Abstract
Thurincin H is an antimicrobial peptide produced by Bacillus thuringiensis SF361. With a helical back bone, the 31 amino acids of thurincin H form a hairpin structure maintained by four pairs of very unique sulfur-to-α-carbon thioether bonds. The production of thurincin H depends on a putative gene cluster containing 10 open reading frames. The gene cluster includes three tandem structural genes (thnA1, thnA2, and thnA3) encoding three identical 40-amino-acid thurincin H prepeptides and seven other genes putatively responsible for prepeptide processing, regulation, modification, exportation, and self-immunity. A homologous thurincin H expression system was developed by transforming a thurincin H-deficient host with a novel expression vector, pGW133. The host, designated B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3, was constructed by deletion of the three tandem structural genes from the chromosome of the natural thurincin H producer. The thurincin H expression vector pGW133 was constructed by cloning the thurincin H native promoter, thnA1, and a Cry protein terminator into the Escherichia coli-B. thuringiensis shuttle vector pHT315. Thirty-three different pGW133 variants, each containing a different point mutation in the thnA1 gene, were generated and separately transformed into B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3. Those site-directed mutants contained either a single radical or conservative amino acid substitution on the thioether linkage-forming positions or a radical substitution on all other nonalanine amino acids. The bacteriocin activities of B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3 carrying different pGW133 variants against three different indicator strains were subsequently compared.
INTRODUCTION
Bacteriocins are ribosomally synthesized, antimicrobial peptides or proteins produced by bacteria that usually have a narrow inhibitory spectrum, although notable exceptions exist (1). Bacteriocins produced by lactic acid bacteria (LAB) have been extensively studied and used as natural food preservatives. Due to the fact that LAB have a generally recognized as safe (GRAS) status, they have been investigated as potential agents for preventing spoilage and enhancing the safety of foods. The LAB bacteriocins can be divided into three main classes: the class I lantibiotics, containing a lanthionine thioether bond linking the sulfur atom of cysteines with the β carbon of other amino acids; the class II non-lanthionine-containing bacteriocins; and the class III heat-labile large proteins (2). In recent years, bacteriocins produced by Bacillus spp. have gained increasing research interest since many of them exhibit a broader antimicrobial spectrum than most lactic acid bacteriocins, and potential applications in the food, agricultural, and pharmaceutical industries in controlling various spoilage and pathogenic microorganisms are anticipated (3, 4). One Bacillus bacteriocin, thurincin H, is an antimicrobial peptide produced by Bacillus thuringiensis SF361, a strain originally isolated from U.S. domestic sunflower honey. It exhibits inhibitory activity against a wide range of Gram-positive bacteria, including different food-borne pathogens and spoilage bacteria, such as Listeria monocytogenes, Bacillus cereus, and Micrococcus spp. (5). Thurincin H contains four pairs of unique sulfur-to-α-carbon thioether bridges (Fig. 1), which is a structure quite different from the structures of the extensively studied group of class I lantibiotics, since the structure of lantibiotics is maintained by sulfur-to-β-carbon bridges (6).
FIG 1.

Structure of thurincin H. Four sulfur-to-α-carbon thioether linkages maintain the hairpin structure.
As elucidated by bioinformatics studies, the thnP-thnI gene cluster is responsible for the production of mature active thurincin H. It is composed of three tandem thurincin H prepeptide genes (thnA1, thnA2, and thnA3), as well as the thnP, thnB, thnD, thnE, thnT, thnR, and thnI genes, which are putatively required for thurincin H prepeptide processing, regulation, modification, exportation, and immunity. It was proposed that the thnA1, thnA2, and thnA3 genes are first translated into three identical 40-amino-acid thurincin H prepeptides and subsequently modified by ThnB, a member of the radical S-adenosylmethionine (SAM) superfamily of enzymes, to form the thioether bonds. The leader peptide is subsequently cleaved, and mature thurincin H is exported to the extracellular environment (5). The mature 31 amino acids of thurincin H feature a helical backbone that is folded over to a hairpin structure maintained by linking the sulfur atoms of the four cysteines (Cys4, Cys7, Cys10, and Cys13) to the α carbons of one asparagine (Asn19), two threonines (Thr22 and Thr25), and one serine (Ser28) (7).
In this study, a homologous thurincin H expression system was constructed by transforming the newly constructed expression vector pGW133 into B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3, in which the three tandem structural genes (thnA1, thnA2, and thnA3) were deleted in frame from the chromosome of the natural thurincin H producer. The expression vector was developed on the basis of the Escherichia coli-B. thuringiensis shuttle vector pHT315 (8). A commonly used expression system, such as Escherichia coli (9, 10) or lactic acid bacteria (11), was not used in the expression of thurincin H since the genes in the thnP-thnI gene cluster are indispensable for the biosynthesis and maturation of the thurincin H. These genes might not exist or could not be expressed in those commonly used hosts.
With the aim of determining the amino acids that are critical for the inhibitory activity of thurincin H, systematic site-directed mutagenesis of thurincin H was performed to explore the bacteriocin activities of 33 constructed variants using the newly developed expression system. Specifically, radical and conservative single-site-directed mutagenesis was performed to mutate the sulfur-to-α-carbon bond forming amino acids. Additionally, radical single-site-directed mutagenesis was performed to mutate all other nonalanine amino acids throughout the peptide.
MATERIALS AND METHODS
Bacterial strains, culture conditions, plasmids, and primers.
