Abstract
Objective:
To clinically investigate the antibacterial effects of a commercially available self-etch 12-methacryloyloxy- dodecylpyridinium bromide (MDPB)-containing adhesive system in comparison with its respective non-MDPB-containing adhesive and to evaluate the pulp responses when in use on human teeth.
Materials and Methods:
Sixty-two viable human teeth scheduled for extraction were used. Class V cavities were prepared on the buccal surfaces of the teeth and filled with the tested materials (Protect Bond/Clearfil AP-X, SE Bond/Clearfil AP-X and Dycal/Ketac Fill Plus) as a control group, according to manufacturer's recommendations. Randomly divided to two groups teeth remained intra-orally for 4 and 8 weeks. After extraction, teeth were decalcified, sectioned and stained using the Mayer's hematoxylin and eosin, and modified Brown-Brenn's technique. Pulp responses were evaluated microscopically under a microscope and remaining dentine thickness measured under a stereomicroscope.
Results:
No statistically significant differences regarding pulp inflammation or bacterial infiltration were found either for the materials tested or for periods of post-operative evaluation.
Conclusions:
The results suggested that for a short period of evaluation there are no quantitative differences, regardless to restoring material used.
Keywords: Adhesive, antibacterial, dentine, 12-methacryloyloxydodecylpyridinium bromide, pulp
INTRODUCTION
The rapid development of adhesive dentistry has resulted to the introduction of new adhesive systems into the clinical procedures. Although biological and mechanical properties of the materials are constantly improving, problems related to polymerization construction of the composite resins may occur. Marginal leakage and post-operative bacterial infiltration may affect the longevity of the restoration and the pulpal reaction. In addition, there is evidence that the removal of the caries is most commonly incomplete.[1,2] The potential of the pulp recovery after placing a resin restoration seems to depend on several factors such as the pre-operative pulp status, the remaining dentine thickness (RDT), the efficacy of a treatment strategy and the pre-operative and post-operative prevention of bacterial infiltration.[3] It has long been recognized that the presence of bacteria along the cavity walls or within the dentinal tubules may be considered as the critical determinant in pulpal inflammatory responses after restorative procedures.[4]
Restorative materials in the new era tend to be “bio-active,” and antibacterial effects are highlighted as one of the most important properties.[5] The incorporation of antibacterial agents into adhesive systems has been proposed to eliminate residual bacteria from dentine. Such an antibacterial monomer, 12-methacryloyloxy-dodecylpyridinium bromide (MDPB), has been developed and incorporated in a self-etch adhesive system, Clearfil SE Bond (Kuraray Europe, GmbH Dusseldorf Germany). The antibacterial adhesive system employing the MDPB-containing primer was commercialized under the name protect bond (Kuraray Europe, GmbH Dusseldorf Germany).
Incorporation of the MDPB antibacterial agent into a dentine adhesive system resulted in strong antibacterial activity against oral streptococci ex vivo.[6,7,8] The pulpal responses have been further evaluated histopathologically in vivo, in the dog's teeth.[9] The results indicated that little or no inflammation was present. Tziafas et al. have further evaluated the repair capacity of the pulp-dentine complex when protect bond system is placed on infected cavities in dog teeth.[10] However, animal test results cannot be directly extrapolated to humans; therefore, a material has to be tested in clinical conditions.
Standardized in vivo studies are using young human subjects which provide teeth with healthy pulps housed in large pulp chambers, for reasons of comparison between test and control procedures.[3,11] Nevertheless, clinical correlation has to be undertaken with caution.[3] Few studies have attempted to assess the pulp's reaction when it is in a compromised state.[3,11] The aim of this study is to evaluate pulp responses of mature human pulps and compare the short-term antibacterial potential of two commercially available self-etching adhesive systems; a containing MDPB (Protect Bond) and a non-MDPB containing (SE Bond) self-etching adhesive system, when applying those adhesives in deep cavities. The working hypothesis is that there is no difference in pulpal responses of mature human pulps in-between teeth treated with those adhesives and the control group and also that the short-term antibacterial potential is equal for each group of the study.
