Abstract
We describe a rare case of a 19-year-old male patient with a history of epilepsy and developmental delay who presented with acute renal failure (ARF) and lactic acidosis (LA) as the first manifestation of T-cell lymphoblastic lymphoma. Renal ultrasound and CT of the abdomen showed renal parenchymal infiltration, and renal biopsy demonstrated T-cell lymphoblastic lymphoma. LA, ARF and electrolyte abnormalities were refractory to the initial treatment of bicarbonate infusion and hydration. However, these abnormalities rapidly normalised after the initiation of chemotherapy, suggesting that the LA and ARF were secondary to lymphomatous renal infiltration.
Background
Patients with cancer occasionally present with acute renal failure (ARF) and lactic acidosis (LA) from multiple aetiologies: tumour lysis syndrome (TLS), neutropenic sepsis, nephrotoxic agents, nutrition deficiency and dehydration. However, ARF from primary renal infiltration and LA as first manifestation of their underlying malignancy are very rare, and LA frequently occurs in the absence of tissue ischaemia. This is classified as type B LA, and its mechanism in patients with cancer is multifactorial including high glucose metabolism affected by elevated levels of growth factors and cytokines in malignant cells, and the lactate accumulation process is facilitated by Warburg effect. Type B LA and ARF associated with malignancy are refractory to hydration and bicarbonate infusion, and they are considered as an oncological emergency requiring accurate diagnosis and prompt treatment with chemotherapeutic agents.
Case presentation
A 19-year-old male patient with a history of epilepsy and developmental delay secondary to hypoxic brain injury was directly admitted from clinic for high-blood pressure and urinary frequency. Two weeks prior to presentation, the patient had reported lightheadedness while standing from a seated position and urinary frequency every 2 h associated with bilateral mid-back pain. Blood pressure on admission was 161/100 mm Hg with heart rate of 99 bpm. The rest of the vitals were normal. Physical examination was remarkable for a palpable spleen in the left upper quadrant. There was no lymphadenopathy or lower extremity swelling. Pertinent laboratory data revealed white cell count of 8.8 K/μL (normal 3.4–10.4 K/μL), haemoglobin 17.7 g/dL (normal 13.5–17.5 g/dL), haematocrit 51.3% (normal 40–51%), platelet 316 K/μL (normal 150–425 K/μL), sodium 139 mmol/L (normal 136–145 mmol/L), potassium 4.5 mmol/L (normal 3.4–5.1 mmol/L), chloride 100 mmol/L (normal 98–107 mmol/L), bicarbonate (HCO3) 17 mmol/L (normal 20–29 mmol/L), blood urea nitrogen 33 mg/dL (normal 8–23 mg/dL), creatinine 2.5 mg/dL (normal 0.67–1.17 mg/dL; the patient's baseline was 0.7), calcium (Ca) 11.1 mg/dL (normal 8.8–10.2 mg/dL; corrected Ca 11.4 mg/dL), magnesium 1.7 mg/dL (normal 1.6–2.6 mg/dL), phosphorus 6.6 mg/dL (normal 2.7–4.9 mg/dL), uric acid 8.8 mg/dL (normal 3.5–8.2 mg/dL) and anion gap was 22 (figure 1). Subsequent venous blood gas demonstrated pH 7.24, partial pressure of carbon dioxide (CO2) 28 mm Hg, partial pressure of oxygen 87 mm Hg and HCO3 17 mmol/L with lactate of 11.2 mmol/L (normal 0.5–2.2 mmol/L). Laboratory studies revealed ARF and LA. Additional workup included: urine eosinophils, metanephrines, aldosterone/renin, ammonia, thyroid stimulating hormone, serum protein electrophoresis with immunofixation, C3, C4, antinuclear antibodies, antineutrophil cytoplasmic antibody and HIV test which were all negative or within normal range. Lactate dehydrogenase and pyruvate were elevated at 733 U/L (normal 135–225 U/L) and 0.293 mmol/L (normal 0.03–0.107 mmol/L), respectively, suggesting possible underlying malignancy and type B LA.1 Subsequent renal ultrasound showed bilateral large kidneys with infiltrative process (figure 2), and CT of the abdomen demonstrated grossly enlarged kidneys with infiltrative changes in the parenchyma, splenomegaly and para-aortic lymphadenopathy (figure 3).
Figure 1.