Both the Bacillus and E. coli strains used in this study were cultivated at 37°C in Trypticase soy broth (TSB) or on Trypticase soy agar (TSA) (BD, Sparks, MD). B. cereus F4552 was used as the indicator strain for testing the antimicrobial activity of the constructed homologous expression systems. E. coli DH5α was used for cloning recombinant plasmids. E. coli K-12 ER2925 (New England Biolabs [NEB], Ipswich, MA) was used to produce demethylated plasmids in preparation for B. thuringiensis transformation, since demethylated plasmids significantly increase the transformation efficiency (12). Erythromycin (25 μg/ml) or ampicillin (100 μg/ml) was supplemented in the TSA plates used to select transformants of B. thuringiensis or E. coli, respectively. B. cereus F4552, B. thuringiensis EG10368, and L. monocytogenes 2289 were used as indicator strains in evaluating the bacteriocin production activity of each constructed mutant. All chemicals and reagents were either autoclaved at 121°C for 15 min or filtered through a polyethersulfone (PES) membrane (pore size, 0.22 μm; Celltreat, China). The strains and plasmids used in this study are listed in Table 1. The primers used in developing expression system are listed in Table 2. The primers used in site-directed mutagenesis are listed in Table 3. All primers used in this study were synthesized by Integrated DNA Technologies (Coralville, IA). All DNA sequencings were performed at the Biotechnology Resource Center at Cornell University (Ithaca, NY) by an Applied Biosystems Automated 3730xl DNA analyzer using BigDye Terminator chemistry and AmpliTaq-FS DNA polymerase. Primers DTC3 and DTC4 were used in DNA sequencing.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 | NEB |
| E. coli K-12 ER2925 | Produces demethylated plasmids; ara-14 leuB6 fhuA31 lacY1 tsx78 glnV44 galK2 galT22 mcrA dcm-6 hisG4 rfbD1 R(zgb210::Tn10)Tets endA1 rpsL136 dam13::Tn9 xylA5 mtl-1 thi-1 mcrB1 hsdR2 | NEB |
| B. thuringiensis SF361 | Thurincin H producer strain carrying the putative thurincin H-producing gene cluster | Lab stock |
| B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3 | Thurincin H producer strain with in-frame deletions of thnA1, thnA2, and thnA3 | This study |
| B. cereus F4552 | Indicator strain sensitive to thurincin H | 5 |
| B. thuringiensis EG10368 | Indicator strain sensitive to thurincin H | 5 |
| Listeria monocytogenes 2289 | Indicator strain sensitive to thurincin H | 5 |
| Plasmids | ||
| pMAD | Thermosensitive vector for efficient allelic replacement carrying bgaB, Eryr Ampr | 17 |
| pMADΔthnA1thnA2thnA3 | pMAD carrying the upstream and downstream DNA sequences of thnA1, thnA2, and thnA3, used for homologous recombination | This study |
| pHT315 | E. coli-B. thuringiensis shuttle vector, 6.5 kb, Eryr Ampr | 8 |
| pGW131 | Carries the thnA1, thnA2, and thnA3 genes with Pnat and Tnat cloned in pHT315 | This study |
| pGW132 | Carries the thnA1 gene with Pnat and Tnat cloned in pHT315 | This study |
| pGW133 | Carries the thnA1 gene with Pnat and Tcry cloned in pHT315 | This study |
TABLE 3.
Primers used in site-directed mutagenesis
| Substitution type and mutation | Primera | Sequenceb |
|---|---|---|
| Radical substitutions (thioether linkage-forming positions) | ||
| C4A | AM 4 | AGTAGTACAACCAAGGGACTGGACTGCATGGAGTTGTTTAGTATGTG |
| C7A | AM 7 | AGGGACTGGACTTGTTGGAGTGCATTAGTATGTGCAGCATGTTCT |
| C10A | AM 10 | GGACTGGACTTGTTGGAGTTGTTTAGTAGCAGCAGCATGTTCTGTG |
| C13A | AM 13 | CTTGTTGGAGTTGTTTAGTATGTGCAGCAGCATCTGTGGAATTATTAAATTTAGTTACT |
| N19A | AM 19 | GTTTAGTATGTGCAGCATGTTCTGTGGAATTATTAGCATTAGTTACTGCGGCAACAG |
| T22A | AM 22 | GTTCTGTGGAATTATTAAATTTAGTTGCAGCGGCAACAGGGGC |
| T25A | AM 25 | AAATTTAGTTACTGCGGCAGCAGGGGCTAGTACTG |
| S28A | AM 28 | ATTTAGTTACTGCGGCAACAGGGGCTGCAACTGCAAGCTAAAATTTAAAAATG |
| Conservative substitutions (thioether linkage-forming positions) | ||
| C4S | CM4 | ACAACCAAGGGACTGGACTAGTTGGAGTTGTTTAG |
| C7S | CM7 | ACTGGACTTGTTGGAGTAGTTTAGTATGTGCAGCATG |
| C10S | CM10 | TGGACTTGTTGGAGTTGTTTAGTAAGTGCAGCATGTTCTG |
| C13S | CM13 | GGAGTTGTTTAGTATGTGCAGCAAGTTCTGTGGAATTATTAAATTTA |
| N19Q | CM19 | AGTATGTGCAGCATGTTCTGTGGAATTATTACAATTAGTTACTGCGGCAAC |
| T22S | CM22 | TCTGTGGAATTATTAAATTTAGTTAGTGCGGCAACAGGGG |
| T25S | CM25 | TATTAAATTTAGTTACTGCGGCAAGTGGGGCTAGTACTGCAAG |
| S28T | CM28 | TTACTGCGGCAACAGGGGCTACAACTGCAAGCTAAAATTTA |
| Radical substitutions (non-thioether linkage-forming positions) | ||
| D1A | AM 1 | ACCAGTAGTACAACCAAGGGCATGGACTTGTTGGAGTTGTT |
| W2A | AM 2 | CACCAGTAGTACAACCAAGGGACGCAACTTGTTGGAGTTGTTTAGTATG |
| T3A | AM 3 | TAGTACAACCAAGGGACTGGGCATGTTGGAGTTGTTTAGTATG |
| W5A | AM 5 | TACAACCAAGGGACTGGACTTGTGCAAGTTGTTTAGTATGTGCAGC |
| S6A | AM 6 | CCAAGGGACTGGACTTGTTGGGCATGTTTAGTATGTGCAGCATGT |
| L8A | AM 8 | CTGGACTTGTTGGAGTTGTGCAGTATGTGCAGCATGTTCTG |
| V9A | AM 9 | ACTGGACTTGTTGGAGTTGTTTAGCATGTGCAGCATGT |
| S14A | AM 14 | GAGTTGTTTAGTATGTGCAGCATGTGCAGTGGAATTATTAAATTTAGTTACTG |
| V15A | AM 15 | TTGTTTAGTATGTGCAGCATGTTCTGCAGAATTATTAAATTTAGTTACTGCGG |
| E16A | AM 16 | GTATGTGCAGCATGTTCTGTGGCATTATTAAATTTAGTTACTGCG |
| L17A | AM 17 | TATGTGCAGCATGTTCTGTGGAAGCATTAAATTTAGTTACTGCGGCAAC |
| L18A | AM 18 | TGCAGCATGTTCTGTGGAATTAGCAAATTTAGTTACTGCGGCAACAG |
| L20A | AM 20 | TGCAGCATGTTCTGTGGAATTATTAAATGCAGTTACTGCGGCAACAG |
| V21A | AM 21 | CAGCATGTTCTGTGGAATTATTAAATTTAGCAACTGCGGCAACAGGG |
| G26A | AM 26 | AAATTTAGTTACTGCGGCAACAGCAGCTAGTACTG |
| T29A | AM 29 | CTGCGGCAACAGGGGCTAGTGCAGCAAGCTAAAATTTAAA |
| S31A | AM 31 | CGGCAACAGGGGCTAGTACTGCAGCATAAAATTTAAAAATGTGAGAGTGT |
Only one of the two primers (one pair) used in each site-directed mutagenesis reaction is listed here. The sequence of the other primer of each pair is complementary to the sequence listed.
