Abstract
Short exposures of Bacillus spores to nutrient germinants can commit spores to germinate when germinants are removed or their binding to the spores' nutrient germinant receptors (GRs) is inhibited. Bacillus subtilis spores were exposed to germinants for various periods, followed by germinant removal to prevent further commitment. Release of spore dipicolinic acid (DPA) was then measured by differential interference contrast microscopy to monitor germination of multiple individual spores, and spores did not release DPA after 1 to 2 min of germinant exposure until ∼7 min after germinant removal. With longer germinant exposures, percentages of committed spores with times for completion of DPA release (Trelease) greater than the time of germinant removal (Tb) increased, while the time Tlag − Tb, where Tlag represents the time when rapid DPA release began, was decreased but rapid DPA release times (ΔTrelease = Trelease − Tlag) were increased; Factors affecting average Trelease values and the percentages of committed spores were germinant exposure time, germinant concentration, sporulation conditions, and spore heat activation, as previously shown for commitment of spore populations. Surprisingly, germination of spores given a 2nd short germinant exposure 30 to 45 min after a 1st exposure of the same duration was significantly higher than after the 1st exposure, but the number of spores that germinated in the 2nd germinant exposure decreased as the interval between germinant exposures increased up to 12 h. The latter results indicate that spores have some memory, albeit transient, of their previous exposure to nutrient germinants.
INTRODUCTION
Spores of Bacillus species are metabolically dormant, can survive for many years in this state (1–3), and are extremely resistant to heat, desiccation, radiation, and many toxic chemicals (4). However, dormant spores contain a set of specific germinant receptors (GRs) present in their inner membrane that continuously monitor the environment, and when appropriate nutrient germinants are present, they bind to specific GRs and trigger the return of the spores to activity through germination followed by outgrowth (1). Some nonnutrient agents, including dodecylamine, exogenous CaDPA (a 1:1 chelate of Ca2+ and dipicolinic acid [DPA]), and high hydrostatic pressure (HP), are also able to initiate spore germination (1, 5). Nutrients trigger germination almost certainly by binding to GRs, since point mutations in at least one GR subunit can increase the apparent affinity of this GR for its cognate germinant and change its germinant specificity (6, 7). Thus, although nutrient-GR binding has not been demonstrated directly, this seems most likely and is assumed throughout this communication. Stimulation of these GRs triggers release of the spore core's large depot (∼10% of spore dry weight) of CaDPA and its replacement by water in stage I of germination (1, 2). These events trigger activation of the cortex-lytic enzymes CwlJ and SleB, either of which can initiate hydrolysis of the spore's peptidoglycan cortex, leading to completion of spore germination. Concomitant with cortex hydrolysis, the spore core becomes fully hydrated, which allows resumption of enzyme activity and initiation of metabolism and macromolecular synthesis in the core and thus spore outgrowth (1, 5). The resistance properties of spores are lost when spores germinate fully and begin outgrowth.
In spores of Bacillus subtilis, there are three functional GRs (GerA, GerB, and GerK), the GerA GR responding to l-alanine or l-valine and the GerB and GerK GRs cooperatively responding to a mixture of l-asparagine, d-glucose, d-fructose, and K+ (AGFK) (8–10). Normally, l-asparagine alone does not trigger B. subtilis spore germination. However, a mutant form of the GerB GR, termed GerB*, displays altered germinant specificity such that the presence of l-asparagine alone triggers the germination of gerB* spores (11). After spores are exposed to nutrient germinants, one of the first measurable events is termed commitment, in which, even if nutrient germinants are removed or their further binding to GRs is blocked, spores that are committed to germinate continue through nutrient germination and release CaDPA 3 to 10 min after commitment (12–15). The interaction of nutrient germinants with their cognate GRs generates an output signal that activates other components of the germination apparatus and commits the spores to germinate, but the signal transduction pathway leading to commitment is not clear. The cascade of events after the commitment step includes (i) the release of CaDPA and its replacement by water, resulting in an elevation of the level of the core water content, and (ii) hydrolysis of the spore's peptidoglycan cortex, allowing the swelling and further hydration of the spore core. However, spores that are not committed to germinate carry out no germination events (12–15).
The germination of individual spores of a population exposed to a constant concentration of a nutrient germinant exhibits significant heterogeneity (16–19), mainly due to variability in the lag time (Tlag) between the addition of nutrient germinants and the start of rapid CaDPA release (Fig. 1A). In contrast, times (ΔTrelease) for the actual release of ≥90% of a spore's CaDPA once its rapid release begins are relatively constant for individual spores (18–20). It is not known precisely what causes the variability in the Tlag times for individual spores in populations, although factors that affect the commitment times of spore populations and Tlag times of individual spores include spore heat activation, germinant concentrations, and GR levels (15, 19, 21). Spores that have higher GR levels germinate faster than spores with lower GR numbers, and spores with extremely low GR levels germinate extremely slowly and are termed superdormant (22, 23).
FIG 1.