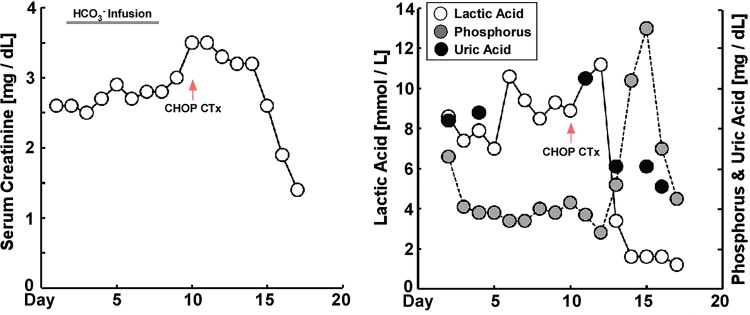
Laboratory values during the hospital course. Patient presented with elevated serum creatinine level and lactic acidosis. HCO3 infusion was started to correct the severe acidosis, however with little effect. CHOP chemotherapy was started at day 10 after diagnosis of T-LBL, and creatinine and lactate level returned to the normal level over the hospital course (CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; HCO3, bicarbonate; T-LBL, T-cell lymphoblastic lymphoma).
Figure 2.
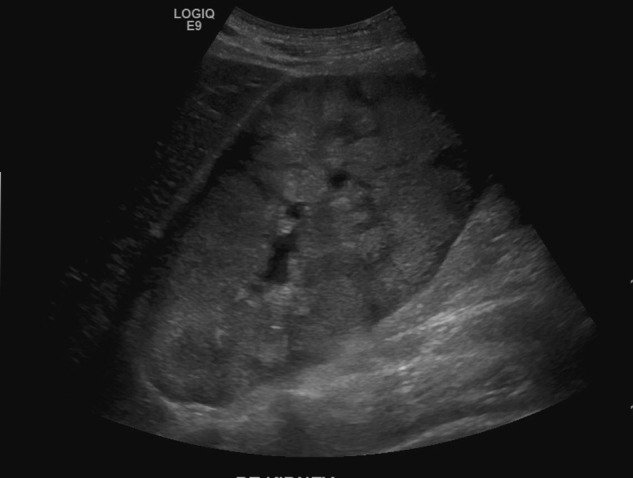
Ultrasonography of the renal showing markedly enlarged right kidney measuring 18 cm with increased echogenicity, decreased cortical medullary differentiation and no evidence of focal cortical thinning, discrete renal mass, calcification or hydronephrosis.
Figure 3.
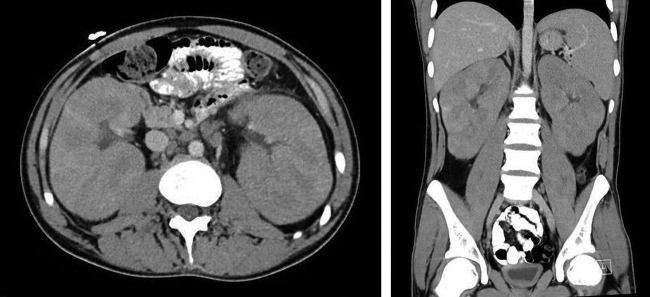
CT of the abdomen and pelvis demonstrating gross enlargement of both kidneys with lymphomatous infiltration and splenomegaly. Renal spans are 18 cm on the right and 16 cm on the left. There is abnormal para-aortic lymphadenopathy at the level of the renal veins, with the largest node in the left para-aortic position measuring 24×15 mm axially. The span of the spleen is 14.5 cm with no focal abnormality.
Bicarbonate infusion was initiated to treat the metabolic acidosis, however creatinine and lactic acid levels continued to trend up without resolution of the metabolic acidosis (figure 1). A diagnostic renal biopsy was performed and demonstrated a monotonous population of small-to-medium sized lymphoid cells with scant cytoplasm diffusely effacing the normal renal parenchyma, and obscured renal architecture with compressed glomeruli from dense lymphocytic infiltration. Immunohistochemistry staining was positive for CD1a, CD3, CD5, CD43 and terminal deoxynucleotidyl transferase, and was negative for CD10, CD20, CD30, CD45, cyclin D1 and Bcl-2. Follow-up bone marrow (BM) biopsy, flow cytometry and peripheral smear did not demonstrate circulating blasts. The BM was normocellular with 3+ iron and no evidence of neoplasm. Thus, confirming the diagnosis of primary T-cell lymphoblastic lymphoma (T-LBL) with primary renal infiltration.2
Outcome and follow-up
Rasburicase, allopurinol and intravenous hydration were started immediately,3–7 and the first course of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy was started on day 10.8–12 Uric acid and potassium levels rose temporarily due to tumour lysis from chemotherapy,6 7 13–17 while serum creatinine, lactate and bicarbonate levels normalised shortly after the initiation of CHOP therapy, suggesting that ARF and LA were secondary to lymphomatous renal infiltration (figure 1). The patient completed two courses of CHOP chemotherapy without further complication and was discharged home with plans for future outpatient chemotherapy.
