Abstract
Bortezomib is a chemotherapeutic agent that acts via proteasome inhibition resulting in cellular apoptosis and inhibition of angiogenesis. Although widely accepted as treatment of multiple myeloma and non-Hodgkin's lymphoma, it has also been shown to be efficacious in a variety of solid tumours such as pancreatic and colonic. Posterior reversible encephalopathy syndrome (PRES) is a neuroradiological syndrome characterised by vasogenic oedema involving the postero-occipital cortical and subcortical white matter resulting in visual disturbances, seizures and altered mental status. Although in most cases PRES is reversible with removal of the provoking condition or drug, if not appropriately recognised and treated it may lead to permanent and life-threatening sequelae such as intracerebral haemorrhage and ischaemic infarction. We report a case of PRES associated with bortezomib therapy and contrast it with four other previously reported cases. Recognition of this potentially severe neurological complication is important with the increasingly widespread use of bortezomib.
Background
Molecularly targeted agents are being increasingly used as therapeutic options for haematological malignancies and solid tumours. Bortezomib, a proteasome inhibitor, has been widely accepted for therapy of multiple myeloma and non-Hodgkin's lymphoma. It also has demonstrated activity against other difficult to treat solid tumours implicating its potential broader use in the future.
Posterior reversible encephalopathy syndrome (PRES) is a neuroradiological syndrome first described by Hinchey et al.1 Clinically, it usually presents with seizures, headaches and altered level of consciousness. Hypertension is associated in 70–80% of cases, however the remaining have normal, or near normal pressure.1 2 Characteristic MRI findings allow for radiographic diagnosis of PRES. Both vasogenic and cytotoxic oedema appear as symmetrical lesions in the posterior regions of the cerebral hemisphere.3 T2-hyperintense signal alterations are seen on MRI in the cortical and subcortical white matter, most commonly in the posterior parieto-occipital region.4 Similar white matter changes have also been reported in brain stem, cerebellum and basal ganglia.5–7
We present a case of PRES associated with bortezomib therapy for multiple myeloma. It is important for clinicians to be aware of how to diagnose and treat this rare but potentially life-threatening complication. Recognition of predisposing factors can allow for early recognition and identification of those patients who may be at highest risk of developing this syndrome.
Case presentation
A 71-year-old woman with a medical history of dyslipidemia and hypertension (well controlled on 3 oral agents) was diagnosed with stage III λ light chain oligosecretory multiple myeloma complicated by renal failure and hypercalcaemia. Her initial treatment of pulse dexamethasone was stopped due to significant fatigue and she was switched to a combination of lenalidomide and weekly dexamethasone, which resulted in clinical remission. Unfortunately, despite good clinical response she developed diarrhoea and peripheral neuropathy such that treatment was ultimately discontinued after only 5 months of therapy. Subsequently, treatment with melphalan, prednisone and bortezomib was initiated on a twice-weekly schedule (Tuesday/Friday×2 weeks). Her treatment interval was increased to weekly dosing after only 1 month of treatment due to myelosuppression . After 6 months of this treatment regimen she had achieved a partial response and further therapy at that time was held.
Three months later, during routine haematology follow-up, she was noted to have a recurrence of easy fatigability. Serum protein electrophoresis revealed an increase in her monoclonal protein and a rising λ light chain level indicative of progressive disease. It was decided that she would complete further treatment with melphalan, prednisone and bortezomib.
After receiving the first dose of repeat bortezomib therapy, she experienced an abrupt onset of frontal throbbing headache that persisted over the course of the night. The following morning her husband witnessed her having a generalised tonic–clonic (GTC) seizure lasting for 3 min. She was taken to a hospital in the periphery where she had two further GTC seizures witnessed by medical staff. She was subsequently transferred to a tertiary care hospital for further investigation and management.
Investigations
On presentation to the emergency department her blood pressure was 175/67 mm Hg. Neurological examination showed altered level of consciousness with no localising features. Glasgow Coma Scale was 6. Nuchal rigidity was not present.
Blood work revealed normal renal and hepatic function. Blood glucose, serum electrolytes and ammonia levels were all within normal limits. All cultures of blood and sputum and viral serology were negative. A lumbar puncture was not completed due to significant thrombocytopenia and the risk of bleeding.