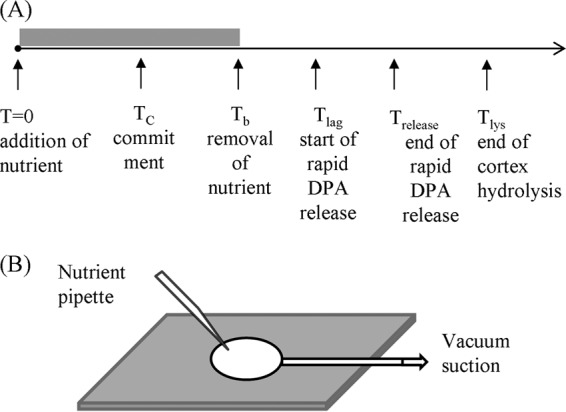
(A) Temporal sequence of events in germination of single spores. The nutrient is added to the spores at T0; the spore commits to germinate at TC; the nutrient is removed and replaced by the buffer at Tb; the spore starts rapid DPA release at Tlag; rapid DPA release ends at Trelease; and the spore completes cortex hydrolysis at Tlys. (B) Schematic of the microscope sample slide. The spores were spread on the surface of a coverslip glued to the sample container. The germinant solution was added with a pipette at T0 and removed with a vacuum suction pump at Tb.
The commitment of spores in populations exposed to nutrient germinants has been well documented (12–15), and a study of the commitment of individual spores exposed to high pressure in a diamond anvil cell has also been reported recently (24). However, the dynamics of the commitment and subsequent germination of individual spores upon exposure to nutrient germinants for various periods have not been studied, and such an analysis would likely indicate the heterogeneity in both commitment and times between individual spores in populations, as well as factors that influence this heterogeneity. In addition, spores that are not committed to germinate but whose GRs have been given a short exposure to nutrient germinants might be at least partially and perhaps reversibly activated for germination, such that these spores would respond more readily upon a 2nd short germinant exposure. Indeed, evidence consistent with such a metastable activated state of GRs was obtained recently in germination induced by high pressures that act via GRs (24).
We report here results of experiments monitoring the dynamic germination of hundreds of individual spores of B. subtilis using differential interference contrast (DIC) microscopy to measure the refractive index of individual spores when they are exposed to specific nutrient germinants for various periods, followed by blocking further commitment. The objectives of the experiments were to (i) examine the influence of various factors on the commitment and subsequent germination of individual spores, including heat activation, germinant concentration, status when commitment is stopped, and GR levels, and (ii) determine if one short germinant pulse giving commitment activates spores for commitment following a second germinant pulse. If observed, the latter finding would imply that spores have some memory, albeit transient, of their past exposures to nutrient germinants, much as spores treated with at least some HPs appear to have a memory of this treatment, allowing these spores to germinate long after the HP has been released (24).
MATERIALS AND METHODS
B. subtilis strains used and spore preparation.
The B. subtilis strains used in this work were isogenic with strain PS832, a prototrophic 168 strain, and were (i) PS533 (wild type) that contains plasmid pUB110 which encodes resistance to kanamycin (25) and (ii) PS3415, spores of which have 18-fold-elevated levels of the modified GerB GR, GerB*, that triggers spore germination with l-asparagine alone (26, 27).
Unless otherwise noted, spores of all B. subtilis strains were prepared at 37°C on 2× SG medium agar plates without antibiotics (28). After incubation for 2 to 3 days, plates were shifted to 23°C for several days to allow completion of lysis of sporulating cells, and spores were then scraped from plates and suspended in 4°C water. Harvested spores were purified by repeated centrifugation and washing with water as well as several sonication treatments. Spores of strain PS533 were also prepared at 37°C in either rich or poor liquid media (2× SG or Spizizen's minimal medium without Casamino Acids, respectively) as described previously (29) and purified essentially as described above. Previous work has shown that spores produced in this minimal medium have significantly lower GR levels than spores made in this rich medium and that rates of germination of these spores are directly related to these GR levels (29). All purified spores were stored at 4°C in water protected from light and were (98%) free of growing and sporulating cells, germinated spores, and cell debris as observed by phase-contrast microscopy.
Spore germination with a constant level of a nutrient germinant or with a nutrient germinant pulse followed by blocking further commitment.
B. subtilis spores were heat activated prior to nutrient germination by incubation in water at 70°C for 30 min and then cooling on ice for at least 15 min prior to germination experiments. Heat-activated PS533 spores were germinated at 37°C with various concentrations of l-valine in 25 mM K-HEPES buffer (pH 7.4) or with various concentrations of l-alanine in 25 mM Tris-HCl buffer (pH 8.3) (8, 9). Heat-activated PS3415 spores were germinated at 37°C with 0.5 or 0.8 mM l-asparagine–25 mM Tris-HCl buffer (pH 8.3). Briefly, 1 μl of heat-activated spores (108 spores per ml in water) was spread on the surface of a glass coverslip glued to a clean, sterile sample container. The spores on the coverslip were dried in a vacuum chamber for 30 min at room temperature so they became firmly adhered to the coverslip. The spore container was then mounted on a microscope heating stage held at 37°C. B. subtilis spores were germinated either with a constant exposure to germinants for 60 to 90 min or for short variable time periods (1 to 10 min) before the germinant was removed (Fig. 1B).