Discussion
T-LBL is a rare subtype of non-Hodgkin lymphoma (NHL) with an incidence of less than 2% (Surveillance, Epidemiology, and End Results, SEER cancer statistics: http://seer.cancer.gov/csr/1975_2010/). According to the WHO classification, T-LBL is differentiated from T-cell acute lymphocytic leukaemia (T-ALL) by a mass in the mediastinum or elsewhere composed of T lymphoblasts and BM with less than 25% of blasts.2 Although 50–70% of T-ALL carry abnormal karyotypes such as translocation at 14q11.2, 7q35 and 7p14–15 involving the T-cell receptors,18 there is no proven association between genetic abnormality and the biological feature of T-ALL or T-LBL. Accordingly, revised WHO classification does not include further subclassification of T-ALL.2
Mediastinum and lymph nodes are the most commonly involved sites in T-LBL and T-ALL. However, patients can present with extranodal involvement including bone, testicle, skin, pancreas and kidney.19 Renal infiltration with lymphoblastic or leukaemic cells was shown to be relatively common, occurring in 36.7% of cases in one autopsy study of 322 patients with lymphoma.20 However, ARF and LA as presenting features of lymphoma or leukaemia is relatively rare.21 22 Many different conditions including TLS, use of nephrotoxic medications and chemotherapeutic agents can also cause ARF in T-LBL or T-ALL and must be ruled out before assuming renal tumour infiltration is the aetiology of ARF. Proper diagnosis of ARF and LA in haematological malignancy is critical in determining appropriate treatment and improving outcomes. Renal biopsy plays a pivotal role in the diagnosis of primary renal lymphoma.6
The mechanism of renal failure from lymphomatous or leukaemic infiltration remains elusive, although several hypothesis including intrarenal obstruction by compression of the tubular lumen from dense tumour cell infiltration, and exacerbation of fibrosis by cytokines released from tumour cells have been suggested.23 Previous case reports of ARF associated with primary tumour infiltration have successfully been treated with chemotherapy and support the hypothesis that tumour cell infiltration has a direct role in ARF.23
In many cases of lymphoma and leukaemia, ARF is associated with LA.16 23–25 LA is classified as types A, B and D LA.23 26 27 Type A LA occurs with acute ischaemia or tissue hypoperfusion, and it is frequently observed in hypovolaemic or septic shock.28 However, type B LA occurs without any evidence of ischaemia or decreased organ perfusion. Instead, it is caused by an underlying systemic pathology including malignancy, diabetes, HIV infection, severe alcoholism and medications such as metformin (table 1).1 Haematological malignancy is one of the most common underlying causes of type B LA, although the mechanism still remains unclear. Several hypothesis such as liver and kidney dysfunction, overexpression of insulin growth factor-1 and hexokinase along with anaerobic glycolysis, tumour necrosis factor-α (TNF-α) secretion, thiamine deficiency and the use of certain chemotherapeutic agents have been postulated as the mechanisms of type B LA (table 1).23 The liver and kidney are the two major organs that convert lactate into pyruvate, and subsequently into glucose. As such, dysfunction of these two organs from any cause may lead to lactate accumulation. However, the low incidence of LA in other liver and kidney diseases suggests the presence of additional unknown factors that contribute to the development of type B LA. In addition, tumour cells have a predilection for anaerobic glycolysis even with adequate oxygen supply, and a significant amount of glucose is converted to lactate.29 This is known as the Warburg effect. Pyruvate dehydrogenase and its cofactor, thiamine, convert pyruvate to acetyl coenzyme A, and also play a pivotal role in glucose metabolism and lactate production (figure 4).30 Thiamine deficiency, frequently observed in patients with cancer, diminishes the activity of pyruvate dehydrogenase, and leads to lactate accumulation.31 Also, TNF-α is a known pyruvate dehydrogenase inhibitor and is often elevated in lymphoma. This may lead to further anaerobic glycolysis and lactate production.32 As an extrinsic factor, certain types of chemotherapeutic agents can exacerbate LA as well. Methotrexate, a dihydrofolate reductase inhibitor, competes with thiamine for intracellular transport, lowering the level of thiamine in the cells, and subsequently diminishes pyruvate dehydrogenase activity.33 In conclusion, LA in a patient with cancer depends on multiple factors, and careful review of nutrition status and chemotherapeutic regimen is important.