MATERIALS AND METHODS
A total of 62 viable caries free single rooted human teeth scheduled for extraction for periodontal reasons from healthy patients between 40 and 50 years of age, males or females were used for the purposes of the study. The vitality of the teeth included in the study evaluated using an electro-pulp tester device. The experimental protocol was conducted according to ethical guidelines for clinical research in humans in the School of Dentistry, Aristotle University of Thessaloniki. Patients were informed with a written statement about the clinical procedures and of the possible inconvenience that they were to experience. Those who agreed to participate in the research signed an acceptance form. The experimental procedures should have no effects on the therapeutic treatment of the patients and should not modify their treatment plans.
Experimental procedures
On the day of the operative procedure all teeth selected for the experiment were scaled and polished with a rubber cup. Local anesthesia was provided prior to cavity preparation (Scandonest 2%, Septodont). Class V cavities (approximately 2.50 mm wide, 3.00 mm long) were prepared on the buccal surface of teeth using a tungsten carbide pear-shaped bur, ISO #330 L (SS. White, Lakewood NJ, USA), at ultra-high speed with a copious water spray. A new bur was employed on every fourth cavity to avoid excessive heating. Cavities were prepared according to the following protocols:
The preparations were cut 0.5-1 mm above the cemento-enamel junction
The floor of the cavity preparations was maintained curved and parallel to the outer buccal surface of the tooth
A bevel was made at the enamel margin of the cavities using a high-speed diamond bur
Teeth were rinsed with sterile distilled water and isolated with a rubber dam and saliva was controlled through high-speed evacuation. All procedures were performed by the same operator.
Teeth randomly divided into six experimental groups, according to materials treated and the time period of evaluation. The calcium hydroxide-based material Dycal (Dentsply) in combination with glass-ionomer cement Ketac Fill Plus (3M-ESPE) was used to form a control group. Chemical compositions of adhesive systems used are shown in Table 1.
Table 1.
Composition of the two-step SE adhesives tested
Teeth were extracted under local anesthesia (Scandonest 2%, Septodont, Cedex, France) and prepared for histological analysis. Immediately after extraction, roots were sectioned midway between the cemento-enamel junction and the apex. Teeth were fixed in 10% neutral-buffered formalin solution for 2 weeks. The specimens were then demineralized in Morse's solution (50% formic acid + 20% sodium citrate) for 2 months. Finally, the teeth were embedded in paraffin and serially sectioned through the pulp at 5 μm thickness. All sections coming through the cavity floor were stained either with Mayer's hematoxylin and eosin, stain to assess soft-tissue organization and tertiary dentine formation or were subjected to modified Brown-Brenn's technique to detect the presence of Gram-positive and Gram-negative microorganisms.
Histological assessment
The stereotypic connective tissue reactions and the bacterial infiltration of the cavity were evaluated according to the following criteria.
Inflammatory cell response
Inflammatory cell infiltration of the pulp tissue was classified as: 0 (none), absence of inflammatory cells; 1 (slight/moderate), a few scattered inflammatory cells or moderate inflammatory cell infiltration; 2 (severe), heavy inflammatory cell infiltration of the coronal pulp around the exposure site (in exposed teeth) or abscess formation.
Tissue disorganization
Disorganization of pulp tissue was classified as: 0 (no), physiological appearance of the pulp dentine interface and central pulp tissue; 1 (slight disorganization), a reduction in cells in the odontoblastic layer beneath the cavities, but normal central pulp (in non-exposed teeth); 2 (severe disorganization) complete disorganization of the odontoblastic layer.
Bacterial infiltration
The presence of stained bacteria in the pulp space or along the cavity walls/within the cut dentinal tubules classified as: 1 (no), absence of bacteria infiltration; 2 (on cavity walls): Presence of bacteria among the cavity walls; 3 (in the pulp): Presence of bacteria inside the pulp tissue or the pulp chamber.
Remaining dentine thickness
All stained sections were evaluated and the RDT was measured by means of a graticule under a stereomicroscope (Olympus CO, Tokyo, Japan) at ×100 magnification, between the cavity floor and the line of interface from the pre-operative circumpulpal dentine to the post-operative formed matrix.[2] The minimum RDT was estimated for every specimen. The 20 adjacent sections were analyzed twice, by two independent observers who were blind to the treatment group. Inter-observation variation was only noticed in scoring tissue disorganization of unexposed teeth. In these cases, the highest disorganization score was finally recorded.