For germination with short pulses of l-alanine or l-valine, two different methods were used to block further commitment. In method A, the germinant solution was removed with a vacuum suction pump unless noted otherwise, although in some initial experiments the solution was removed with a pipette, and then the sample chamber was rinsed 5 to 6 times using a vacuum suction pump (Fig. 1B) unless noted otherwise with ∼200 μl of prewarmed (37°C) 25 mM K-HEPES buffer (pH 7.4) (l-valine germination) or Tris-HCl buffer (pH 8.3) (l-alanine and l-asparagine germination) such that the resultant germinant concentration was too low to trigger further germination; finally, the sample chamber was filled with 37°C 25 mM K-HEPES buffer (pH 7.4) or Tris-HCl buffer (pH 8.3). It took 1 to 2 min using a pipette and less than 30 s with a vacuum suction pump to complete the above process. In method B, the germinant solution was removed as described above, and then the sample chamber was filled with a prewarmed mixture of 10 mM d-alanine with either 25 mM K-HEPES buffer (pH 7.4) or 25 mM Tris-HCl buffer (pH 8.3). d-Alanine blocks further spore commitment due to l-valine or l-alanine via the GerA GR but does not block subsequent germination events following commitment (12–15). Notably, wild-type spores incubated at 37°C in a mixture of 10 mM d-alanine and 25 mM Tris-HCl buffer (pH 8.3) exhibited no germination in 1 h as observed by DIC microscopy (data not shown).
Spore germination with two short germinant pulses.
B. subtilis PS533 spores were given an initial exposure to 10 mM l-valine–25 mM K-HEPES buffer (pH 7.4) at 37°C for various short times; the germinant was then removed, and the spores were rinsed using a vacuum suction pump as described for method A. The spores were then incubated for ∼45 min at 37°C in K-HEPES buffer alone and then given a 2nd exposure to 10 mM l-valine–25 mM K-HEPES buffer (pH 7.4) for 2.5 min, followed by germinant removal and rinsing and incubation at 37°C in 25 mM K-HEPES buffer for an additional 45 min.
PS3415 spores were exposed to 0.5 mM or 0.8 mM l-asparagine–25 mM Tris-HCl buffer (pH 8.3) at 37°C for 1.0 min. The germinant was then removed by method A, the spores were incubated at 37°C in 25 mM Tris-HCl buffer (pH 8.3) for 30 min to 12 h, and the spores were exposed again for 1.0 min to 0.5 or 0.8 mM l-asparagine–25 mM Tris-HCl buffer (pH 8.3) followed by germinant removal by method A and further incubation at 37°C in 25 mM Tris-HCl buffer (pH 8.3) for 45 min.
Monitoring spore germination and data analysis.
Germination of individual B. subtilis spores adhered to a microscope coverslip was monitored by DIC microscopy as described previously (18, 19). After the addition of germinant solution to the spores, a digital charge-coupled-device (CCD) camera (12 bit; 1,392 by 1,040 pixels) was used to record DIC images at a rate of 15 s per frame for 90 min. These images were analyzed with a computation program in Matlab to locate each spore's position and to calculate the average pixel intensity of an area of 20 by 20 pixels that covered the whole individual spore on the DIC image (18, 19). The DIC image intensity of each individual spore was plotted as a function of the incubation time (with a resolution of 15 s), and the initial intensity (the first DIC image recorded at T0 after the addition of germinant solution) was normalized to 1. Usually, it took 30 to 60 s to record the first DIC image at T0, so T0 was actually 0.5 to 1.0 min after the germinant solution was added. The intensity at the end of measurements was normalized to zero. Invariably, the latter value had been constant for ≥10 min at the end of measurements.
From the time-lapse DIC image intensity, the time of completion of the rapid fall of ∼75% of the total fall in a spore's DIC image intensity during germination could be determined; this time is concomitant with the time of completion of spore CaDPA release (defined as Trelease) (18, 30, 31). For this work, a spore is defined as germinated if CaDPA release is complete as monitored by DIC microscopy, although germination is not technically complete until the spore cortex has been hydrolyzed. CaDPA release kinetics during germination of individual spores were further quantified by the parameters Tlag and ΔTrelease (Fig. 1A), where Tlag is the time between the mixing of spores with germinants and the initiation of most of the CaDPA release and ΔTrelease = (Trelease − Tlag) (30, 31). The parameter Ilag is also used to describe the germination of individual spores and is defined as the intensity of the DIC image of a spore at Tlag, and Irelease is defined as the DIC image intensity at Trelease, Tlys (Fig. 1A) is the time when spore cortex hydrolysis is completed as determined by the completion of the fall in the spore's DIC image intensity, and ΔTlys = (Tlys − Trelease) (30, 31).
For germination with short germinant exposures, the time between the addition and the removal of the germinant was defined as Tb, at which time the further commitment of spores to germination was blocked by method A or method B (Fig. 1A). Individual spores are identified as committed to germinate if they complete their rapid CaDPA release after Tb such that they have Trelease > Tb. We also defined the time between the addition and the removal of a second germinant pulse as Tb, and ΔTb, which is Trelease − Tb, is the interval between the CaDPA release time of individual spores and the germinant removal time for each germinant exposure.
RESULTS
Commitment and germination of individual B. subtilis wild-type spores with a nutrient germinant.
