Abstract
Objective:
The aim of the present study is to evaluate the biocompatibility of glass ionomer cements (GICs) with and without chlorhexidine (CHX) as well as coated with varnish or not using in vitro cytotoxicity test.
Materials and Methods:
Biocompatibility of Fuji IX, Fuji IX with varnish, Fuji IX with 1% CHX diacetate and Fuji IX with 1% CHX diacetate with varnish was determined with in vitro cytotoxicity assay by using L929 mouse connective tissue fibroblasts. After 72 h, cell viabilities were evaluated by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay to determine the effects of the cements on the mitochondrial function and microscopic images were taken by scanning electron microscopy.
Results:
Statistical analysis was performed by one-way analysis of variance followed by the Bonferroni post-hoc test at a significance level of P < 0.05. 72 h after treatment, there were statistically significant differences between Fuji IX and Fuji IX-CHX (P < 0.001). In addition, the reduction of the cytotoxicity by coating the GICs with varnish was indicative and increased the cell viability ratio (P < 0.001).
Conclusions:
Fuji IX coated with varnish was found to be the most biocompatible one among others. Thus adding CHX significantly reduced the cell viability, it is assumed that, due to the leakage of CHX and the other components of the GICs to the cell culture medium, the cell viabilities were decreased, so it is highly recommended to use varnish not only to reduce the water loss from the GICs, but also to reduce the cytotoxicity of the GICs.
Keywords: Biocompatibility, chlorhexidine, glass ionomer cements
INTRODUCTION
Glass ionomer cements (GICs) are widely used dental materials first introduced to dentistry in 1972 by Wilson and Kent.[1] GICs are especially used as restorative materials in children dentistry, as well as liners and bases.[2] To improve the physical efficacy of the GICs a variety of filling materials were used such as bioactive glass and hydroxyapatite.[3] In addition to improve the anti-bacterial properties of GICs, anti-bacterial materials such as cetylpyridinium chloride, cetrimide and benzalkonium chlorhexidine (CHX) were used.[4,5,6] Nowadays, novel anti-bacterial agents like titanium dioxide nanoparticles are also used as anti-bacterial filling materials.[3]
Amongst the anti-bacterial applications CHX is accepted to be the gold standard in dentistry.[6] As well as fluoride is a possible agent for preventing dental caries.[7] Fluoride containing GICs has gained great importance because of the sustained levels of fluoride release over-time.[7] For this reason, most GICs include fluoride however fluoride is not enough to combat the bacterial growth.[8] For this reason, to increase the anti-bacterial properties of GICs, the controlled release of CHX from GICs that include fluoride is a potent solution.[6]
The low molecular weight of CHX results in slow release, which is also desired to control the bacterial growth for a longer duration compared to flouride.[8,9] In an experimental CHX release study, 10% of the total CHX diacetate loaded in the GICs (11.28%) were released after 240 days.[5] Fluoride can also be encapsulated into chitosan microparticles to achieve controlled and long term release.[10] Furthermore adding 1% CHX diacetate into Fuji IX GICs reduced the bacterial growth.[11]
Thus, to increase the anti-bacterial properties of GICs, formulations that include both fluoride and CHX are promising. However, it is also important to determine the biocompatibility properties of new formulations. Adding CHX to GICs may alter the in vitro biocompatibility properties. CHX has cytotoxic effects on a variety of eukaryotic cells, which can alter membrane permeability, protein synthesis, lysosomal enzyme release, mitochondrial function disturbance, intracellular Ca increase and oxidative stress.[12,13,14]
In this study, biocompatibilities of GICs that include fluoride either with CHX or without CHX were evaluated. Various cells are used for in vitro test systems to evaluate the biocompatibility of GICs such as osteoblasts, fibroblasts, bone marrow and osteoclasts.[15] In this study, L929 mouse connective tissue fibroblasts were used for in vitro cytotoxicity studies due to its fibroblastic nature to be a model of gingival fibroblasts.[16]
MATERIALS AND METHODS
Test materials, chemicals and reagents
L929 mouse connective tissue fibroblasts were obtained from HUKUK (Foot and Mouth Disease Institute, Animal Cell Culture Collection, Ankara, Turkey). Cell culture medium Roswell Park Memorial Institute (RPMI 1640), L-glutamine, gentamicine, fetal bovine serum (FBS) were obtained from Biochrom (Germany). Trypsin and MTT (3-4,5-dimethyldiazol-2-yl]-2, 5 diphenyl tetrazolium bromid) were from Sigma (Germany) and Trypan blue and dimethyl sulphoxide (DMSO) (dimethylsulfoxide were from Applichem (Germany). The test materials were Fuji IX and Fuji IX-CHX (GC Corporation, Japan).