Reactionary dentine formation
The thickness of reactionary dentine was measured beneath the site where minimum RDT was measured and also at the opposite pulp wall [Figure 1, arrows].
Figure 1.
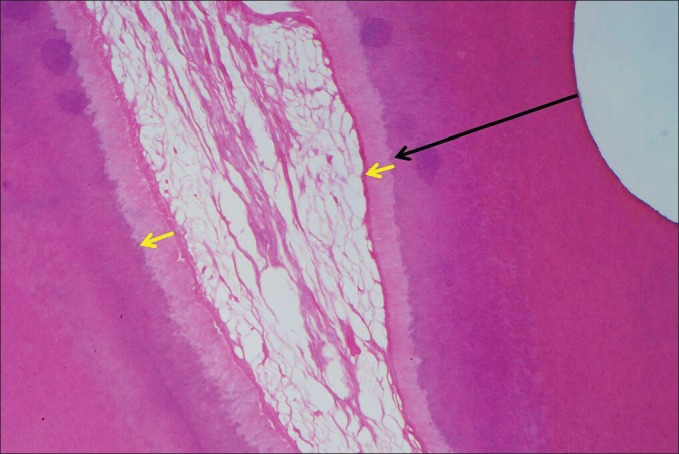
Measurement sites of remaining dentine thickness (big arrow), reactionary dentine thickness adjacent to cavity and to the opposite dentine walls (small arrows)
Statistical methods
The comparison of the natural log-transformed values of the minimum RDT between the different groups was performed using the two-way ANOVA. The assumptions of normality and the homogeneity of variances were tested by the Shapiro-Wilk test (sample size < 50) and Levene's test, respectively. The Kruskal-Wallis test was used to compa re pulp degeneration, inflammatory cell response and tissue necrosis between the three materials within each time period. Pair wise comparisons were performed with the Mann-Whitney test, whereas Bonferroni's method was applied for the adjustment of Type I error. In addition, the Mann-Whitney test was used to compare 4-week and 8-week period samples for each material. All P values were also computed by the Monte Carlo method based on 10,000 sampled tables. The analysis was performed with SPSS 15.0 (Statistical Package for Social Sciences, Chicago, Illinois, USA) and the level of statistical significance was set at P < 0.05.
Regarding reactionary dentine thickness, mean and standard deviation were used for the description of the differences% (measurement 1 − measurement 2)/measurement 2 × 100) between the thickness below the RDT site (measurement 1) and the thickness at the opposite pulp wall (measurement 2). Data were fractions hence the Arcsine of the square root of each value were computed. The assumption of normality was tested by the Shapiro Wilk test (N < 50) and the assumption of homogeneity of variances was tested by the Levene's test. Kruskal-Wallis test and Mann-Whitney test were used for the analysis of the transformed data. The analysis was performed on the Statistical Package for the Social Sciences (SPSS 16.0, IBM) software and the level of statistical significance was set at P < 0.05.
RESULTS
The teeth used in this study were mainly from periodontal patients aged between 40 and 50 years old. As expected some reticular degeneration was observed in the pulp tissue. This fact may affect the pulp healing potential or the inflammation intensity.
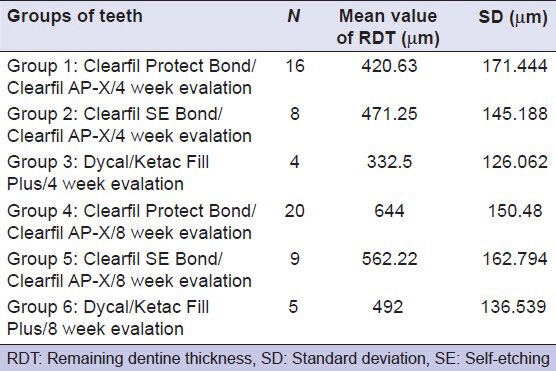
The minimum RDT was measured and compared among the six groups. In Table 2, the mean and standard deviation of the minimum RDT in each group of teeth are presented.