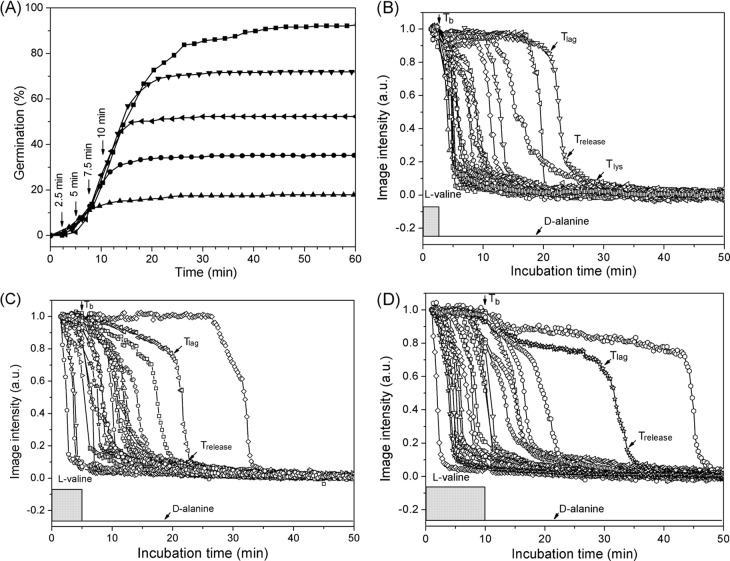
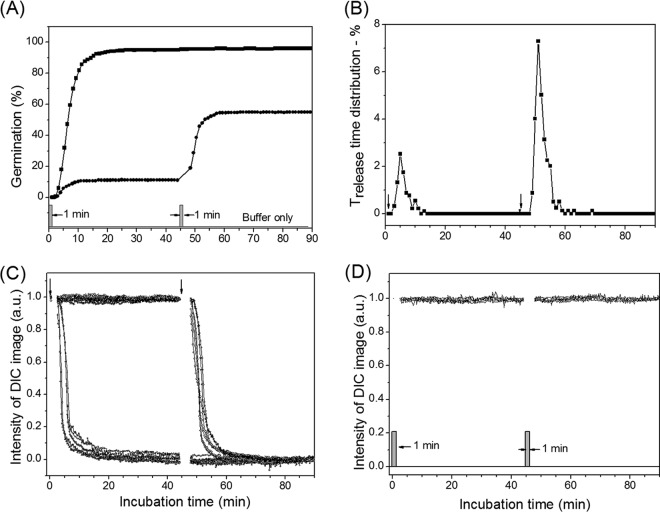
As noted above, while there have been several studies of the commitment of populations of spores of Bacillus species during nutrient germination, there have been no such studies in which the commitment of significant numbers of individual Bacillus spores has been examined. Consequently, we used DIC microscopy to monitor the germination of multiple individual wild-type B. subtilis spores exposed to either l-alanine or l-valine to trigger germination via the GerA GR, followed by removal of the germinant and addition of d-alanine to block further commitment (method B) (15). Figure 2A shows the extent of germination of multiple individual spores as a function of time when the spores were exposed to 10 mM l-valine for 60 min as well as given various short l-valine exposures, followed by germinant removal, replacement with 10 mM d-alanine to inhibit spore commitment, and further incubation (method B) (15). Following germinant removal and d-alanine addition (Fig. 2A, arrows), the extent of germination continued to increase for ∼10 min and reached a constant value, similar to the results observed in previous studies with spore populations (15). The spores that released CaDPA after d-alanine addition were spores that had become committed to germinate during the l-valine pulse. Panels B, C, and D of Fig. 2 show time-lapse DIC image intensities of individual spores exposed to l-valine pulses of 2.5, 5, and 10 min, respectively, as shown in Fig. 2A. Table 1 lists the average kinetic germination parameters of >60 individual germinated spores given various l-valine exposure times. The results indicate that with the shortest l-valine exposure (Tb = 2.5 min), almost all spores that germinated completed CaDPA release after d-alanine addition, i.e., Trelease > Tb (Table 1), and thus all these spores, ∼18% of the total, had been committed only during the short l-valine pulse. These committed spores had an average value of Trelease − Tb of 7.2 ± 7.7 min; thus, Trelease times, and also Tlag times, were clearly heterogeneous whereas the average ΔTrelease value was more constant (Table 1). As the germinant pulse time Tb increased, some spores released CaDPA before d-alanine addition, while another portion became committed only during Tb and released CaDPA after Tb. The percentage of total spores that released CaDPA after Tb increased from 18% to 47% as Tb increased from 2.5 to 10 min (Table 2). The average Tlag values for the spores that released CaDPA after Tb decreased as Tb times also increased, whereas average Trelease − Tb values were relatively similar, indicating that as Tb was increased, many committed spores had begun to release CaDPA at the time of d-alanine addition (Tlag = ∼Tb). In addition, for spores with values of Trelease > Tb, which would include spores that committed late in the Tb period, the Trelease values for these spores increased ∼50% going from spores given a 2.5-min l-valine exposure to those given a 10-min l-valine exposure (Table 1 and Discussion).
FIG 2.
Germination of PS533 spores (wild type) with l-valine either continuously or with various short exposures. (A) Heat-activated spores were germinated with 10 mM l-valine either continuously (◼) or for exposures of 2.5 (▲), 5.0 (•), 7.5 (◀), or 10 (▼) min followed by blocking of further commitment by method B. (B to D) DIC image intensities of ∼20 individual spores germinating with 10 mM l-valine for 2.5 (B), 5.0 (C), and 10 min (D) before blocking further commitment by method B. The DIC image intensity values at 50 min were subtracted from the intensities of images at various times and the values were normalized to the spores' intensity values at T0. In all cases, spore germination was monitored and germination parameters were determined as described in Materials and Methods, and times for Tb, Tlag, Trelease, and Tlys for a few germinating individual spores are noted in various panels. a.u., arbitrary units.
TABLE 1.