Codons for the mutated amino acids are underlined.
TABLE 2.
Primers used in developing the expression system
| Primer | Sequencea | Restriction enzyme |
|---|---|---|
| DEL1 | GATGGAGGATCCAATACCAATTTTGTTTTTAGACTTTCTTTCATGCTTATCCATAT | BamHI |
| DEL2 | GATGGAAAGCTTTTGTACTACTGGTGTTTCCATTTGACC | HindIII |
| DEL3 | GATGGAAAGCTTGGAGCTAGTACTGCAAGTTAAAATTTAAAAATGTGAGAG | HindIII |
| DEL4 | GAAGGACCATGGTGCATATCAATTTGCACAAATGTTTTCGACTT | NcoI |
| DTC1 | CATTGTTAAATATGGATTCCTTAATTTGCTCCTTTATCTGTTG | NA |
| DTC2 | CGCTCTCGTTTGGGTTTTTATGAACTACC | NA |
| TH01 | GATGGACTGCAGGTAAATATATGTCACAAAATATTAAAGAAACACACACAAAATGTTTG | PstI |
| TH02 | GATGGTGAATTCGTTTTTAGTTTATGTATTACAAAAATCCCATACTCGTTTTCG | EcoRI |
| TH03 | ATGGAAACACCAGTAGTACAACCAAGG | NA |
| TH04 | TTAGCTTGCAGTACTAGCCCCTGT | NA |
| TH05 | GATGGACTGCAGGTAAATATATGTCACAAAATATTAAAGAAACACACACAAAATGTTTGAAATTTTTTGATTGTTTTTAGAAAACATAGGGAGTTATACTTTAGTCGCACCACACAATACAGAAAAGGGGGTAGGTCAAATGGAAACACCAGTAGTACAACCAAGG | PstI |
| TH06 | GATGGTGAATTCGTTTTTAGTTTATGTATTACAAAAATCCCATACTCGTTTTCGATAAATTTATAGGACTTATTCTTAACATAACTGTTTGTCATTAGAATAAGTCCTAATTTAATTTCACCATAAACACTCTCACATTTTTAAATTTTAGCTTGCAGTACTAGCCCCTGT | EcoRI |
| TH07 | GATGGTGAATTCTAGTAAAACGGACATCACTCCGTTTCAATGGAGGTGATGTCCGTTTTATTTAATTTCACCATAAACACTCTCACATTTTTAAATTTTA | EcoRI |
| DTC3 | CTGCAAGGCGATTAAGTTGGGTAAC | NA |
| DTC4 | CGGATAACAATTTCACACAGGAAACAGCTA | NA |
Underlining and italics indicate the restriction enzyme sites.
Bacteriocin activity assays.
Bacteriocin activity on TSA plates was detected using a deferred antagonism assay as previously described (13), with modifications. B. thuringiensis SF361 (on TSA) and B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3 carrying different plasmids (on TSA with 25 μg/ml erythromycin) were incubated for 12 h at 37°C. Single colonies were subsequently spotted on TSA and incubated at 37°C for 15 h. Fifty microliters of the overnight indicator strain culture was inoculated into 8 ml soft TSA (50°C, 0.75% agar) and overlaid on top of the plates. After incubation at 25°C for 12 h, the diameters of the inhibition zones around the colonies were measured.
Bacteriocin activity in liquid medium was detected by a previously described microtiter plate assay (14) modified for the current study. In brief, using untreated, clear, flat-bottom 96-well microplates (Thermo Scientific, Nunc, Denmark), 50 μl of bacteriocin diluted 2-fold in the appropriate buffer was mixed with 150 μl of a 1.33% (vol/vol) B. cereus F4552 overnight culture in TSB and incubated at 37°C for 8 h. The absorbance at 600 nm (A600) of each well was measured using a Synergy HT multimode microplate reader (BioTek, Winooski, VT). One arbitrary unit (AU) was defined as the amount of bacteriocin in the 50-μl sample that caused 50% growth inhibition compared with the growth of the control group.
General DNA manipulation.