Cylindrical specimens (5 mm in diameter × 5 mm thick) of each GIC material were prepared by using teflon molds. All cement pastes were dispensed and evenly mixed according to the manufacturers’ instructions. All specimens were shaped as described by Lin et al., 2008. Briefly, GICs were filled into the cylindrical teflon molds (which were autoclaved for sterilization before), sandwiched between 2 glass plates and separated by cellophane strips. Excess cement was removed and the specimens were allowed to completely set according to the time recommended by the manufacturer.[17] There were four experimental groups; Fuji IX, Fuji IX with varnish, Fuji IX-CHX and Fuji IX-CHX with varnish containing three samples were tested for three replicates.
In vitro cytotoxicity test
L929 mouse connective tissue fibroblasts were routinely cultivated in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (100 mg/mL) at 37°C and 5% CO2. 50000 cells were seeded onto 12 mm diameter round shaped coverslips (Menzel-Glaser, Germany), which are placed in 24 well plates and incubated for 24 h at 37°C. After overnight attachment of the cells, 0.4 μm, 0.5 cm2 polycarbonate tissue culture inserts (Nunc, Germany) were placed on the coverslips and the GICs specimens were placed on the tissue culture inserts. After 72 h, the inserts and cements were discarded from the culture plates and cell viabilities were determined by using MTT assay to evaluate the effects of the cements on the mitochondrial function [Figure 1]. In addition, cells remained attached on the coverslips were scanned by electron microscopy [Figure 2].
Figure 1.
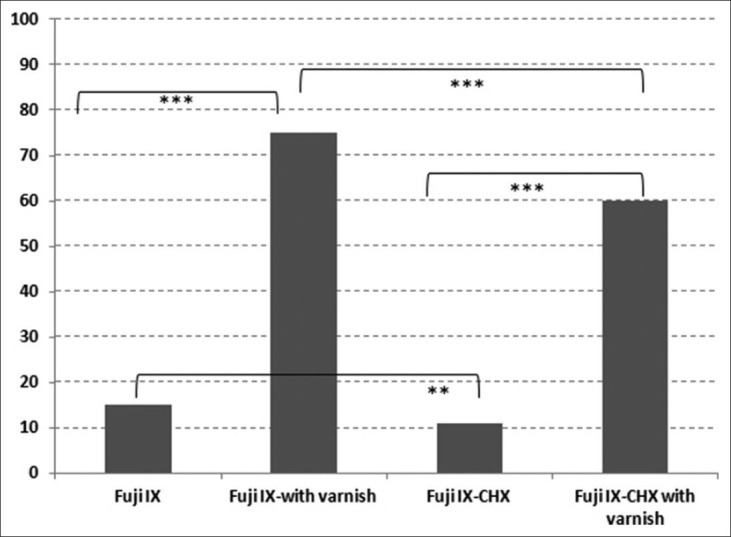
Cell viability rates after treatment with glass ionomer cements samples
Figure 2.
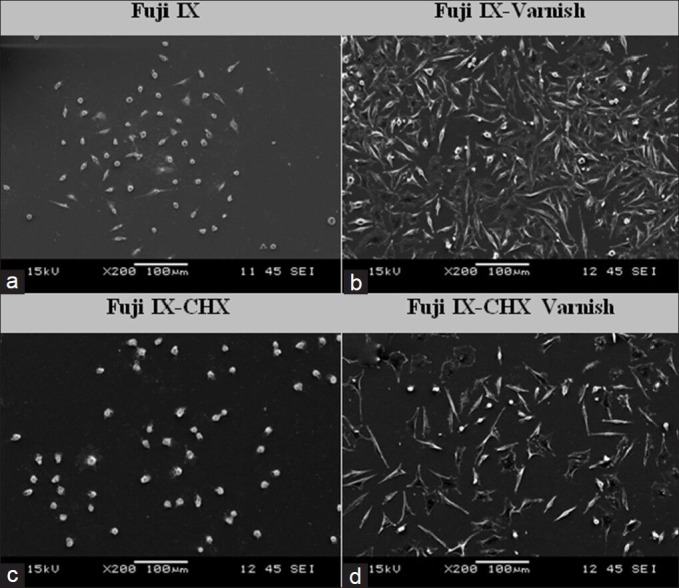
Scanning electron microscopy images taken after 72 h treatment period. (a) Fuji IX. (b) Fuji IX-varnish. (c) Fuji IX- chlorhexidine (CHX)-varnish. (d) Fuji IX-CHX-varnish (×200 magnification)
MTT is a colorimetric assay, which measures the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide to a purple formazan product. After discarding the exposure medium, 0.5 mg/mL of MTT were added to each well and incubated at 37°C, and 5% CO2 for 4 h. After that, 200 μl DMSO was added to each well to dissolve the formazan salts. The absorbance was immediately determined at 570 nm using an ultraviolet-visible single beam spectrophotometer (Jenway, 6400, UK).