Mean values and standard deviations of parameters of the germination of PS533 spores with 10 mM l-valine for different periods, followed by the addition of 10 mM d-alaninea
| Time of D-ala addition (min) | Tlag (min)c | Trelease (min)c | ΔTrelease (min)c | ΔTlys (min) | Ilag | Irelease | No. of germinated spores (% germinated b) |
|---|---|---|---|---|---|---|---|
| No D-ala (60) | 12.4 ± 9.0 | 16.5 ± 9.2 | 4.0 ± 1.1 | 12.2 ± 3.9 | 0.92 ± 0.05 | 0.21 ± 0.06 | 200 (93) |
| 2.5 | 6.3 ± 6.8 (NA/6.3 ± 6.8) | 9.7 ± 7.7 (NA/9.7 ± 7.7) | 3.4 ± 1.5 (NA/3.4 ± 1.5) | 10.6 ± 4.4 | 0.93 ± 0.05 | 0.20 ± 0.06 | 90 (18) |
| 5.0 | 6.3 ± 4.3 (1.7 ± 0.3/6.8 ± 4.2) | 10.7 ± 5.1 (3.6 ± 0.8/11.4 ± 4.8) | 4.3 ± 1.7 (1.9 ± 0.7/4.6 ± 1.5) | 13.4 ± 4.1 | 0.94 ± 0.05 | 0.21 ± 0.06 | 202 (35) |
| 7.5 | 7.4 ± 3.9 (4.0 ± 1.6/7.8 ± 3.9) | 12.1 ± 4.4 (6.0 ± 1.3/12.9 ± 3.9) | 4.7 ± 1.8 (2.0 ± 1.1/5.0 ± 1.6) | 13.3 ± 5.0 | 0.94 ± 0.05 | 0.21 ± 0.12 | 102 (57) |
| 10 | 8.0 ± 5.1 (3.7 ± 1.5/9.4 ± 5.0) | 13.3 ± 5.8 (1.9 ± 2.1/15.5 ± 5.0) | 5.3 ± 2.1 (3.2 ± 1.0/6.0 ± 1.8) | 13.0 ± 4.0 | 0.94 ± 0.05 | 0.21 ± 0.05 | 262 (72) |
Spores of B. subtilis strain PS533 (wild type) were germinated with 10 mM l-valine for different periods, followed by blocking further commitment by method B involving germinant removal and then d-alanine (D-ala) addition, further incubation in buffer for 60 min, and determination of germination parameters as described in Materials and Methods. Data are from the experiment described in the Fig. 2 legend, and all values shown are from >90 individual spores that germinated.
The percentage of germination is defined as the ratio of the number of the germinated spores to the number of dormant spores at T0.
The values in the parentheses are for spores that committed early (Trelease < Tb) and those that committed late (Trelease > Tb). The kinetic parameters (Tb, Tlag, Trelease, and Tlys) are as defined in the Fig. 1A legend. NA, not available.
TABLE 2.
Mean values and standard deviations of germination parameters of committed B. subtilis PS533 spores (wild type) that had a Trelease time longer than the Tb timea
| Spore prepn (activation) | Treatment | Tlag − Tb (min) | Trelease − Tb (min) | ΔTrelease (min) | ΔTlys (min) | No. of committed spores (% committedb) |
|---|---|---|---|---|---|---|
| Plates (heat activated) | 10 mM l-valine, 2.5 min | 3.8 ± 6.8 | 7.2 ± 7.7 | 3.4 ± 1.5 | 10.6 ± 4.4 | 61 (18) |
| 10 mM l-valine, 5.0 min | 1.8 ± 4.2 | 6.4 ± 4.8 | 4.6 ± 1.5 | 13.7 ± 3.8 | 87 (30) | |
| 10 mM l-valine, 7.5 min | 0.3 ± 4.0 | 5.4 ± 4.0 | 5.1 ± 1.6 | 13.3 ± 4.7 | 44 (45) | |
| 10 mM l-valine, 10 min | −0.4 ± 5.1 | 5.5 ± 5.0 | 6.0 ± 1.8 | 13.2 ± 4.0 | 120 (47) | |
| Rich liquid (heat activated) | 10 mM l-valine, 5 min | −0.4 ± 3.8 | 3.0 ± 3.7 | 3.1 ± 1.0 | 9.6 ± 4.6 | 78 (30) |
| 3.3 mM l-valine, 5 min | −0.3 ± 3.2 | 2.6 ± 3.1 | 2.9 ± 1.0 | 12.3 ± 6.0 | 128 (47) | |
| 1 mM l-valine, 5 min | −0.1 ± 3.4 | 2.7 ± 3.3 | 2.9 ± 1.0 | 11.9 ± 4.6 | 61 (22) | |
| 0.33 mM l-valine, 5 min | −0.6 ± 0.8 | 2.0 ± 1.5 | 2.6 ± 0.9 | 8.4 ± 4.1 | 30 (11) | |
| Poor liquid (heat activated) | 10 mM l-valine, 40 min | −4.2 ± 16.4 | 14.1 ± 14.6 | 18.3 ± 7.9 | 19.7 ± 6.5 | 117 (40) |
| 3.3 mM l-valine, 40 min | 3.8 ± 15.3 | 17.3 ± 13.7 | 12.5 ± 4.7 | 16.2 ± 7.0 | 35 (8.5) | |
| 1 mM l-valine, 40 min | NA | NA | NA | NA | 4 (0.9) | |
| Rich liquid (not heat activated) | 10 mM l-valine, 5 min | −0.1 ± 3.8 | 2.8 ± 4.1 | 2.9 ± 0.7 | 9.2 ± 3.9 | 56 (18) |
| 3.3 mM l-valine, 5 min | 0.5 ± 5.7 | 3.4 ± 6.0 | 2.8 ± 0.9 | 9.1 ± 4.1 | 42 (14) | |
| 1 mM l-valine, 5 min | 0.0 ± 1.7 | 2.5 ± 1.8 | 2.5 ± 0.6 | 12.3 ± 4.2 | 27 (8) |
Spores were prepared either on plates or in rich or poor liquid media and were germinated with various l-valine concentrations with or without prior heat activation as described in Materials and Methods. Data for spores prepared on plates are from the experiments represented in Fig. 2, and data for spores made in liquid are from the experiments represented in Fig. 3. Germination was for various times, and further commitment was blocked by method B, spores were incubated further through 60 min, and germination parameters for individual spores were determined as described in Materials and Methods.
