perform because of likely low accrual rates and the need for prolonged follow-up times to assess clinical outcomes. Inconsistent views revealed by this review, whilst recognizing its limitations, indicate that there may be a need for production of consensus guidelines for the treatment of keratocyst in NBCCS and then it might be the cause of recurrence would be decreased.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi PS, Deshmukh V, Golgire S. Gorlin-Goltz syndrome. Dent Res J (Isfahan) 2012;9:100–6. doi: 10.4103/1735-3327.92963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leger M, Quintana A, Tzu J, Yee H, Kamino H, Sanchez M. Nevoid basal cell carcinoma syndrome. Dermatol Online J. 2011;17:23. [PubMed] [Google Scholar]
- 4.Tom WL, Hurley MY, Oliver DS, Shah MR, Bree AF. Features of basal cell carcinomas in basal cell nevus syndrome. Am J Med Genet A. 2011;155A:2098–104. doi: 10.1002/ajmg.a.34127. [DOI] [PubMed] [Google Scholar]
- 5.Shumway BS, Kalmar JR, Allen CM, Rawal YB. Basal cell carcinoma of the buccal mucosa in a patient with nevoid basal cell carcinoma syndrome. Int J Surg Pathol. 2011;19:348–54. doi: 10.1177/1066896908329596. [DOI] [PubMed] [Google Scholar]
- 6.Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908–12. doi: 10.1056/NEJM196005052621803. [DOI] [PubMed] [Google Scholar]
- 7.Meerkotter VA, Shear M. Multiple primordial cysts associated with bifid rib and ocular defects. oral surg oral med oral pathol. 1964;18:498–503. doi: 10.1016/0030-4220(64)90399-8. [DOI] [PubMed] [Google Scholar]
- 8.Díaz-Fernández JM, Infante-Cossío P, Belmonte-Caro R, Ruiz-Laza L, García-Perla-García A, Gutiérrez-Pérez JL. Basal cell nevus syndrome. Presentation of six cases and literature review. Med Oral Patol Oral Cir Bucal. 2005;10(Suppl 1):E57–66. [PubMed] [Google Scholar]
- 9.Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol. 2005;152:1108–24. doi: 10.1111/j.1365-2133.2005.06587.x. [DOI] [PubMed] [Google Scholar]
- 10.Evans DG, Ladusans EJ, Rimmer S, Burnell LD, Thakker N, Farndon PA. Complications of the naevoid basal cell carcinoma syndrome: Results of a population based study. J Med Genet. 1993;30:460–4. doi: 10.1136/jmg.30.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanley S, Ratcliffe J, Hockey A, Haan E, Oley C, Ravine D, et al. Nevoid basal cell carcinoma syndrome: Review of 118 affected individuals. Am J Med Genet. 1994;50:282–90. doi: 10.1002/ajmg.1320500312. [DOI] [PubMed] [Google Scholar]
- 12.Pindborg JJ, Hansen J. Studies on odontogenic cyst epithelium. 2. clinical and roentgenologic aspects of odontogenic keratocysts. Acta Pathol Microbiol Scand. 1963;58:283–94. [PubMed] [Google Scholar]
- 13.Wang XX, Zhang J, Wei FC. Familial multiple odontogenic keratocysts. J Dent Child (Chic) 2007;74:140–2. [PubMed] [Google Scholar]
- 14.Reisner KR, Riva RD, Cobb RJ, Magidson JG, Goldman HS, Sordill WC. Treating nevoid basal cell carcinoma syndrome. J Am Dent Assoc. 1994;125:1007–11. doi: 10.14219/jada.archive.1994.0202. [DOI] [PubMed] [Google Scholar]
- 15.Madras J, Lapointe H. Keratocystic odontogenic tumour: Reclassification of the odontogenic keratocyst from cyst to tumour. J Can Dent Assoc. 2008;74:165–165h. [PubMed] [Google Scholar]
- 16.Hansen J. Keratocysts in the jaws. Trans Int Conf Oral Surg. 1967:128–34. [PubMed] [Google Scholar]
- 17.Browne RM. The odontogenic keratocyst. Clinical aspects. Br Dent J. 1970;128:225–31. doi: 10.1038/sj.bdj.4802449. [DOI] [PubMed] [Google Scholar]