Table 2.
Mean value of the minimum RDT and SD values
Levene's method was used to test the working hypothesis that the error variance of the dependent variable (RDT) is equal across the groups. This hypothesis was not rejected (P = 0.070). In addition, a two-way ANOVA was conducted to compare the mean values of the RDT between the groups of materials (P = 0.447) and the mean values between the levels of time (P = 0.482).
Bacterial infiltration of the pulp was not present in any specimen of this experiment. In a few number of specimens bacteria were found along the cavity walls or within the cut dentinal tubules [Figure 2]. A small number of pulps demonstrated slight to moderate inflammatory cell response. No pulp demonstrated severe inflammation. Slight tissue disorganization of coronal pulp in proximity to cavity walls was present in few specimens, but of no statistical importance. The majority of specimens demonstrated no tissue disorganization or pulp inflammation [Figures 3–5]. Tests showed no statistical difference of importance regarding to inflammatory cell response and tissue necrosis for all groups of the specimens.
Figure 2.
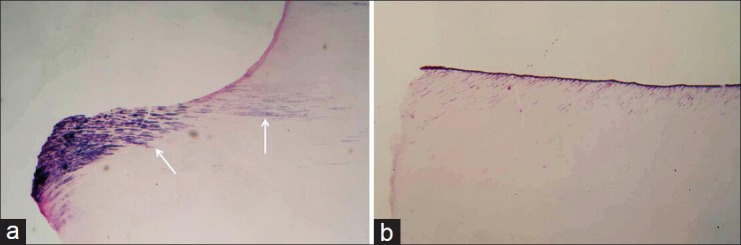
Presence (a) or absence of bacteria (b) in cut dentinal walls treated with protect bond, observed 8 weeks post-operatively (Brown-Brenn's modified technique, original magnification ×40)
Figure 3.
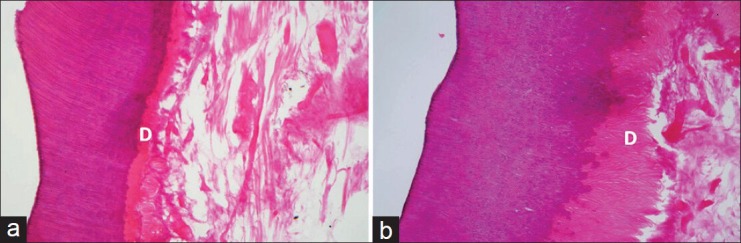
Cavities treated with SE Bond, evaluated at 4 weeks (a) and 8 weeks (b). Degeneration of the pulp mesenchyma is noticeable but dentinoblastic layer (D) is preserving its architecture (H and E, ×100)
Figure 5.
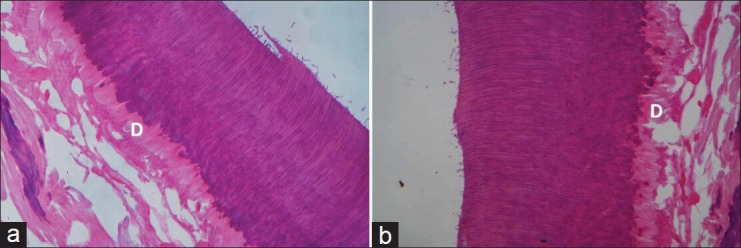
No inflammation of dentinoblastic layer (D) was observed in cavities treated with Ca (OH)2 at 4 weeks (a) and 8 weeks (b). Pulp degeneration is also advanced (H and E, ×100)
Figure 4.