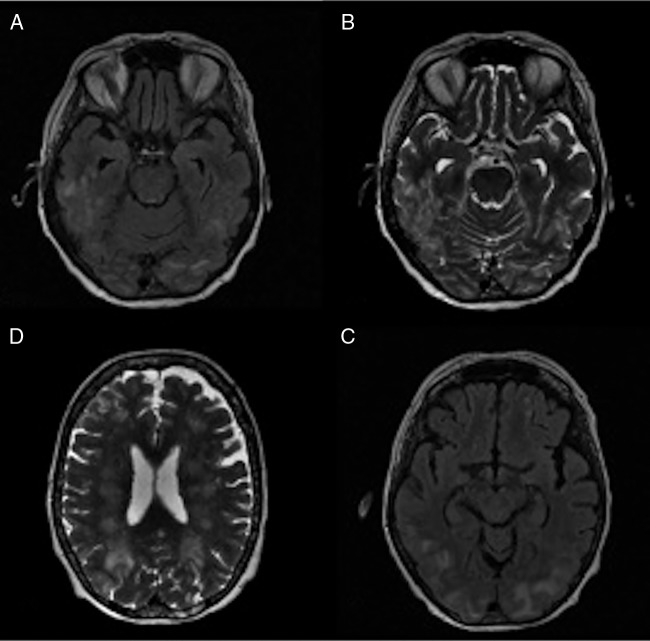
A CT scan was carried out initially and showed hypodense areas in the white matter primarily in the occipital lobes. MRI showed diffuse bilateral oedema throughout the cerebral white matter involving the pons and cerebellum, consistent with a diagnosis of PRES (figure 1).
Figure 1.
Cerebral MR. (A) Extensive bilateral symmetric fluid attenuated inversion recovery (FLAIR) hyperintensities in the parieto-occipital regions and posteroinferior temporal lobes. (B) T2-weighted image showing multifocal patchy cortical/subcortical hyperintensities in the posteroinferior temporal lobes and occipital lobes bilaterally representing vasogenic oedema. (C) T2-weighted image showing multifocal patchy frontoparieto-occipital cortical/subcortical hyperintensities bilaterally with extensive white matter involvement. (D) Extensive bilateral symmetric FLAIR hyperintensities in the parieto-occipital regions and posteroinferior temporal lobes.
Treatment
Prior to transfer to the tertiary centre the patient was loaded with phenytoin and intubated for airway protection. On arrival she was given intravenous labetalol to lower her blood pressure (25% reduction which was achieved in the first 2 h). She was taken to the intensive care unit (ICU) for mechanical ventilation, invasive monitoring and further supportive care. Throughout the remainder of her admission her blood pressure was reasonably controlled between 115 and 160 systolic. She was discharged on oral phenytoin in addition to her other usual home medications. Further treatment with bortezomib was discontinued.
Outcome and follow-up
Following her discharge the patient had no seizure activity. She was taken off phenytoin therapy 4 months after discharge. Her haematologist considered returning to therapy with melphalan, prednisone and bortezomib but ultimately a decision was made not to retry bortezomib therapy, given her previous life-threatening episode of PRES and the risk of recurrence with re-exposure to bortezomib. She had no repeat episodes of PRES. She passed away approximately 5 months later from complications of progressive treatment refractory multiple myeloma.
Discussion
Bortezomib is a proteasome complex inhibitor that induces cell cycle arrest, apoptosis and inhibition of angiogenesis via interruption of cell signalling.5 It is used as first-line treatment of multiple myeloma for those patients who are not amenable to stem cell transplantation. It has also been used in the treatment of non-Hodgkin's lymphoma. Although not approved for use in solid organ tumours, it has been shown in early studies to have some antineoplastic effects against pancreatic and colon cancer.8 Common adverse effects include gastrointestinal symptoms, anaemia, thrombocytopenia and fatigue. The most common adverse neurological effect is peripheral neuropathy, which can be dose limiting. Central nervous system complications are typically not associated with bortezomib. We present a temporally linked case of PRES associated with bortezomib use. Discontinuation of bortezomib treatment resulted in resolution of PRES. There have now been four other cases that implicate bortezomib as a causative agent for PRES (summarised in table 1).9–12 In contrast to the other four cases, this case is unique in that the patient had previously received bortezomib with no significant complications, and it was not until a repeat treatment cycle with bortezomib 3 months later that PRES occurred, suggesting that previous successful tolerance does not reduce the future risk of PRES. In addition this case is novel for the severe degree of PRES that was caused by bortezomib, which is distinct from the previous cases of mild-to-moderate PRES. This is the first report of a patient becoming comatose and requiring prolonged intubation and invasive mechanical ventilation in the ICU. Risk factors for development of PRES include hypertension, renal dysfunction, immunosuppression and treatment with chemotherapeutic agents.13 The patient presented here had all of these risk factors with the exception of renal dysfunction. This is an important complication to describe given that this is a first-line treatment in many haematological and solid tumour malignancies; if patients develop PRES it can result in significant morbidity and mortality.