The general molecular cloning methods used in this study were performed as previously described by Sambrook and Russell (15), unless otherwise indicated. PrimeSTAR Max DNA polymerase (R045; TaKaRa) was used for all PCRs, including colony PCR, using a total 50-μl mixture volume. The PCR protocol included a template denaturation step at 98°C (1 min), followed by 30 cycles of denaturing at 98°C (10 s), annealing at 55°C (10 s), and polymerization at 72°C (30 to 60 s) and a final extension at 72°C (7 min). Generally, to construct recombinant plasmids, the PCR products were purified, double digested with high-fidelity restriction enzymes (NEB) for 2 h at 37°C, and ligated to the double-digested plasmids overnight at 16°C by T4 ligase. Recombinant plasmids were transformed using commercial competent E. coli DH5α by the heat shock method according to the standard protocol (NEB). Plasmids were purified from E. coli DH5α using a QIAprep Spin miniprep kit (Qiagen) and passed through E. coli K-12 ER2925 to produce a demethylated plasmid in preparation for electroporation into B. thuringiensis strains (12). Recombinant plasmids were transformed into B. thuringiensis strains as previously described (16). The sequences of all recombinant plasmids were confirmed by DNA sequencing.
Thurincin H-nonproducing host construction.
The thurincin H-nonproducing host (B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3) was constructed by use of the in-frame deletion of thnA1, thnA2, and thnA3 from the wild-type producer B. thuringiensis SF361 using a homologous recombination method through the thermosensitive suicide plasmid pMAD, as described by Arnaud et al. (17). pMAD is a plasmid which carries the bgaB gene encoding a thermostable galactosidase for screening for blue and white colonies (17). A 984-bp BamHI/HindIII DNA fragment and an 895-bp HindIII/NcoI DNA fragment corresponding to the regions upstream and downstream of thnA1, thnA2, and thnA3, respectively, were amplified by PCR with primers DEL1 and DEL2 and primers DEL3 and DEL4, respectively, and cloned into pMAD. For the ligation of the amplified inserts into the pMAD vector, the upstream and downstream regions and digested pMAD were mixed in the same reaction mixture at a ratio of 3:3:1. The recombinant plasmid pMADΔthnA1thnA2thnA3 was transformed into E. coli DH5α, passed through E. coli K-12 ER2925, and subsequently transformed into B. thuringiensis SF361 as previously described (16). Transformants were inoculated in 5 ml TSB, incubated for 3 h at 42°C followed by 3 h at 30°C, and plated on TSA containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 50 μg/ml). White colonies were selected for further confirmation. Using primers designed according to the chromosomal region flanking the thnA1, thnA2, and thnA3 genes (primers DTC1 and DTC2), DNA fragments were amplified from the wild-type producer by colony PCR, and the selected white colonies were compared for size differences. The presence of the expected sequence in the deletion mutant was also confirmed by DNA sequencing.
To determine if all other genes necessary for thurincin H production still functioned properly after the deletion, a complementation experiment was performed. Specifically, a fragment containing the native promoter (Pnat), three tandem structural genes (thnA1, thnA2, and thnA3), and the native terminator (Tnat) was amplified from B. thuringiensis SF361 using primers TH01 and TH02 and cloned into pHT315, resulting in plasmid pGW131. pGW131 was transformed into B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3, and the obtained transformants were selected on TSA (with erythromycin at 25 μg/ml). The bacteriocin production of the deletion mutant and transformants was detected using the deferred antagonism assay described above (13) and compared to that of the wild-type producer.
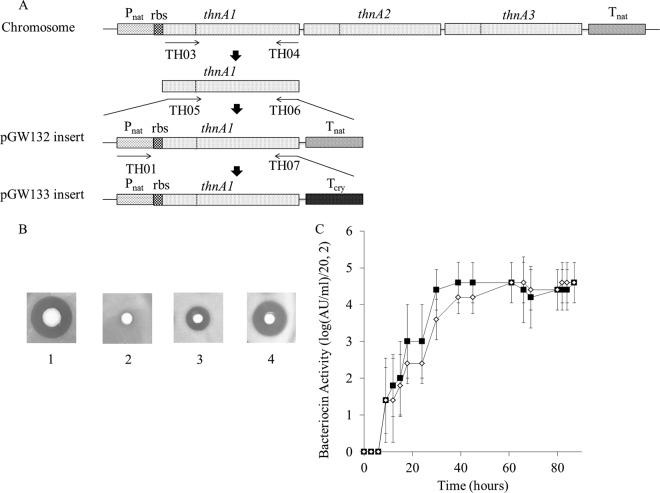
Construction of homologous expression vectors.
In preparation for site-directed mutagenesis, two plasmids each carrying a single copy of the structural gene thnA1 under the control of the native promoter were constructed. First, thnA1 was amplified using primers TH03 and TH04 and purified using a QIAquick PCR purification kit (Qiagen). Using the purified thnA1 gene as a template, an insertion fragment containing Pnat, the thnA1 gene, and Tnat was amplified with primers TH05 and TH06 and cloned into pHT315, resulting in the pGW132 plasmid. Second, using the purified pGW132 inserts as the template, another fragment containing Pnat, thnA1, and the Cry protein terminator (Tcry) (18) was amplified with primers TH01 and TH07 and cloned into pHT315, resulting in the plasmid pGW133. In plasmid pGW133, the reverse primer contained the sequence of the Cry terminator loop. These two constructed plasmids (pGW132 and pGW133) were used separately to transform B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3. The antimicrobial activity of both transformants was detected by the modified deferred antagonism assay described above.
Thurincin H production in broth by B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 was compared to that by the wild-type producer. Fresh overnight colonies of B. thuringiensis SF361 on TSA and B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 on TSA (with erythromycin at 25 μg/ml) were inoculated into 5 ml of TSB and incubated at 37°C for 12 h with shaking at 225 rpm. A 1.5-ml aliquot of this incubation was added to 150 ml TSB and incubated at 37°C for up to 90 h with shaking at 225 rpm. Samples were taken every 3 h, centrifuged (8,000 × g, 4°C, 5 min), filtered, and stored at −20°C. The antimicrobial activity of each sample was quantified by the microtiter plate method described above. Five independent experiments were performed.
Site-directed mutagenesis.