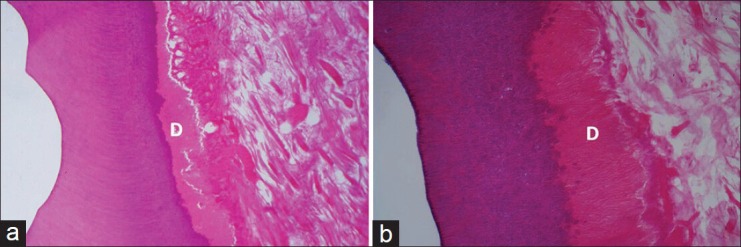
Cavities treated with protect bond, 4 weeks (a) and 8 weeks (b) post-operatively. Similar to those treated with SE Bond, dentinoblastic layer (D) appears intact and with no signs of inflammation. Pulp mesenchyma is degenerated (H and E, ×100)
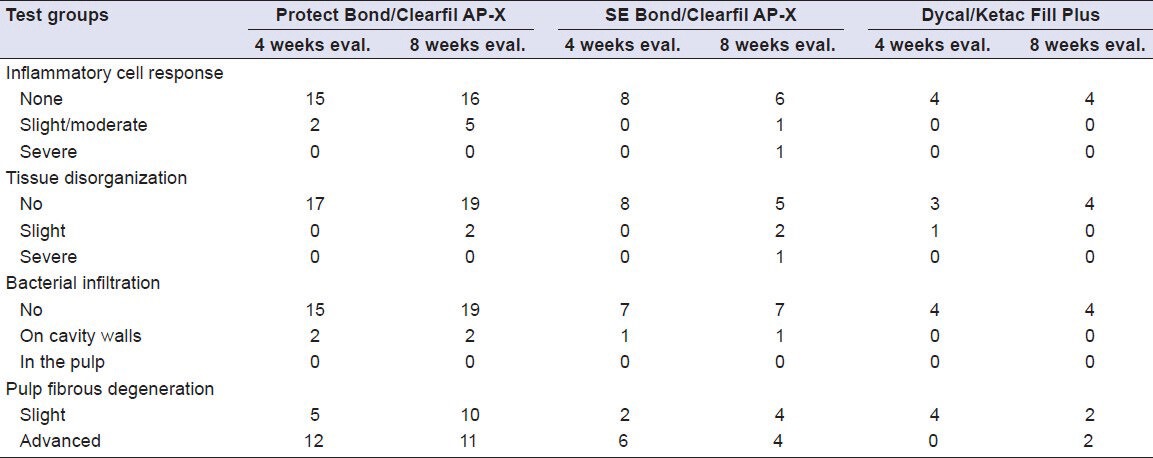
Histopathological findings of this study are presented in detail in Table 3.
Table 3.
Frequency of scores for each group of teeth after treatment with the test materials
DISCUSSION
The results of this study showed that there were no statistically significant differences between groups of material tested regarding inflammatory pulp response and bacterial infiltration of the cavity walls. This is considering confirming study's working hypothesis. Inflammatory pulp response was not dependent of the time elapsed from the restoration to extraction nor the restoration material used. Most of the tooth pulp, demonstrated slight or no inflammation. Pulp inflammation that occurs during a restoration procedure is considered to be a result of: (a) mechanical irritation of dentinoblastic processes and temperature rising during preparation, (b) bacterial infiltration in cavity walls and dentinal tubuli or pulp tissue and (c) chemical irritation of non-polymerized small sized monomers, which can penetrate toward pulp chamber through dentinal tubuli.[11]
Preventing of bacterial infiltration in cavity walls has been a challenge for manufacturers. The use of self etching adhesive system such as Clearfil SE Bond has been shown to provide a short-term, mild antibacterial effect, which has been supposed to be due to the acidic nature of those materials.[5,6,11,12] In addition, an antibacterial agent (MDPB) that co-polymerizes with other resin monomers has been added in Clearfil protect bond, in order to achieve long-term residual bacteria inhibition and protection against bacterial infiltration. In vitro studies have shown that non-light-activated material demonstrates greater antibacterial activity.[13] This decrease of antibacterial activity caused by polymerization, is supposed to occur due to entrapment of the antibacterial molecule in the resin matrix.[13] It seems that the remaining antibacterial effect is caused by the unpolymerized adhesive because there is never complete conversion of monomers to polymers mainly due to the presence of an oxygen-inhibited layer.[9] On the other side, the acidity of the unpolymerized monomers contained in self etch adhesives could cause inflammatory reactions when in direct contact or in close proximity with pulp tissue.