- 18.Browne RM, Miller WA. Rupture strength of capsules of odontogenic cysts in man. Arch Oral Biol. 1969;14:1351–4. doi: 10.1016/0003-9969(69)90209-x. [DOI] [PubMed] [Google Scholar]
- 19.Vedtofte P, Praetorius F. Recurrence of the odontogenic keratocyst in relation to clinical and histological features. A 20-year follow-up study of 72 patients. Int J Oral Surg. 1979;8:412–20. doi: 10.1016/s0300-9785(79)80079-4. [DOI] [PubMed] [Google Scholar]
- 20.Forssell K. The primordial cyst. A clinical and radiographic study. Proc Finn Dent Soc. 1980;76:129–74. [PubMed] [Google Scholar]
- 21.Niemeyer K, Schlien HP, Habel G, Mentler C. Therapeutic results and long-term observations of 62 patients with keratocysts. Dtsch Zahnarztl Z. 1985;40:637–40. [PubMed] [Google Scholar]
- 22.Myoung H, Hong SP, Hong SD, Lee JI, Lim CY, Choung PH, et al. Odontogenic keratocyst: Review of 256 cases for recurrence and clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:328–33. doi: 10.1067/moe.2001.113109. [DOI] [PubMed] [Google Scholar]
- 23.Wright JM. The odontogenic keratocyst: Orthokeratinized variant. Oral Surg Oral Med Oral Pathol. 1981;51:609–18. doi: 10.1016/s0030-4220(81)80011-4. [DOI] [PubMed] [Google Scholar]
- 24.Forssell K, Forssell H, Kahnberg KE. Recurrence of keratocysts. A long-term follow-up study. Int J Oral Maxillofac Surg. 1988;17:25–8. doi: 10.1016/s0901-5027(88)80224-8. [DOI] [PubMed] [Google Scholar]
- 25.Fickling BW. Cysts of the jaw: A long-term survey of types and treatment. Proc R Soc Med. 1965;58:847–54. doi: 10.1177/003591576505811P101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forssell K, Sorvari TE, Oksala E. An analysis of the recurrence of odontogenic keratocysts. Proc Finn Dent Soc. 1974;70:135–40. [PubMed] [Google Scholar]
- 27.Toller P. Origin and growth of cysts of the jaws. Ann R Coll Surg Engl. 1967;40:306–36. [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlfors E, Larsson A, Sjögren S. The odontogenic keratocyst: A benign cystic tumor? J Oral Maxillofac Surg. 1984;42:10–9. doi: 10.1016/0278-2391(84)90390-2. [DOI] [PubMed] [Google Scholar]
- 29.Shear M. The aggressive nature of the odontogenic keratocyst: Is it a benign cystic neoplasm? Part 1. Clinical and early experimental evidence of aggressive behaviour. Oral Oncol. 2002;38:219–26. doi: 10.1016/s1368-8375(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 30.Shear M. The aggressive nature of the odontogenic keratocyst: Is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002;38:323–31. doi: 10.1016/s1368-8375(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 31.Shear M. The aggressive nature of the odontogenic keratocyst: Is it a benign cystic neoplasm? Part 3. Immunocytochemistry of cytokeratin and other epithelial cell markers. Oral Oncol. 2002;38:407–15. doi: 10.1016/s1368-8375(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 32.Stoelinga PJ. Long-term follow-up on keratocysts treated according to a defined protocol. Int J Oral Maxillofac Surg. 2001;30:14–25. doi: 10.1054/ijom.2000.0027. [DOI] [PubMed] [Google Scholar]
- 33.Stoelinga PJ. Recurrent odontogenic keratocyst within the temporalis muscle. Br J Oral Maxillofac Surg. 1992;30:277–8. doi: 10.1016/0266-4356(92)90277-p. [DOI] [PubMed] [Google Scholar]
- 34.Stoelinga PJ, Peters JH, van de Staak WJ, Cohen MM., Jr Some new findings in the basal-cell nevus syndrome. Oral Surg Oral Med Oral Pathol. 1973;36:686–92. doi: 10.1016/0030-4220(73)90141-2. [DOI] [PubMed] [Google Scholar]