Scanning electron microscopy analysis
In order to examine the morphology, especially the shape and attachment properties of the L929 cells on the coverslips, SEM were used. Briefly, cells on the coverslips were fixed with 2.5% glutaraldehyde in 0.1M cacodylate buffer (pH 7.4) for 30 min, post-fixed in 1% osmium tetraoxide in distilled water for 30 min, dehydrated through a graded ethanol series, desiccated for 5 min in hexamethyldizilasane, followed by air drying for 30 min and incubation in a desiccator with phosphorus pentaoxide overnight. Finally, samples were coated with a thin layer of gold by ion sputtering. Samples were then examined using a JEOL JSM-6060 SEM (Tokyo, Japan).
Statistical analysis
All assays were repeated at least three times to ensure reproducibility and three replicates of each group were performed in each test. The significance of difference between GIC types with and without CHX as well as varnish coated or not was analyzed by one-way analysis of variance followed by Benferroni's post-hoc comparision by Graphpad Prism version 5.0 for Windows (Graphpad Software, USA). A P < 0.05 was considered to be statistically significant.
RESULTS
GICs are widely used in dentistry so their biocompatibility is a very important issue. In this study, the biocompatibilities of Fuji IX with and without CHX as well as with and without varnish coating were examined by L929 cells. The cell viability ratios were determined by MTT assay while the morphologies of the cells were scanned by SEM.
Cell viability rates after incubated with different GIC samples were determined by MTT assay. The absorbances of the control cells not incubated with any GIC were considered to be one hundred. For each GIC sample, the absorbances were converted into percentages relative to control cells. There were statistically significant differences among GICs with and without CHX after 72 h (P < 0.001) [Figure 1]. The highest cell viability rate was achieved by Fuji IX with varnish (75%), the lowest cell viability rate was achieved by Fuji IX-CHX without varnish (11%). Cell viabilities of Fuji IX compared with Fuji IX with varnish and Fuji IX-CHX compared to Fuji IX-CHX with varnish were significantly lower (P < 0.001) [Figure 1].
Fuji IX without varnish demonstrated 15% cell viability while Fuji IX with varnish demonstrated 75% cell viability with a significant difference (P < 0.001). Fuji IX-CHX without varnish demonstrated 11% cell viability while Fuji IX-CHX with varnish demonstrated 60% cell viability (P < 0.001). In addition, cell viabilities of the GICs that are containing CHX were significantly lower compared with GICs without CHX. The cell viability ratio difference of Fuji IX without varnish compared with Fuji IX-CHX without varnish was significant (P < 0.05). Similarly, there was a statistically significant difference among Fuji IX with varnish compared with Fuji IX-CHX with varnish (P < 0.001).
Cell morphologies were examined by SEM, 72 h after treatment; ×200 magnifications of the images are shown on Figure 2. Fuji IX and Fuji IX-CHX with varnish the morphologies of the cells were elongated flattened healthy fibroblastic nature and the confluency ratio of the cells on the coverslip was reflecting almost the same ratios obtained by MTT assay. However, Fuji IX and Fuji IX-CHX without varnish, the cells were detached from the surface with a round unhealthy nature, which were also correlating with the cell viability rates obtained by MTT assay.
The cytotoxicities of GICs in vitro were mostly attributed to the leachable materials from them. On Figure 3 (×750 magnification), the leachable components from Fuji IX (A) and Fuji IX-CHX (C) without varnish were shown like small dots. However, using varnish prevents the leakage of the GIC components to the cell culture medium, there were not any debris as seen on the Fuji IX (B) and Fuji IX-CHX (D).
Figure 3.