Bacterial infiltration of the cavity walls has been estimated histologically with the use of a modified Brown and Brenn's technique. Patients participating in this study suffered from severe periodontitis caused by poor hygienic habits. Restorations that remain in such an environment are expected to be exposed to a large amount of bacteria, which potentially could proliferate beneath the composite. On the contrary, histological examination showed that only a small number of bacteria could be found in cavity walls of few specimens. This probably demonstrates the protection from bacterial infiltration that provides the adhesive with the antibacterial molecule MDPB, but it also shows the short-term antibacterial action of the non-MDPB-containing adhesive, which occurs due to its acidity. These antibacterial properties of the adhesives used in this study have been shown previously in vitro.[13] Bacterial infiltration was not present either in the control group specimens. The antibacterial properties of Ca (OH)2 and glass-ionomer cements have been well-demonstrated in the past.[14] The presence of residual bacteria within the dentinal tubules or along the cavity walls is considered to be the most significant factor related to the pulp reaction under a resin-based restorative material in non-exposed cavities. Previous studies have shown that there a precise method for clinical diagnosis of carious dentine is still not available and in most cases of caries treatment, there is infected dentine left on the cavity walls.[15,16] Use of materials with antibacterial properties could inhibit the residual bacteria or prevent the colonization of the tooth-restoration interface post-operatively with beneficial results for the vitality of the pulp and the longevity of the restoration. The present experiment tests the short-term performance against bacterial infiltration of such an adhesive antibacterial factor-containing material in comparison with its predecessor adhesive from the same manufacturer. It is likely to assume that inflammation, in cases that occurred, may be the result of clinical procedures or result of diffusion of uncured residual monomers or oligomers with cytotoxic effects towards the pulp tissue, rather than a result of a release of acids and toxins by bacteria, since the presence of bacteria in cavity walls has been observed only in few cavities.
In the present study, RDT values ranged between 332.50 and 562.22 μm and in-between the groups of teeth there were no statistically significant differences (P < 0.05) observed. Chemical irritation of the pulp occurs due to the ability of monomers to penetrate into the pulp tissue. In vitro studies have shown that uncured residual monomers containing in primer or/and the adhesive such as hydroxyethylmethacrylate (HEMA), 10-methacryloxydecylihydrogen phosphate (MDP), bisphenol A diglycidylethermethacrylate (Bis-GMA), may penetrate through the dentine toward the pulp. This seems to be dependent of the RDT and of the dentine permeability.[17,18] The potential for this penetration increases as the RDT decreases. Should be noted that dentine permeability depends also on the width of dentinal tubuli, which is significantly smaller in elder people.[9] Teeth used in this study were the origin from mature individuals aged between 40 and 50 years old and it is likely to assume that permeability of dentine is reduced.
Pre-operative pulp tissue condition could be affected by the fact that the patients were older and suffered from periodontitis. In order to calibrate the results, a histological estimation of the pre-operative pulp condition depending on the fibrous degeneration was introduced in the study. The statistical analysis of the results has shown that-with the exception of the groups 1 and 3 - no statistically significant differences were found in pulp degeneration between the groups. These results indicate that the teeth used for this study had similar pulp tissue condition prior to the restoration procedures.
Our results are in agreement with the results of previous studies, which tested the same materials in dog teeth pulps.[10,19,20,21] In conclusion, pulp inflammation or tissue necrosis is irrelevant with application of contemporary adhesives in deep non-exposed cavities. The application of an adhesive containing antibacterial monomer (MDPB), demonstrates no difference regarding pulp cell response when compared with an adhesive without antibacterial monomer and when placed in non-exposed cavities.