Table 1.
Aetiologies and mechanisms of type B lactic acidosis in cancer23
| Aetiologies of type B lactic acidosis | Mechanisms of type B acidosis in cancer |
|---|---|
| Diabetic ketoacidosis Metformin Haematological malignancy Severe alcoholism HIV infection |
Liver and kidney dysfunction IGF-1 overexpression Hexokinase overexpression TNF-α Thiamine deficiency Methotrexate treatment |
IGF-1, insulin-like growth factor 1; TNF-α, tumour necrosis factor-α.
Figure 4.
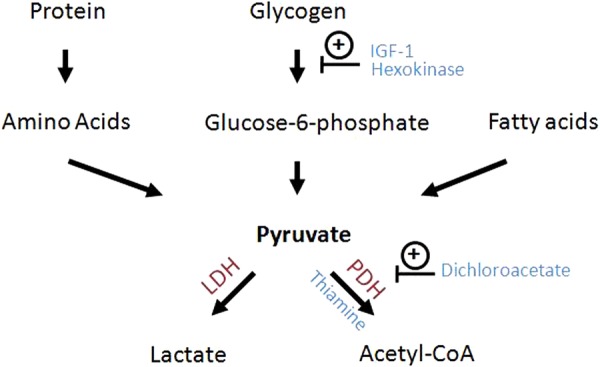
Glucose metabolism pathway. PDH converts pyruvate to acetyl-CoA, which enters into Krebs cycle to generate ATP. However, cancer cells have predilection to anaerobic glycolysis leading to lactate accumulation.29 This process is facilitated by overexpression of IGF-1, hyperactivation of hexokinase and thiamine deficiency, which are frequently observed in the malignancy (CoA, coenzyme A; IGF-1, insulin-like growth factor 1; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase complex).
Treatment of lymphomatous renal infiltration-induced ARF and type B LA is different from that of TLS or type A LA, which needs aggressive volume resuscitation, haemofiltration, haemodialysis or medications such as allopurinol or rasburicase.17 Type B LA and kidney dysfunction are usually refractory to the above treatments, and correction of the underlying pathology is the essential treatment. Accordingly, chemotherapy is the treatment of choice in type B LA associated with malignancy. Thiamine supplementation may also be used if there is concern for thiamine deficiency. In severe LA with pH lower than 7.2, bicarbonate infusion may be used, although it will not alter the course of the disease and it might actually exacerbate the acidosis via CO2 retention.34
In conclusion, ARF and LA as an initial manifestation of primary lymphoma or leukaemia are rare and renal biopsy plays an essential role in the diagnosis of primary renal lymphoma or leukaemia. Type B LA associated with malignancy is a result of multiple mechanisms including thiamine deficiency, growth factors, cytokines and the Warburg effect. The main treatment of type B LA and ARF in patients with lymphoma/leukaemia is chemotherapy with careful review of nutritional status and medications.
Learning points.
The majority of haematological malignancies including lymphoma and leukaemia are associated with lactic acidosis (LA), a poor prognostic indicator.
Acute kidney injury and type B LA secondary to lymphomatous or leukaemic renal infiltration are oncological emergencies, and chemotherapy is the treatment of choice.
Sodium bicarbonate infusion should be avoided as it can exacerbate metabolic acidosis. Although, it may be used with severe acidosis (pH lower than 7.2) and haemodynamic instability.