Table 1.
Cases of PRES associated with bortezomib therapy
| Age/sex | Diagnosis | Therapy | Clinical features | Imaging | Outcome | Reference |
|---|---|---|---|---|---|---|
| 66, M | Waldenstrom macroglobinaemia | 2 cycles/Bort | Altered level of consciousness, GTC seizures | Hyperintense subcortical white matter of occipital lobes (MRI) | No persistent neurological deficit | Kelly et al10 |
| 62, F | Multiple myeloma | 2 cycles/Bort+Dex | Headache, visual disturbances, impaired speech | Hypodense lesions in bilateral parieto-occipital lobes (CT) | No persistent neurological deficit | Kager et al9 |
| 58, F | Multiple myeloma | 1 cycle/Bort+Dex | Bilateral painless vision loss | Hyperintense lesions in bilateral occipital lobes (MRI) | Improvement in vision, incomplete recovery | Terwiel et al12 |
| 54, F | Multiple myeloma | 1 cycle/Bort+Dex | Altered level of consciousness, GTC seizure | High-intensity signal in subcortical white matter of thalamus and occipital lobes (MRI) | No persistent neurological deficit | Oshikawa et al11 |
| 71, F | Multiple myeloma | 1 cycle of second course/Bort+Pred+Melph | Altered mental status, seizures | Hyperintense parieto-occipital and posteroinferior temporal lobe (MRI) | No persistent neurological deficit | Present case |
Bort, bortezomib; Dex, dexamethasone; F, female; GTC, generalised tonic–clonic; M, male; Melph, melphalan; Pred, prednisone; PRES, posterior reversible encephalopathy syndrome.
There are two potential mechanisms for PRES reported in the literature. The first relates to uncontrolled hypertension in conjunction with failed cerebral autoregulation leading to the typical MRI changes of PRES.1 6 7 As blood pressures rise above the upper limit of the autoregulatory capacity of vessels in the brain, the blood–brain barrier breaks down causing vasodilation and oedema. Since white matter is less densely packed than cortex, changes are primarily seen here.2 6 This mechanism is well recognised in the literature6 but is contradicted by reports of PRES occurring with lower degrees of hypertension,3 14 and also by the observation that severity of oedema does not correlate with the degree of hypertension.14 A second theory has been used to explain the occurrence with chemotherapy and immunosuppressive agents, and suggests that cerebral vasoconstriction as a result of sudden changes in pressure lead to the changes seen. Vasoconstriction results in ischaemia and subsequently cytotoxic oedema. This theory is supported by the observation that MRI changes occur in watershed areas between vascular territories that are at greater risk of ischaemia. Also, studies using cerebral angiography on patients with clinical and radiological evidence of PRES show vasoconstriction in the posterior and middle cerebral arteries.15 Evidence against this theory, however, is that PRES is frequently reversible with removal of the causative agent, whereas cytotoxic oedema results in irreversible brain defects.2 3
In addition to hypertension, other conditions associated with PRES include eclampsia, transplantation (allograft, bone marrow and solid organ), immunosuppressive agents and autoimmune disorders.1 2 In recent years, cytotoxic chemotherapies are becoming increasingly recognised as a predisposing factor associated with PRES.3 12 16 In most cases, PRES is rapidly reversible with recognition and removal of the causative agent, however, failure to do so may result in profound and permanent nervous system damage. Similar to the previous four cases of PRES associated with bortezomib therapy, the case presented shares a number of features. The current case supports the observation that bortezomib-induced PRES is more common in women, with four of the five current cases occurring in female patients. This is also consistent with cases reported for other molecular targeting agents.13 17 Our patient had a full neurological recovery with discontinuation of bortezomib. In addition she did not have any seizure recurrence with discontinuation of phenytoin therapy, an observation also shared by four of the five cases. Moderate hypertension was noted in each of the four other cases similarly to the present case.9–12 In contrast to the other cases, our patient had received a course of treatment with bortezomib therapy successfully 3 months prior to presentation without being complicated by PRES. It was only on re-exposure to bortezomib 3 months after her last dose of bortezomib that she developed PRES. A possible explanation for this may be that the patient’s blood pressure control had been better during the first course. Although she had a history of hypertension, she had previously been well controlled on medical therapy according to the available information. Whether or not her hypertension had worsened between courses of bortezomib is not entirely clear, as the patient was not recording her blood pressure at home. An alternate explanation that is equally feasible is that bortezomib-induced PRES may be dose dependent, similar to that seen with cyclosporine-induced PRES.7
In summary this case represents one of a small number of PRES associated with bortezomib therapy. Although a rare complication, the growing literature suggests that patients undergoing therapy should potentially be screened to determine if they fit the demographic of patients at highest risk such as female patients, and those with a history of hypertension. Although the majority of the cases did occur with the initial dose of bortezomib, the case we present is novel in that bortezomib-induced PRES occurred on the second course. This suggests that a previous successful tolerance of bortezomib does not predict that bortezomib will not induce PRES in the future. PRES is a very important complication of therapy to recognise given the increasing use of bortezomib and the potential for serious neurological injury or death. Based on the severe case of PRES presented here and the review of previous cases, a larger study to better define the incidence and prevalence of bortezomib-induced PRES is warranted to better define the safety profile of this frequently used chemotherapeutic.