The percentage of committed spores is defined as the ratio of the number of the committed spores with Trelease > Tb to the total number of dormant spores at T0.
Effects of nutrient concentration, sporulation conditions, and heat activation on the commitment of individual B. subtilis spores.
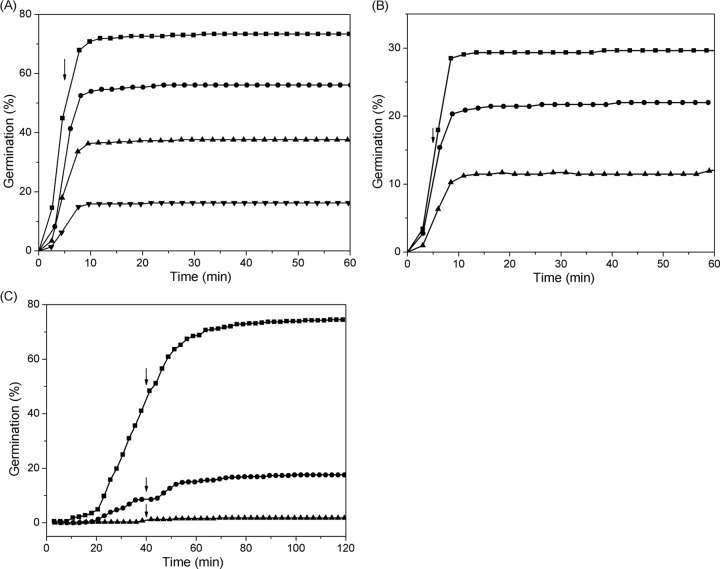
The experiments described above indicated that some individual spores given brief exposures to a nutrient germinant became committed and that these individual committed spores showed less heterogeneity in their average Trelease values than spores germinated during a long germinant exposure (Tables 1 and 2). It has been found that nutrient germinant concentrations can have marked effects on the kinetics of commitment of spore populations (15), so it was of interest to examine the effects of germinant concentration on the kinetics of commitment of individual spores. Figure 3A shows the extent of germination of multiple individual heat-activated PS533 spores made in a rich liquid medium and exposed to various l-valine concentrations for 5 min, followed by replacement with d-alanine and further incubation. As the l-valine concentration was increased from 0.33 to 3.3 mM, the proportion of spores that had committed but not released CaDPA during Tb increased from 11% to 47%, while average values of Tlag − Tb, Trelease − Tb, and ΔTrelease were relatively constant (Table 2). It was notable that when the l-valine concentration increased to 10 mM (a saturating concentration), the percentage of committed spores that had not released CaDPA during Tb fell to 30% (Table 2). For various germinant concentrations, the percentage of committed spores that had not released CaDPA during Tb was calculated as the ratio of the number of these committed spores to the total number of dormant spores at T0. Since at higher germinant concentrations more spores released their CaDPA during Tb (∼43% for a 5-min exposure to 10 mM l-valine [Fig. 3A]), the number of ungerminated spores at the time of d-alanine addition was much less than that at T0. Thus, with 10 mM l-valine the actual proportion of committed spores (defined as the ratio of the number of germinated spores after addition of d-alanine at 5 min to the number of ungerminated spores prior to the addition of d-alanine) was increased to 54%. Similar effects of nutrient concentration on spore commitment were observed with PS533 spores in a poor liquid medium and for unactivated PS533 spores in a rich liquid medium in which either GR levels (poor-medium spores [29]) or GR responsiveness (unactivated spores) is much lower than with heat-activated spores in the rich medium (Fig. 3B and C and Table 2). As the l-valine concentration increased, the percentage of committed spores increased, while the average values of Trelease and ΔTrelease were nearly unchanged.
FIG 3.
Germination of PS533 spores (wild type) made in rich and poor liquid media and with and without heat activation. (A and B) Germination of ∼400 heat-activated (A) and ∼400 unactivated (B) individual spores made in rich medium with 0.33 (▼), 1.0 (▲), 3.3 (•), or 10 (◼) mM l-valine for 5.0 min, followed by blocking further commitment by method B, was monitored as described in Materials and Methods. (C) Germination of ∼500 heat-activated individual spores made in the poor medium with 1.0 (▲), 3.3 (•), or 10 (◼) mM l-valine for 40 min, followed by blocking further commitment by method B, was monitored as described in Materials and Methods. Vertical arrows denote the times of germinant removal and its replacement with d-alanine.
Sporulation conditions also affected the commitment of individual spores. Thus, compared to spores made on rich medium plates, spores made in a similar rich liquid medium had higher percentages of committed spores for the same l-valine concentrations and exposure times (Tb) and germinated earlier after the addition of d-alanine (smaller Trelease − Tb values) (Table 2). The committed spores made in rich liquid medium also had shorter rapid CaDPA release times than spores made on the same rich medium but on plates (Table 2). In contrast, spores that were made in the poor liquid medium that had low GR levels (29) had much lower rates of commitment such that the l-valine pulse duration had to be increased to 40 min in order to observe significant numbers of committed spores with various l-valine concentrations. In addition, the committed spores that were prepared in the poor liquid medium had longer Trelease and ΔTrelease times than the spores made in rich liquid medium. Presumably, the spores made in the poor medium not only had low levels of the GerA GR (29) but also had low levels of some other germination component(s).