A total of 33 different pGW133 plasmids, each with a different mutated thnA1 gene, were generated by using a QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA). Mutagenesis reactions were performed in a Bio-Rad T100 thermal cycler by using the PfuUltra HF DNA polymerase in the kit according to the manufacturer's guidelines, with modifications. Each reaction mixture contained 2.5 μl 10× reaction buffer, 18.5 ng plasmid template, 62.5 ng of each oligonucleotide primer, 0.5 μl deoxynucleoside triphosphate mix, 0.75 μl QuickSolution reagent, and 0.5 μl QuikChange Lightning enzyme and was brought up to 25 μl with sterile Milli-Q water (Millipore Corporation, Billerica, MA). The mutagenesis reaction was performed with a program of the following conditions: one initial cycle of 95°C for 2 min, followed by 18 cycles of a set of reactions composed of 20 s at 95°C, 10 s at 60°C, and 4 min at 68°C and with one final cycle of 68°C for 5 min. The resulting products were digested for 5 min at 37°C using the endonuclease DpnI to eliminate the methylated and hemimethylated DNA containing the nonmutated sequence originating from the template plasmids. The nicked vector containing the expected mutation was transformed into E. coli XL10-Gold ultracompetent cells, and the nick was sealed by E. coli DNA repair systems. Plasmids were purified, passed through E. coli K-12 ER2925, and transformed into B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3. The DNA sequences of all mutated plasmids were verified by DNA sequencing.
Antimicrobial activity assay on plates.
The inhibitory activities of the 33 site-directed mutants obtained were compared to the inhibitory activity of B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 by the deferred antagonism assay using B. cereus F4552, B. thuringiensis EG10368, and L. monocytogenes 2289 as the indicator strains, as described above. At least four independent sets of experiments were performed on each strain.
Statistical analysis.
For the production of thurincin H in TSB, the bacteriocin activities of the cell-free supernatants produced by the wild-type producer and B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 at each time point were compared by the t test (P < 0.05). Statistical analyses were conducted using SigmaPlot (version 12.0) software (Systat Software Inc., San Jose, CA).
RESULTS
Thurincin H-deficient expression host engineered from wild-type producer.
The structural genes thnA1, thnA2, and thnA3 were deleted in frame on the basis of the double-crossover homologous recombination method using the thermosensitive plasmid pMAD. The deletion of thnA1, thnA2, and thnA3 from the white colonies was first confirmed by PCR, the amplicon from which was run on agarose gels, which revealed that the product from each deletion mutant was smaller than that from the wild type (Fig. 2A). Subsequent confirmation was made by DNA sequencing (data not shown).
FIG 2.

In-frame deletion of structural genes (thnA1, thnA2, and thnA3) by homologous recombination. (A) Confirmation of deletion by PCR with primers DTC1 and DTC2. Lane M, BenchTop pGEM DNA markers (Promega); lane 1, wild type; lane 2, deletion mutant. Numbers on the left are in base pairs. (B) Bacteriocin activity assay. Colony 1, wild type; colony 2, deletion mutant; colony 3, B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW131. Images are representative of those obtained from multiple independent experiments with reproducible results.
Deletion mutants with the expected DNA sequence lost the ability to inhibit the sensitive indicator strain B. cereus F4552 (Fig. 2B, colony 2). As further verification, pGW131 containing the native promoter (Pnat), thnA1, thnA2, thnA3, and the thurincin H native terminator (Tnat) was constructed and was used to transform the deletion mutants, in order to test if it could complement the production of the thurincin H prepeptide. The results showed that B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3 carrying pGW131 (Fig. 2B, colony 3) exhibited a zone of inhibition similar in size to that of the wild type (Fig. 2B, colony 1), indicating that the structural genes in pGW131 successfully complemented the mature thurincin H production and secretion pathway.
Expression vector construction and optimization.
The plasmid construct pGW132 containing Pnat, thnA1, and Tnat was preliminarily constructed to express thurincin H in preparation for site-directed mutagenesis (Fig. 3A). However, B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW132 (Fig. 3B, colony 3) exhibited a significantly smaller inhibition zone than the wild type (Fig. 3B, colony 1).
FIG 3.
Construction of different expression vectors and measurement of their bacteriocin production levels. (A) Scheme of plasmid pGW132 and pGW133 construction. rbs, ribosome binding site. (B) Bacteriocin assay of pGW132 and pGW133 on solid TSA medium. Colony 1, wild type; colony 2, B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3; colony 3, B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW132; colony 4, B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133. Images are representative of those from multiple independent experiments with reproducible results. (C) Bacteriocin activity of the natural producer and B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 in TSB. ■, B. thuringiensis SF361; ◇, B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133. Means and standard deviations are presented.
To improve the homologous expression level of thurincin H, Tnat was replaced by a CryIAa protein terminator (Tcry) originally found in B. thuringiensis subsp. kurstaki HD1, leading to the construction of pGW133 (Fig. 3A). The result indicated that the bacteriocin activity of B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 was significantly increased compared with that of pGW132, reaching a level of expression similar to that by the wild type (Fig. 3B, colony 4).
To evaluate the bacteriocin production of B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 in liquid broth, it was incubated in TSB without antibiotics and the level of bacteriocin production was compared with that of the wide-type producer under the same conditions. There was no significant difference in bacteriocin activity in the supernatant between B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 and the natural producer for all samples tested (Fig. 3C). No selective antibiotics were added to the TSB medium during the 90 h of production, since pHT315 has been reported to be stably maintained in the B. thuringiensis host without selective pressure (8, 19).
Site-directed mutagenesis.
Mature thurincin H is composed of 6 alanines at non-sulfur-to-α-carbon bond-forming positions (A11, A12, A23, A24, A27, and A30), in addition to 25 other amino acids throughout the rest of the peptide (Fig. 1). The 33 site-directed mutations at single amino acids on plasmid pGW133 were categorized into three groups: (i) mutations consisting of 8 radical substitutions at thioether bond-forming positions (group 1), (ii) mutations consisting of 8 conservative substitutions at thioether bond-forming positions (group 2), and (iii) mutations consisting of 17 radical substitutions at non-thioether bond-forming positions (group 3) (Table 3). Mutants with those mutations were systematically generated to elucidate the tolerance of the biosynthesis, regulation, and transportation pathways toward each amino acid substitution in leaderless thurincin H. The results of the change in bacteriocin activity were measured by the deferred antagonism assay and are summarized in Table 4.