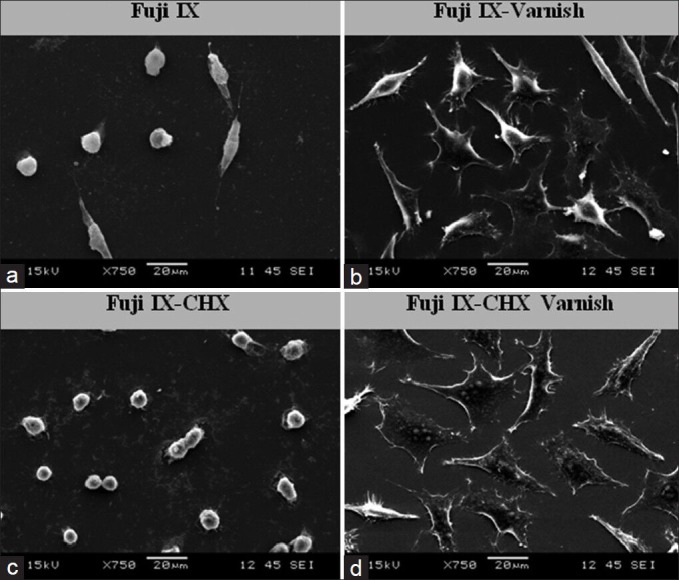
Scanning electron microscopy images taken after 72 h treatment period to show glass ionomer cements competent release. (a) Fuji IX. (b) Fuji IX-Varnish. (c) Fuji IX-chlorhexidine. (CHX)-varnish. (d) Fuji IX-CHX-varnish (×750 magnification)
DISCUSSION
To increase the anti-bacterial properties of GICs different formulations are under development. Amongst them formulations that include both fluoride and CHX are promising. However, it is also important to determine the biocompatibility properties of new formulations. Adding CHX to GICs may alter the in vitro biocompatibility properties.
In this study, the biocompatibilities of Fuji IX with and without CHX as well as with and without varnish coating were examined by L929 cells. The highest cell viability ratio was obtained by Fuji IX with varnish followed by Fuji IX-CHX with varnish (P < 0.001). The lowest cell viability ratio was obtained by Fuji IX-CHX without varnish followed by Fuji IX without varnish (P < 0.05). Thus adding CHX significantly reduces the cell viability (P < 0.05). However using varnish significantly increases the cell viability (P < 0.001).
Especially, varnishes were applied to the GICs when they were used as restorative materials in clinical use to prevent water loss from the immature GICs.[18] In addition, this study shows coating the GICs with varnish reduce the cytotoxicities of GICs by preventing the component leakage from them.
In this study, adding CHX to GICs reduced the cell viability. Apart from anti-bacterial studies, various studies were done to determine in vitro and in vivo cytotoxicities of CHX. It was shown that CHX has serious toxic effects on several cells such as gingival fibroblasts, endothelial cells, alveolar osteoblast cells and odontoblast-like cells in vitro[14,19,20] and on rat blood and kidney cells in vivo.[21]
In this study, Fuji IX coated with varnish is found to be the most biocompatible one among others. It is assumed, due to the leakage of CHX and the other components of the GICs the cell viabilities are decreased.
Several modifications are carried out to enhance the mechanical and physical properties of GICs as well as anti-bacterial properties. To increase the anti-bacterial properties of GICs, formulations that include CHX are promising. However, it is also important to determine the biocompatibility properties of new formulations. Adding CHX to GICs may alter the in vitro biocompatibility properties of GICs.
Various in vitro tests are used to evaluate the biocompatibility of GICs but Hatton et al., 2006 stated that GICs are classified as bioactive rather than bioinert. Thus, the results of the in vitro tests are not reflecting the real in vivo situation.[15]
CONCLUSION
In dentistry, in vitro cell culture studies are important tools to evaluate the cytotoxicities of restorative dental materials before in vivo applications. However, in vitro test systems have several limitations in testing surface bioactive materials like GICs,[15] thus the correlation of in vitro laboratory tests with in vivo clinical trials are inevitable. To achieve this correlation, clinical trials, which last at least 10-20 years must be done to see the real efficacy of the dental materials on the target tissue and patient because of the limitations of the in vitro test systems.[21,22,23]
ACKNOWLEDGEMENTS
This study was partially funded by ‘Ege University, Directorate of Administrative and Financial Affairs by a grant number 2006-DIS-015.
We would like to thank GC Cooperation, Japan for providing the restorative materials.
Footnotes
Source of Support: This study was partially funded by ‘Ege University, Directorate of Administrative and Financial Affairs by a grant number 2006-DIS-015.