- 35.Altini M, Lurie R, Shear M. A case report of keratoameloblastoma. Int J Oral Surg. 1976;5:245–9. doi: 10.1016/s0300-9785(76)80020-8. [DOI] [PubMed] [Google Scholar]
- 36.Voorsmit RA, Stoelinga PJ, van Haelst UJ. The management of keratocysts. J Maxillofac Surg. 1981;9:228–36. doi: 10.1016/s0301-0503(81)80049-5. [DOI] [PubMed] [Google Scholar]
- 37.Emerson TG, Whitlock RI, Jones JH. Involvement of soft tissue by odontogenic keratocysts (primordial cysts) Br J Oral Surg. 1972;9:181–5. doi: 10.1016/s0007-117x(71)80032-x. [DOI] [PubMed] [Google Scholar]
- 38.Partridge M, Towers JF. The primordial cyst (odontogenic keratocyst): Its tumour-like characteristics and behaviour. Br J Oral Maxillofac Surg. 1987;25:271–9. doi: 10.1016/0266-4356(87)90065-9. [DOI] [PubMed] [Google Scholar]
- 39.Jackson IT, Potparic Z, Fasching M, Schievink WI, Tidstrom K, Hussain K. Penetration of the skull base by dissecting keratocyst. J Craniomaxillofac Surg. 1993;21:319–25. doi: 10.1016/s1010-5182(05)80490-1. [DOI] [PubMed] [Google Scholar]
- 40.Chuong R, Donoff RB, Guralnick W. The odontogenic keratocyst. J Oral Maxillofac Surg. 1982;40:797–802. doi: 10.1016/0278-2391(82)90177-x. [DOI] [PubMed] [Google Scholar]
- 41.Worrall SF. Recurrent odontogenic keratocyst within the temporalis muscle. Br J Oral Maxillofac Surg. 1992;30:59–62. doi: 10.1016/0266-4356(92)90139-a. [DOI] [PubMed] [Google Scholar]
- 42.DeGould MD, Goldberg JS. Recurrence of an odontogenic keratocyst in a bone graft. Report of a case. Int J Oral Maxillofac Surg. 1991;20:9–11. doi: 10.1016/s0901-5027(05)80686-1. [DOI] [PubMed] [Google Scholar]
- 43.Payne TF. An analysis of the clinical and histopathologic parameters of the odontogenic keratocyst. Oral Surg Oral Med Oral Pathol. 1972;33:538–46. doi: 10.1016/0030-4220(72)90366-0. [DOI] [PubMed] [Google Scholar]
- 44.Donatsky O, Hjørting-Hansen E. Recurrence of the odontogenic keratocyst in 13 patients with the nevoid basal cell carcinoma syndrome. A 6-year follow-up. Int J Oral Surg. 1980;9:173–9. doi: 10.1016/s0300-9785(80)80016-0. [DOI] [PubMed] [Google Scholar]
- 45.Woolgar JA, Rippin JW, Browne RM. The odontogenic keratocyst and its occurrence in the nevoid basal cell carcinoma syndrome. Oral Surg Oral Med Oral Pathol. 1987;64:727–30. doi: 10.1016/0030-4220(87)90176-9. [DOI] [PubMed] [Google Scholar]
- 46.Woolgar JA, Rippin JW, Browne RM. A comparative study of the clinical and histological features of recurrent and non-recurrent odontogenic keratocysts. J Oral Pathol. 1987;16:124–8. doi: 10.1111/j.1600-0714.1987.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 47.Woolgar JA, Rippin JW, Browne RM. A comparative histological study of odontogenic keratocysts in basal cell naevus syndrome and control patients. J Oral Pathol. 1987;16:75–80. doi: 10.1111/j.1600-0714.1987.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez FV, Keszler A. Comparative study of keratocysts, associated and non-associated with nevoid basal cell carcinoma syndrome. J Oral Pathol. 1988;17:39–42. doi: 10.1111/j.1600-0714.1988.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 49.Brannon RB. The odontogenic keratocyst. A clinicopathologic study of 312 cases. Part I. Clinical features. Oral Surg Oral Med Oral Pathol. 1976;42:54–72. doi: 10.1016/0030-4220(76)90031-1. [DOI] [PubMed] [Google Scholar]
- 50.Kinard BE, Chuang SK, August M, Dodson TB. How well do we manage the odontogenic keratocyst. J Oral Maxillofac Surg. 2013;71:1353–8. doi: 10.1016/j.joms.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 51.Stoelinga PJ, Bronkhorst FB. The incidence, multiple presentation and recurrence of aggressive cysts of the jaws. J Craniomaxillofac Surg. 1988;16:184–95. doi: 10.1016/s1010-5182(88)80044-1. [DOI] [PubMed] [Google Scholar]