TABLE 4.
Inhibitory activities of site-directed mutants
| Group | Mutation | Inhibitory activitya |
||
|---|---|---|---|---|
| B. cereus F4552 | B. thuringiensis EG10368 | L. monocytogenes 2289 | ||
| 1 (radical substitutions, thioether linkage-forming positions) | C4A | − | − | − |
| C7A | − | − | − | |
| C10A | − | − | − | |
| C13A | − | − | − | |
| N19A | − | − | − | |
| T22A | − | − | − | |
| T25A | ++++ | ++++ | − | |
| S28A | +++++ | +++++ | ++ | |
| 2 (conservative substitutions, thioether linkage-forming positions) | C4S | − | − | − |
| C7S | − | − | − | |
| C10S | − | − | − | |
| C13S | − | − | − | |
| N19Q | +++ | +++ | − | |
| T22S | ++++ | ++++ | − | |
| T25S | ++++ | ++++ | − | |
| S28T | ++++ | ++++ | − | |
| 3 (radical substitutions, no thioether linkage-forming positions) | D1A | +++ | +++ | − |
| W2A | − | − | − | |
| T3A | + | + | − | |
| W5A | − | − | − | |
| S6A | +++++ | +++++ | ++ | |
| L8A | − | − | − | |
| V9A | +++ | +++ | − | |
| S14A | +++++ | +++++ | ++ | |
| V15A | +++ | +++ | ||
| E16A | ++ | ++ | − | |
| L17A | − | − | − | |
| L18A | ++ | ++ | − | |
| L20A | − | − | − | |
| V21A | + | + | − | |
| G26A | + | + | − | |
| T29A | − | − | − | |
| S31A | +++++ | +++++ | ++ | |
| No substitution (positive control) | +++++ | +++++ | ++ | |
The inhibitory activity of the producer was graded from − (no activity) to +++++ (the highest activity).
In mutants with group 1 and 2 mutations, the inhibitory activity was completely lost as a result of any of the eight single cysteine-alanine/cysteine-serine mutations. However, in the thioether acceptor sites of mutants with group 1 mutations, bacteriocin activity disappeared in the N19A and T22A mutants, was partially retained in the T25A mutant, and was completely retained in the S28A mutant. Remarkably, in mutants with group 2 mutations, relatively high partial bacteriocin activities were retained in all four thioether acceptor sites (N19Q, T22S, T25S, and S28T). This result indicated that all four cysteines (C4, C7, C10, and C13), the donors of the thioether bonds, are critical in maintaining bacteriocin activity. However, the thurincin H pathway does have limited tolerance toward amino acid substitutions in the thioether acceptor positions (N19, T22, T25, and S28).
In mutants with group 3 mutations, bacteriocin activity was completely abolished when the amino acids at W2, W5, L8, L17, L20, and T29 were replaced by alanine. Partial activity was lost when E16, G26, D1, V 9, V15, V21, L18, and T3 were mutated. Very high activity was retained when the amino acids at S6, S14, and S31 were replaced by alanine. Tryptophan could not be substituted at any position, probably because the featured indole functional group plays an essential role. The substitutability of leucine and threonine depended on their positions. Remarkably, all four serine-to-alanine mutations (S28A, S6A, S14A, and S31A) maintained high levels of bacteriocin activity, regardless of their positions in the peptide.
DISCUSSION
In-frame deletion and complementation.
A deletion mutant of the wild-type producer was chosen as the expression host in our study since there are always potential technical limitations to the expression of proteins or peptides in other hosts. For example, the structural gene of the protein might contain codons rarely used in the desired host (20) or the proteins expressed in the desired host might be degraded by native proteolytic enzymes (21–23). In this particular case of extensively modified thurincin H, the sulfur-to-α-carbon bonds were very unique and to date are reported to exist in only three other bacterial peptides (24–26). These bonds are not reported in proteins expressed by commonly used expression hosts, such as E. coli (27) or lactic acid bacteria (11), rendering the deletion mutant a logical host for expression of site-directed mutants. The pGW131 complementation experiments indicated that the thurincin H prepeptide translated from the plasmids was correctly modified by the chromosomally encoded radical SAM enzyme, processed, and exported to the extracellular environment. The deletion mutant remained immune to thurincin H.
Construction of the homologous expression vector.
To construct a plasmid for site-directed mutagenesis, the expression vector has to carry only one structural gene for the mutagenesis reaction and express sufficient mature thurincin H to facilitate downstream studies. The plasmid pGW132 was constructed accordingly, but the bacteriocin level was lower than that of the wild type, in spite of its high copy number (15 copies/cell) (8). The thurincin H native terminator in pGW132 was replaced with a more stable Cry protein terminator, resulting in pGW133. The substituted terminator construct showed an increase in thurincin H production, likely as a result of enhanced mRNA stability (18, 28). Even with a high number of copies (8), the level of production by pGW131 and pGW133 was not higher than that by the wild type. This might be caused by limited regulation and posttranslational modification systems or self-toxicity, which is also a limiting factor for the natural producer to express higher levels of bacteriocin, despite the fact that the immunity protein usually protects the producer up to a certain level (29, 30).
In homologous expression of less extensively modified bacteriocins, only the mature bacteriocin-encoding sequence was incorporated into the expression vector (9, 27). However, the leader peptide of the thurincin H precursor was included in our constructs, since the leader peptide of the bacteriocins can play essential roles in the formation of lanthionine/methyllanthionine in lantibiotics, as well as keep the bacteriocin prepeptide in an inactive state and facilitate exportation (31). Similarly, the formation of sulfur-to-α-carbon thioether bonds in subtilosin A was found to be dependent on its leader peptide too (32). Some bacteriocins can be produced only in liquid medium under certain conditions (33–35), but thurincin H from B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3/pGW133 reached high production levels under the same conditions used for the wild type. This system could be used to produce high levels of thurincin H variants that could be purified for further characterization.