Footnotes
Contributors: SY, CNW and FA were involved in patient care, and all authors participated in the writing of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vernon C, Letourneau JL. Lactic acidosis: recognition, kinetics, and associated prognosis. Crit Care Clin 2010;26:255–83, table of contents [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937–51 [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol 2008;26:2767–78 [DOI] [PubMed] [Google Scholar]
- 4.Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood 2001;97:2998–3003 [DOI] [PubMed] [Google Scholar]
- 5.Galardy PJ, Hochberg J, Perkins SL, et al. Rasburicase in the prevention of laboratory/clinical tumour lysis syndrome in children with advanced mature B-NHL: a Children's Oncology Group Report. Br J Haematol 2013;163:365–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011;364:1844–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004;127:3–11 [DOI] [PubMed] [Google Scholar]
- 8.Abouyabis AN, Shenoy PJ, Sinha R, et al. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol 2011;2011:623924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisselbrecht C, Lepage E, Molina T, et al. Shortened first-line high-dose chemotherapy for patients with poor-prognosis aggressive lymphoma. J Clin Oncol 2002;20:2472–9 [DOI] [PubMed] [Google Scholar]
- 10.Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 2004;104:634–41 [DOI] [PubMed] [Google Scholar]
- 11.Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood 2004;104:626–33 [DOI] [PubMed] [Google Scholar]
- 12.Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 2006;20:1533–8 [DOI] [PubMed] [Google Scholar]
- 13.Boles JM, Dutel JL, Briere J, et al. Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemia. Cancer 1984;53:2425–9 [DOI] [PubMed] [Google Scholar]
- 14.Hsu HH, Huang CC. Acute spontaneous tumor lysis in anaplastic large T-cell lymphoma presenting with hyperuricemic acute renal failure. Int J Hematol 2004;79:48–51 [DOI] [PubMed] [Google Scholar]
- 15.Hsu HH, Chan YL, Huang CC. Acute spontaneous tumor lysis presenting with hyperuricemic acute renal failure: clinical features and therapeutic approach. J Nephrol 2004;17:50–6 [PubMed] [Google Scholar]
- 16.Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin's lymphoma. Am J Med 1993;94:133–9 [DOI] [PubMed] [Google Scholar]
- 17.Hochberg J, Cairo MS. Tumor lysis syndrome: current perspective. Haematologica 2008;93:9–13 [DOI] [PubMed] [Google Scholar]
- 18.Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest 2012;122:3398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin P, Jones D, Dorfman DM, et al. Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. Am J Surg Pathol 2000;24:1480–90 [DOI] [PubMed] [Google Scholar]
- 20.Miyake O, Namiki M, Sonoda T, et al. Secondary involvement of genitourinary organs in malignant lymphoma. Urol Int 1987;42:360–2 [DOI] [PubMed] [Google Scholar]
- 21.Sellin L, Friedl C, Klein G, et al. Acute renal failure due to a malignant lymphoma infiltration uncovered by renal biopsy. Nephrol Dial Transplant 2004;19:2657–60 [DOI] [PubMed] [Google Scholar]
- 22.Obrador GT, Price B, O'Meara Y, et al. Acute renal failure due to lymphomatous infiltration of the kidneys. J Am Soc Nephrol 1997;8:1348–54 [DOI] [PubMed] [Google Scholar]
- 23.Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore) 2007;86:225–32 [DOI] [PubMed] [Google Scholar]
- 24.Seidemann K, Meyer U, Jansen P, et al. Impaired renal function and tumor lysis syndrome in pediatric patients with non-Hodgkin's lymphoma and B-ALL. Observations from the BFM-trials. Klin Padiatr 1998;210:279–84 [DOI] [PubMed] [Google Scholar]
- 25.Montesinos P, Lorenzo I, Martín G, et al. Tumor lysis syndrome in patients with acute myeloid leukemia: identification of risk factors and development of a predictive model. Haematologica 2008;93:67–74 [DOI] [PubMed] [Google Scholar]
- 26.Luft FC. Lactic acidosis update for critical care clinicians. J Am Soc Nephrol 2001;12(Suppl 17):S15–19 [PubMed] [Google Scholar]
- 27.Uribarri J, Oh MS, Carroll HJ. D-lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine (Baltimore) 1998;77:73–82 [DOI] [PubMed] [Google Scholar]
- 28.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580–637 [DOI] [PubMed] [Google Scholar]
- 29.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891–9 [DOI] [PubMed] [Google Scholar]
- 31.Naito E, Ito M, Yokota I, et al. Thiamine-responsive lactic acidaemia: role of pyruvate dehydrogenase complex. Eur J Pediatr 1998;157:648–52 [DOI] [PubMed] [Google Scholar]
- 32.Zell R, Geck P, Werdan K, et al. TNF-alpha and IL-1 alpha inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: evidence for primary impairment of mitochondrial function. Mol Cell Biochem 1997;177:61–7 [DOI] [PubMed] [Google Scholar]
- 33.Zhao R, Gao F, Goldman ID. Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. Am J Physiol Cell Physiol 2002;282:C1512–17 [DOI] [PubMed] [Google Scholar]
- 34.Sabatini S, Kurtzman NA. Bicarbonate therapy in severe metabolic acidosis. J Am Soc Nephrol 2009;20:692–5 [DOI] [PubMed] [Google Scholar]