Conflict of Interest: None declared
REFERENCES
- 1.Wilson AD, Kent BE. The glass-ionomer cement: A new translucent dental filling material. J Appl Chem Biotechnol. 1971;21:313–8. [Google Scholar]
- 2.Czarnecka B, Nicholson JW. Ion release by resin-modified glass-ionomer cements into water and lactic acid solutions. J Dent. 2006;34:539–43. doi: 10.1016/j.jdent.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J Dent. 2011;39:589–98. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Botelho MG. Inhibitory effects on selected oral bacteria of antibacterial agents incorporated in a glass ionomer cement. Caries Res. 2003;37:108–14. doi: 10.1159/000069019. [DOI] [PubMed] [Google Scholar]
- 5.Palmer G, Jones FH, Billington RW, Pearson GJ. Chlorhexidine release from an experimental glass ionomer cement. Biomaterials. 2004;25:5423–31. doi: 10.1016/j.biomaterials.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–53. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu H, Yamamato H, Nomachi M, Yasuda K, Matsuda Y, Murata Y, et al. Fluorine uptake into human enamel around a fluoride-containing dental material during cariogenic pH cycling. Nucl Instrum Methods Phys Res B. 2007;260:201–6. [Google Scholar]
- 8.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–96. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Verraedt E, Pendela M, Adams E, Hoogmartens J, Martens JA. Controlled release of chlorhexidine from amorphous microporous silica. J Control Release. 2010;142:47–52. doi: 10.1016/j.jconrel.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Keegan GM, Smart JD, Ingram MJ, Barnes LM, Burnett GR, Rees GD. Chitosan microparticles for the controlled delivery of fluoride. J Dent. 2012;40:229–40. doi: 10.1016/j.jdent.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Deepalakshmi M, Poorni S, Miglani R, Rajamani I, Ramachandran S. Evaluation of the antibacterial and physical properties of glass ionomer cements containing chlorhexidine and cetrimide: An in-vitro study. Indian J Dent Res. 2010;21:552–6. doi: 10.4103/0970-9290.74217. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo E, Dominguez C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro. 2001;15:271–6. doi: 10.1016/s0887-2333(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro DA, Scolastici C, De Lima PL, Marques ME, Salvadori DM. Genotoxicity of antimicrobial endodontic compounds by single cell gel (comet) assay in Chinese hamster ovary (CHO) cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:637–40. doi: 10.1016/j.tripleo.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Giannelli M, Chellini F, Margheri M, Tonelli P, Tani A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol In Vitro. 2008;22:308–17. doi: 10.1016/j.tiv.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Hatton PV, Hurrell-Gillingham K, Brook IM. Biocompatibility of glass-ionomer bone cements. J Dent. 2006;34:598–601. doi: 10.1016/j.jdent.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Zange R, Kissel T. Comparative in vitro biocompatibility testing of polycyanoacrylates and poly (D, L-lactide-co-glycolide) using different mouse fibroblast (L929) biocompatibility test models. Eur J Pharm Biopharm. 1997;44:149–57. [Google Scholar]
- 17.Lin YC, Lai YL, Chen WT, Lee SY. Kinetics of fluoride release from and reuptake by orthodontic cements. Am J Orthod Dentofacial Orthop. 2008;133:427–34. doi: 10.1016/j.ajodo.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson JW, Czarnecka B. Kinetic studies of the effect of varnish on water loss by glass-ionomer cements. Dent Mater. 2007;23:1549–52. doi: 10.1016/j.dental.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Rajabalian S, Mohammadi M, Mozaffari B. Cytotoxicity evaluation of persica mouthwash on cultured human and mouse cell lines in the presence and absence of fetal calf serum. Indian J Dent Res. 2009;20:169–73. doi: 10.4103/0970-9290.52894. [DOI] [PubMed] [Google Scholar]
- 20.de Souza LB, de Aquino SG, de Souza PP, Hebling J, Costa CA. Cytotoxic effects of different concentrations of chlorhexidine. Am J Dent. 2007;20:400–4. [PubMed] [Google Scholar]
- 21.Grassi TF, Camargo EA, Salvadori DM, Marques ME, Ribeiro DA. DNA damage in multiple organs after exposure to chlorhexidine in Wistar rats. Int J Hyg Environ Health. 2007;210:163–7. doi: 10.1016/j.ijheh.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.de Souza Costa CA, Hebling J, Garcia-Godoy F, Hanks CT. In vitro cytotoxicity of five glass-ionomer cements. Biomaterials. 2003;24:3853–8. doi: 10.1016/s0142-9612(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 23.Bayne SC. Correlation of clinical performance with ‘in vitro tests’ of restorative dental materials that use polymer-based matrices. Dent Mater. 2012;28:52–71. doi: 10.1016/j.dental.2011.08.594. [DOI] [PubMed] [Google Scholar]