Site-directed mutagenesis.
Genetic engineering has been used as a tool to construct bacteriocin mutants with new features. For example, replacing the threonine at residue 6 with isoleucine in subtilosin A not only enhanced its bactericidal activity but also rendered the mutant hemolytic (36). Deferred antagonism assays were conducted to evaluate the bacteriocin activities of B. thuringiensis SF361 ΔthnA1 ΔthnA2 ΔthnA3 carrying different pGW133 variants with mutated thurincin H structural genes. The method could detect only the active bacteriocin that had been secreted into the extracellular environment and therefore reflects the tolerance of the entire biosynthesis, regulation, and transportation pathway toward each different amino acid point mutation. In the mutants that lost activity, it is possible that any step in biosynthesis was affected; for example, the mutation could have completely abolished bacteriocin production, the recognition of the radical SAM enzyme which modifies thurincin H derivatives (32), or transportation. However, the bacteriocin activity loss caused by thioether bond amino acid substitutions, as in groups 1 and 2, was highly likely a result of interference with thioether bond formation. It was reported that the three pairs of sulfur-to-α-carbon thioether bonds in subtilosin A were sensitive to single amino acid substitutions, and none with thioether bonds were formed if any of the six amino acids (C4, C7, C13, F22, T28, and F31) in the sulfur-to-α-carbon bond-forming positions was replaced by alanine in vivo. However, that study detected thioether bond formation using only high-performance liquid chromatography and high-resolution mass spectrometry and did not directly report the bacteriocin activity of the mutants (32). In the thurincin H variants, partial activity was retained with some of the mutants containing substitutions in the four thioether bonds (T25A, S28A, N19Q, T22S, T25S, S28T). It is also possible that less than four pairs of thioether bonds were formed or the bacteriocin activity was lost due to the amino acid substitutions in the four pairs of thioether bonds being formed. Besides their potential for use in inhibitory activity studies, those 33 thurincin H variants also provide a library for the screening of new features or to better improve the character of thurincin H, as was done for subtilisin E (37).
Conclusions.
A homologous expression system was developed to sufficiently express a high level of thurincin H from one structural gene. This structural gene was under the control of the native promoter and included a modified Cry protein terminator, which significantly improved the expression level through enhancement of mRNA stability. Our research analyzed a complete, systematic system for site-directed mutagenesis of thurincin H, which is representative of the bacteriocins produced by Bacillus spp. with unique sulfur-to-α-carbon thioether bonds. These thurincin H variants could be sufficiently purified, and new features of the variants, such as changes in their three-dimensional structures, inhibitory spectra, and thermal stability, could be explored in future research.
ACKNOWLEDGMENTS
We acknowledge Didier Lereclus of the Institut National de la Recherche Agronomique in France for sending us plasmid pHT315 and Barbara M. Bowen in Martin Wiedmann's group in the Food Science Department at Cornell University for her technical assistance.
This research was supported by USDA-CSREES (project 2008-51110-0688).
Footnotes
Published ahead of print 28 March 2014
REFERENCES
- 1.Cotter PD, Ross RP, Hill C. 2013. Bacteriocins—a viable alternative to antibiotics? Nat. Rev. Microbiol. 11:95–105. 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- 2.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788. 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- 3.Abriouel H, Franz CM, Ben Omar N, Galvez A. 2011. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35:201–232. 10.1111/j.1574-6976.2010.00244.x [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Kim HY. 2011. Lantibiotics, class I bacteriocins from the genus Bacillus. J. Microbiol. Biotechnol. 21:229–235 [PubMed] [Google Scholar]
- 5.Lee H, Churey JJ, Worobo RW. 2009. Biosynthesis and transcriptional analysis of thurincin H, a tandem repeated bacteriocin genetic locus, produced by Bacillus thuringiensis SF361. FEMS Microbiol. Lett. 299:205–213. 10.1111/j.1574-6968.2009.01749.x [DOI] [PubMed] [Google Scholar]
- 6.Willey JM, van der Donk WA. 2007. Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 61:477–501. 10.1146/annurev.micro.61.080706.093501 [DOI] [PubMed] [Google Scholar]
- 7.Sit CS, van Belkum MJ, McKay RT, Worobo RW, Vederas JC. 2011. The 3D solution structure of thurincin H, a bacteriocin with four sulfur to alpha-carbon crosslinks. Angew. Chem. Int. Ed. Engl. 50:8718–8721. 10.1002/anie.201102527 [DOI] [PubMed] [Google Scholar]
- 8.Arantes O, Lereclus D. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115–119. 10.1016/0378-1119(91)90495-W [DOI] [PubMed] [Google Scholar]
- 9.Ingham AB, Sproat KW, Tizard ML, Moore RJ. 2005. A versatile system for the expression of nonmodified bacteriocins in Escherichia coli. J. Appl. Microbiol. 98:676–683. 10.1111/j.1365-2672.2004.02502.x [DOI] [PubMed] [Google Scholar]
- 10.Lohans CT, Vederas JC. 2012. Development of class IIa bacteriocins as therapeutic agents. Int. J. Microbiol. 2012:386410. 10.1155/2012/386410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez JM, Martinez MI, Horn N, Dodd HM. 2003. Homologous production of bacteriocins by lactic acid bacteria. Int. J. Food Microbiol. 80:101–116. 10.1016/S0168-1605(02)00153-8 [DOI] [PubMed] [Google Scholar]