- 52.Ramaglia L, Morgese F, Pighetti M, Saviano R. Odontogenic keratocyst and uterus bicornis in nevoid basal cell carcinoma syndrome: Case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:217–9. doi: 10.1016/j.tripleo.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Schussel JL, Stramandinoli RT, Dissenha JL, Avila LF, Sassi LM. Retrospective study of 25 cases of keratocystic odontogenic tumor: Epidemiology and treatment. J Contemp Dent Pract. 2011;12:100–3. doi: 10.5005/jp-journals-10024-1016. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro O, Junior, Borba AM, Alves CA, de Gouveia MM, Coracin FL, Guimarães J., Júnior Keratocystic odontogenic tumors and Carnoy's solution: Results and complications assessment. Oral Dis. 2012;18:548–57. doi: 10.1111/j.1601-0825.2012.01907.x. [DOI] [PubMed] [Google Scholar]
- 55.Bartake A, Shreekanth N, Prabhu S, Gopalkrishnan K. Non-syndromic recurrent multiple odontogenic keratocysts: A case report. J Dent (Tehran) 2011;8:96–100. [PMC free article] [PubMed] [Google Scholar]
- 56.Borgonovo AE, Di Lascia S, Grossi G, Maiorana C. Two-stage treatment protocol of keratocystic odontogenic tumour in young patients with Gorlin-Goltz syndrome: Marsupialization and later enucleation with peripheral ostectomy. A 5-year-follow-up experience. Int J Pediatr Otorhinolaryngol. 2011;75:1565–71. doi: 10.1016/j.ijporl.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Mello LA, Gurgel CA, Ramos EA, de Souza RO, Schlaepfer-Sales CB, de Azevedo RA, et al. Keratocystic odontogenic tumour: An experience in the Northeast of Brazil. Srp Arh Celok Lek. 2011;139:291–7. doi: 10.2298/sarh1106291a. [DOI] [PubMed] [Google Scholar]
- 58.Goldberg LH, Landau JM, Moody MN, Kazakevich N, Holzer AM, Myers A. Resolution of odontogenic keratocysts of the jaw in basal cell nevus syndrome with GDC-0449. Arch Dermatol. 2011;147:839–41. doi: 10.1001/archdermatol.2011.50. [DOI] [PubMed] [Google Scholar]
- 59.Karagozoglu KH, Van Hagen JM, Baart JA, Van Der Waal I. Genetic screening for nevoid basal cell carcinoma syndrome in patients with a solitary keratocystic odontogenic tumour is not useful. Minerva Stomatol. 2011;60:1–4. [PubMed] [Google Scholar]
- 60.Ba K, Li X, Wang H, Liu Y, Zheng G, Yang Z, et al. Correlation between imaging features and epithelial cell proliferation in keratocystic odontogenic tumour. Dentomaxillofac Radiol. 2010;39:368–74. doi: 10.1259/dmfr/27538271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reyes-Macias JF, Bagán Sebastián JV. Gorlin-Goltz syndrome literature review and case report. Rev Eur Odontoestomatol. 2002;14:105–12. [Google Scholar]
- 62.Matsumoto MA, Ribeiro DA. Inducible nitric oxide expression correlates with the level of inflammation in periapical cysts. Eur J Dent. 2007;1:212–5. [PMC free article] [PubMed] [Google Scholar]
- 63.Agaram NP, Collins BM, Barnes L, Lomago D, Aldeeb D, Swalsky P, et al. Molecular analysis to demonstrate that odontogenic keratocysts are neoplastic. Arch Pathol Lab Med. 2004;128:313–7. doi: 10.5858/2004-128-313-MATDTO. [DOI] [PubMed] [Google Scholar]
- 64.Kapoor S, Sikka P, Saxena K. Publication of research article: An art or science. Ann Med Health Sci Res. 2013;3:96–8. doi: 10.4103/2141-9248.109459. [DOI] [PMC free article] [PubMed] [Google Scholar]