Learning points.
Posterior reversible encephalopathy syndrome (PRES) is a potentially life-threatening condition that may result in permanent neurological deficits if not diagnosed and treated in a timely fashion. It can be provoked by chemotherapeutic agents such as bortezomib.
Bortezomib usage is increasing and thus even rare complications of treatment such as PRES may become more prevalent.
Previous usage of bortezomib without complication does not ensure that patients are safe from the risk of PRES with future use.
Patients who may be at the highest risk include women and patients with a history of hypertension.
Immediate cessation of bortezomib is paramount in treatment of bortezomib-induced PRES.
Footnotes
Contributors: NAN and KP contributed to the data collection, writing and review of the final submitted version of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500 [DOI] [PubMed] [Google Scholar]
- 2.Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartynski WS, Zeigler Z, Spearman MP, et al. Etiology of cortical and white matter lesions in cyclosporin-A and FK-506 neurotoxicity. AJNR Am J Neuroradiol 2001;22:1901–14 [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller-Mang C, Mang T, Pirker A, et al. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 2009;51:373–83 [DOI] [PubMed] [Google Scholar]
- 5.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008;112:1593–9 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Gor D, Walicki D, et al. Spectrum and potential pathogenesis of reversible posterior leukoencephalopathy syndrome. J Stroke Cerebrovasc Dis 2012;21:873–82 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RB, Bravo SM, Klufas RA, et al. Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. AJR Am J Roentgenol 1995;165:627–31 [DOI] [PubMed] [Google Scholar]
- 8.Cusack JC. Rationale for the treatment of solid tumors with the proteasome inhibitor bortezomib. Cancer Treat Rev 2003;29(Suppl 1):21–31 [DOI] [PubMed] [Google Scholar]
- 9.Kager LM, Kersten MJ, Kloppenborg RP, et al. Reversible posterior leucoencephalopathy syndrome associated with bortezomib in a patient with relapsed multiple myeloma. BMJ Case Rep 2009;2009;pii: bcr06.2009.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly K, Kalachand R, Murphy P. Bortezomib-induced reversible posterior leucoencephalopathy syndrome. Br J Haematol 2008;141:566. [DOI] [PubMed] [Google Scholar]
- 11.Oshikawa G, Kojima A, Doki N, et al. Bortezomib-induced posterior reversible encephalopathy syndrome in a patient with newly diagnosed multiple myeloma. Intern Med 2013(52):111–14 [DOI] [PubMed] [Google Scholar]
- 12.Terwiel E, Hanrahan R, Lueck C, et al. Reversible posterior encephalopathy syndrome associated with bortezomib. Intern Med J 2010;40:69–71 [DOI] [PubMed] [Google Scholar]
- 13.Femia G, Hardy TA, Spies JM, et al. Posterior reversible encephalopathy syndrome following chemotherapy with oxaliplatin and a fluoropyrimidine: a case report and literature review. Asia Pac J Clin Oncol 2012;8:115–22 [DOI] [PubMed] [Google Scholar]
- 14.Bartynski WS, Boardman JF, Zeigler ZR, et al. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol 2006;27:2179–90 [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y, Niwa H, Iida T, et al. Post-transfusion reversible posterior leukoencephalopathy syndrome with cerebral vasoconstriction. Neurology 1997;49:1174–5 [DOI] [PubMed] [Google Scholar]
- 16.Rajasekhar A, George TJ., Jr Gemcitabine-induced reversible posterior leukoencephalopathy syndrome: a case report and review of the literature. Oncologist 2007;12:1332–5 [DOI] [PubMed] [Google Scholar]
- 17.Truman N, Nethercott D. Posterior reversible encephalopathy syndrome (PRES) after treatment with oxaliplatin and 5-fluorouracil. Clin Colorectal Cancer 2013;12:70–2 [DOI] [PubMed] [Google Scholar]