The thickness of reactionary dentine formation was also measured beneath the RDT site and to the opposite pulp chamber wall on a straight line [Figure 1], in order to evidence possible “rebound” response of odontoblasts. This response is assumed to be caused by activation of odontoblastic cells due to inflammation. Data in this study are in compliance with the previous experiment data were no evidence of such a “rebound” response was present.[15]
CONCLUSIONS
The majority of clinical studies concerning the influence of restorative materials to the pulp tissue are performed at healthy young pulps with large pulp chambers and good healing potential. However, the everyday clinical reality abstains from this situation, since pulps of restored teeth have been previously undergone bacterial attack, causing alterations in pulpal mesenchyma and pulp volume. The present study is an attempt of estimating the safety of restoring procedures with self-etch adhesives in proximity to compromised pulp tissue. Within the limitations of a research performed at teeth with pulps in a compromised situation, results showed that placing self-etch adhesives in proximity to such pulps is a safe procedure. The pulp inflammatory response seems to be non-MDPB-containing-dependent and so is the bacterial infiltration of the cavity walls since acidity of a self-etch adhesive system seems to provide a short-term antibacterial action.[13]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Anderson MH, Loesche WJ, Charbeneau GT. Bacteriologic study of a basic fuchsin caries-disclosing dye. J Prosthet Dent. 1985;54:51–5. doi: 10.1016/s0022-3913(85)80069-x. [DOI] [PubMed] [Google Scholar]
- 2.Kidd EA, Banerjee A. What is absence of caries? In: Albbrektsson T, Bratthall D, Per-Olof J, Glantz PO, editors. Tissue Preservation in Caries Treatment. New Malden, UK: Quintessence Publ. Co. Ltd; 2001. pp. 69–79. [Google Scholar]
- 3.Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467–80. doi: 10.1177/10454411000110040501. [DOI] [PubMed] [Google Scholar]
- 4.Bergenholtz G, Cox CF, Loesche WJ, Syed SA. Bacterial leakage around dental restorations: Its effect on the dental pulp. J Oral Pathol. 1982;11:439–50. doi: 10.1111/j.1600-0714.1982.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 5.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 6.Imazato S, Kuramoto A, Kaneko T, Ebisu S, Russell RR. Comparison of antibacterial activity of simplified adhesive systems. Am J Dent. 2002;15:356–60. [PubMed] [Google Scholar]
- 7.Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 2004;29:369–75. [PubMed] [Google Scholar]
- 8.Imazato S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin Oral Investig. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 10.Tziafas D, Koliniotou-Koumpia E, Tziafa C, Papadimitriou S. Effects of a new antibacterial adhesive on the repair capacity of the pulp-dentine complex in infected teeth. Int Endod J. 2007;40:58–66. doi: 10.1111/j.1365-2591.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohmori K, Maeda N, Kohno A. Evaluation of antibacterial activity of three dentin primers using an in vitro tooth model. Oper Dent. 1999;24:279–85. [PubMed] [Google Scholar]
- 12.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 13.Gondim JO, Duque C, Hebling J, Giro EM. Influence of human dentine on the antibacterial activity of self-etching adhesive systems against cariogenic bacteria. J Dent. 2008;36:241–8. doi: 10.1016/j.jdent.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Stanley HR, Lundy T. Dycal therapy for pulp exposures. Oral Surg. 1973;35:389–401. doi: 10.1016/0030-4220(72)90300-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Walton RE, Osborne JW. Pulp response to bases and cavity depths. Am J Dent. 1992;5:64–8. [PubMed] [Google Scholar]
- 16.Shovelton DS. A study of deep carious dentine. Int Dent J. 1968;18:392–405. [PubMed] [Google Scholar]
- 17.Bouillaguet S, Virgillito M, Wataha J, Ciucchi B, Holz J. The influence of dentine permeability on cytotoxicity of four dentine bonding systems, in vitro. J Oral Rehabil. 1998;25:45–51. doi: 10.1046/j.1365-2842.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- 18.Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Cavity remaining dentin thickness and pulpal activity. Am J Dent. 2002;15:41–6. [PubMed] [Google Scholar]
- 19.Koliniotou-Koumpia E, Dionysopoulos P, Koumpia E. In vivo evaluation of microleakage from composites with new dentine adhesives. J Oral Rehabil. 2004;31:1014–22. doi: 10.1111/j.1365-2842.2004.01323.x. [DOI] [PubMed] [Google Scholar]
- 20.Koliniotou-Koumpia E, Papadimitriou S, Tziafas D. Pulpal responses after application of current adhesive systems to deep cavities. Clin Oral Investig. 2007;11:313–20. doi: 10.1007/s00784-007-0121-4. [DOI] [PubMed] [Google Scholar]
- 21.Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J Dent. 2005;33:639–47. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]