- 12.Macaluso A, Mettus AM. 1991. Efficient transformation of Bacillus thuringiensis requires nonmethylated plasmid DNA. J. Bacteriol. 173:1353–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birri DJ, Brede DA, Forberg T, Holo H, Nes IF. 2010. Molecular and genetic characterization of a novel bacteriocin locus in Enterococcus avium isolates from infants. Appl. Environ. Microbiol. 76:483–492. 10.1128/AEM.01597-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daba H, Pandian S, Gosselin JF, Simard RE, Huang J, Lacroix C. 1991. Detection and activity of a bacteriocin produced by Leuconostoc mesenteroides. Appl. Environ. Microbiol. 57:3450–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 16.Lereclus D, Arantes O, Chaufaux J, Lecadet M. 1989. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 51:211–217 [DOI] [PubMed] [Google Scholar]
- 17.Arnaud M, Chastanet A, Débarbouillé M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 70:6887–6891. 10.1128/AEM.70.11.6887-6891.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong HC, Chang S. 1986. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc. Natl. Acad. Sci. U. S. A. 83:3233–3237. 10.1073/pnas.83.10.3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okay S, Tefon BE, Ozkan M, Ozcengiz G. 2008. Expression of chitinase A (chiA) gene from a local isolate of Serratia marcescens in Coleoptera-specific Bacillus thuringiensis. J. Appl. Microbiol. 104:161–170. 10.1111/j.1365-2672.2007.03570.x [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson C, Govindarajan S, Minshull J. 2004. Codon bias and homologous protein expression. Trends Biotechnol. 22:346–353. 10.1016/j.tibtech.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Murashima K, Chen CL, Kosugi A, Tamaru Y, Doi RH, Wong SL. 2002. Homologous production of Clostridium cellulovorans engB, using protease-deficient Bacillus subtilis, and preparation of active recombinant cellulosomes. J. Bacteriol. 184:76-81. 10.1128/JB.184.1.76-81.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen CL, Stephenson K, Jørgensen ST, Harwood C. 2000. Cell-associated degradation affects the yield of secreted engineered and homologous proteins in the Bacillus subtilis expression system. Microbiology 146(Pt 10):2583–2594 http://mic.sgmjournals.org/content/146/10/2583.full.pdf [DOI] [PubMed] [Google Scholar]
- 23.Narayanan N, Chou CP. 2009. Alleviation of proteolytic sensitivity to enhance recombinant lipase production in Escherichia coli. Appl. Environ. Microbiol. 75:5424–5427. 10.1128/AEM.00740-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawulka KE, Sprules T, Diaper CM, Whittal RM, McKay RT, Mercier P, Zuber P, Vederas JC. 2004. Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to α-carbon cross-links: formation and reduction of α-thio-α-amino acid derivatives. Biochemistry 43:3385–3395. 10.1021/bi0359527 [DOI] [PubMed] [Google Scholar]
- 25.Sit CS, McKay RT, Hill C, Ross RP, Vederas JC. 2011. The 3D structure of thuricin CD, a two-component bacteriocin with cysteine sulfur to α-carbon cross-links. J. Am. Chem. Soc. 133:7680–7683. 10.1021/ja201802f [DOI] [PubMed] [Google Scholar]
- 26.Liu WT, Yang YL, Xu Y, Lamsa A, Haste NM, Yang JY, Ng J, Gonzalez D, Ellermeier CD, Straight PD, Pevzner PA, Pogliano J, Nizet V, Pogliano K, Dorrestein PC. 2010. Imaging mass spectrometry of intraspecies metabolic exchange revealed the cannibalistic factors of Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 107:16286–16290. 10.1073/pnas.1008368107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richard C, Drider D, Elmorjani K, Marion D, Prevost H. 2004. Homologous expression and purification of active divercin V41, a class IIa bacteriocin encoded by a synthetic gene in Escherichia coli. J. Bacteriol. 186:4276–4284. 10.1128/JB.186.13.4276-4284.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agaisse H, Lereclus D. 1995. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J. Bacteriol. 177:6027–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinzmann S, Entian KD, Stein T. 2006. Engineering Bacillus subtilis ATCC 6633 for improved production of the lantibiotic subtilin. Appl. Microbiol. Biotechnol. 69:532–536. 10.1007/s00253-005-0023-9 [DOI] [PubMed] [Google Scholar]
- 30.Kim WS, Hall RJ, Dunn NW. 1998. Improving nisin production by increasing nisin immunity/resistance genes in the producer organism Lactococcus lactis. Appl. Microbiol. Biotechnol. 50:429–433. 10.1007/s002530051316 [DOI] [Google Scholar]
- 31.Riley MA, Gillor O. 2007. Research and applications in bacteriocins. Horizon Bioscience Press, Norfolk, United Kingdom [Google Scholar]
- 32.Fluhe L, Knappe TA, Gattner MJ, Schafer A, Burghaus O, Linne U, Marahiel MA. 2012. The radical SAM enzyme AlbA catalyzes thioether bond formation in subtilosin A. Nat. Chem. Biol. 8:350–357. 10.1038/nchembio.798 [DOI] [PubMed] [Google Scholar]
- 33.Quadri LE, Kleerebezem M, Kuipers OP, de Vos WM, Roy KL, Vederas JC, Stiles ME. 1997. Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J. Bacteriol. 179:6163–6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado-Barragan A, Ruiz-Barba JL, Jimenez-Diaz R. 2009. Knockout of three-component regulatory systems reveals that the apparently constitutive plantaricin-production phenotype shown by Lactobacillus plantarum on solid medium is regulated via quorum sensing. Int. J. Food Microbiol. 130:35–42. 10.1016/j.ijfoodmicro.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 35.Gobbetti M, Raffaella DC. 2013. Bacterial communication in foods. Springer, New York, NY [Google Scholar]
- 36.Huang T, Geng H, Miyyapuram VR, Sit CS, Vederas JC, Nakano MM. 2009. Isolation of a variant of subtilosin A with hemolytic activity. J. Bacteriol. 191:5690–5696. 10.1128/JB.00541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takagi H, Takahashi T, Momose H, Inouye M, Maeda Y, Matsuzawa H, Ohta T. 1990. Enhancement of the thermostability of subtilisin E by introduction of a disulfide bond engineered on the basis of structural comparison with a thermophilic serine protease. J. Biol. Chem. 265:6874–6878 [PubMed] [Google Scholar]