In addition to the germinant concentration and sporulation conditions, heat activation also affected the commitment of individual B. subtilis spores. Spores that were not heat activated had lower percentages of committed spores for the same nutrient concentration and exposure duration than did heat-activated spores (Fig. 3A and B and Table 2). However, the kinetic parameters Trelease, ΔTrelease, and ΔTlys of the committed unactivated spores were nearly identical to those for heat-activated spores (Table 2).
Effects of d-alanine on the commitment of spores upon a short germinant exposure.
When spores were exposed to l-alanine for a period as short as 2 min (Tb), all the spores that subsequently germinated had longer Trelease times than Tb. It is known that removal of l-alanine or l-valine from B. subtilis spores followed by incubation in buffer with low pH or the addition of d-alanine (method B) can stop further commitment (13, 15). In this work, we also found that while method A was able to quickly stop commitment after a germinant is replaced by buffer with the same pH, when the buffer was replaced by germinant again, spore commitment resumed. However, with method B, the addition of d-alanine to an l-alanine or l-valine germination not only quickly halted further commitment but also blocked further germination even when the d-alanine was replaced after 5 to 6 rinses by l-alanine or l-valine (data not shown).
Germination of wild-type spores given two l-valine exposures.
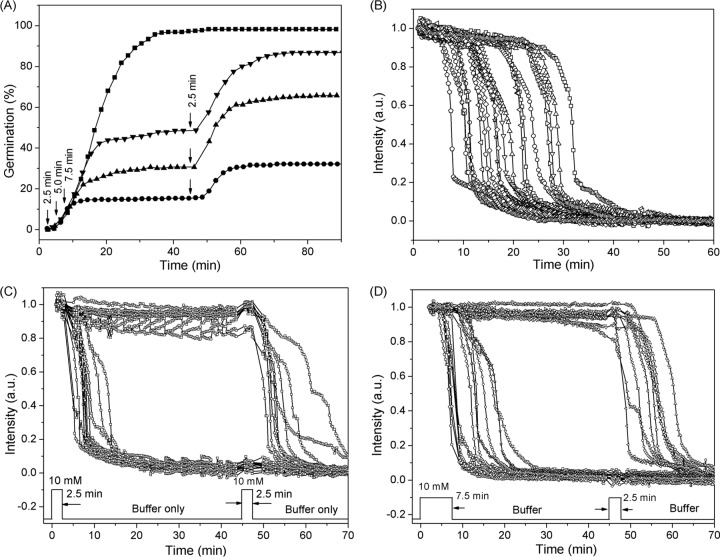
The experiments whose results are shown in Fig. 2B and Table 2 indicated that exposure of PS533 spores to l-valine for short periods caused a portion of the spores to be committed to germinate after the removal of the germinant and its replacement with buffer. However, a large percentage of the spores were not committed after short l-valine exposures. A potentially important question then is whether the latter fraction of spores would exhibit commitment and germination if they were given a 2nd l-valine exposure at a later time and, if so, would the commitment and germination due to the 2nd l-valine exposure occur in a manner independent of the 1st exposure? If the percentage of spores that released CaDPA after the 2nd l-valine exposure were higher than with the 1st exposure, this would suggest that the germination due to the 2nd exposure is not independent of the 1st one. Thus, in addition to causing commitment of some spores, which as far as we know is irreversible, the 1st l-valine exposure may also convert spores to a metastable state that can either decay to the ground state over time or be readily be triggered to generate a committed spore by a 2nd l-valine exposure.
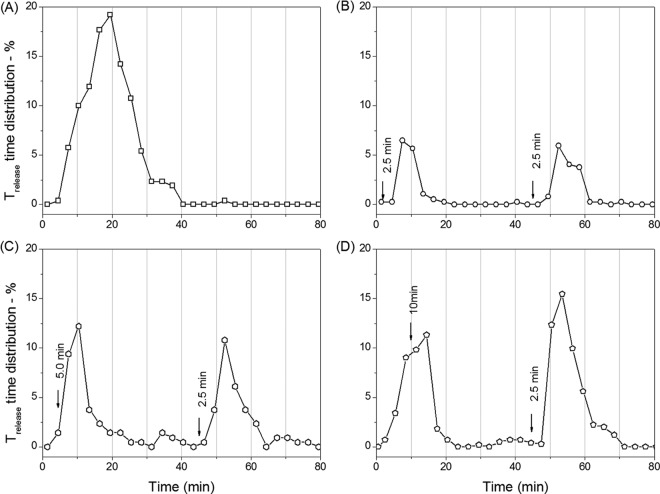
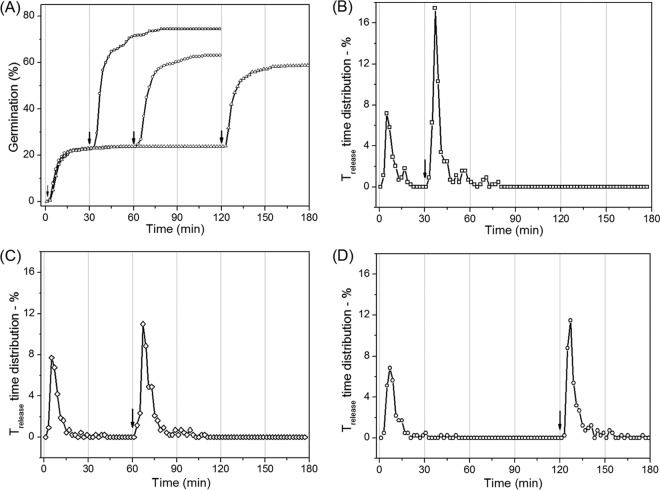
To test the possibilities outlined above, we examined germination of PS533 spores given two short exposures to l-valine (Fig. 4 and 5 and Table 3). Figure 3A shows the extent of germination of wild-type PS533 spores given 2.5 to 7.5 min of exposure to 10 mM l-valine, followed by l-valine removal, replacement with buffer, and incubation until 45 min, when a 2nd 2.5-min l-valine exposure was given, followed again by l-valine removal, replacement by buffer, and incubation until 90 min. Panels B to D of Fig. 4 show the time-lapse DIC intensities of individual spores germinating with either a constant l-valine exposure or two separate exposures, with the 1st one either 2.5 or 7.5 min. Figure 5 shows the distribution of individual germinated spores that had various CaDPA release times when the 1st l-valine exposure increased from 2.5 to 10 min while the 2nd l-valine exposure was constant at 2.5 min. The results indicated that when the duration of the 1st l-valine exposure was equal to the duration of the 2nd exposure, the percentages of the spores that germinated due to the 1st and 2nd exposures were identical (Fig. 4A and 5B and Table 3). However, since the ∼15% of the initial spores germinated due to the 1st exposure left only ∼85% of spores ungerminated, the actual percentage of spores that germinated due to the 2nd l-valine exposure was ∼18%, slightly higher than that seen with the first nutrient exposure. When the 1st l-valine exposure was increased to 5.0, 7.5, or 10 min while keeping the 2nd exposure at 2.5 min, more spores (30%, 39% or 42%, respectively) germinated due to the 1st exposure (Fig. 4A and Table 3). Surprisingly, the second exposure resulted in the germination of very slightly more of the initial spores (32%, 40%, and 46% of the spores) (Fig. 4A and 5C to D and Table 3). Furthermore, when the percentages of spores that had germinated due to the 1st l-valine exposures of 5, 7.5, and 10 min are taken into account, the percentages of available ungerminated spores that germinated due to the 2nd l-valine exposure were 45%, 65%, and 79%, respectively. These results suggested that ungerminated spores exposed once to l-valine are more receptive to commitment and subsequent germination upon a 2nd l-valine exposure than are the initial dormant spores.
FIG 4.
Germination of PS533 spores (wild type) with continuous l-valine exposure or two short l-valine pulses. (A) Germination of spores with 10 mM l-valine for 90 min (◼) or for 2.5 (•), 5.0 (▲), or 7.5 (▼) min followed by germinant removal by method A, further incubation at 37°C for 45 min, and a second 2.5-min exposure to 10 mM l-valine (vertical arrows), followed again by germinant removal by method A and further incubation until 90 min, although there were no significant changes after 70 min. Values shown are from data for ∼400 individual spores determined as described in Materials and Methods. (B) Time-lapse DIC image intensities of ∼20 individual spores germinating with 10 mM l-valine for 60 min. (C and D) Time-lapse DIC image intensities of ∼20 individual spores germinating with 10 mM l-valine for 2.5 (C) or 7.5 min (D), after which the germinant was removed and the spores were rinsed 6 times with buffer, incubated in buffer until 45 min, and reexposed to 10 mM l-valine for 2.5 min, followed by rinsing again and incubation in buffer for 45 min at 37°C. DIC image intensities of individual spores were followed as described in Materials and Methods, and there were only a few notable changes after 70 min.
FIG 5.
Percentages of Trelease times of PS533 spores (wild type) as a function of germination time with either continuous l-valine exposure or two short l-valine exposures. Spores were germinated with 10 mM l-valine for 90 min with no germinant removal (A) or for 2.5 (B), 5.0 (C), and 10 (D) min followed by germinant removal by method A, further incubation until 45 min, a second 2.5-min exposure to 10 mM l-valine, germinant removal again by method A, and further incubation until 90 min, although there were no changes after 80 min. Germination of ∼400 individual spores was followed as described for panels A to D, and the percentages of spores with Trelease values in 3.0-min windows were summed and plotted against the germination time.
TABLE 3.
Mean values and standard deviations of germination parameters of B. subtilis spores that germinated due to the 1st and 2nd exposures to a nutrient germinanta
| Spore, germinant | Exposure time in min (exposure category) | Tlag − Tb (min) | Trelease − Tb (min) | ΔTrelease (min) | No. of germinated spores examined (% germinationb) |
|---|---|---|---|---|---|
| PS533, 10 mM l-valine | 2.5 (1st) | 2.2 ± 1.9 | 7.4 ± 3.0 | 4.8 ± 1.9 | 55 (14.8) |
| 2.5 (2nd) | 2.3 ± 3.2 | 7.9 ± 3.6 | 5.6 ± 2.3 | 57 (15.4) | |
| PS533, 10 mM l-valine | 5 (1st) | 4.2 ± 8.1 | 8.5 ± 8.0 | 4.2 ± 1.8 | 76 (30) |
| 2.5 (2nd) | 4.0 ± 6.1 | 8.4 ± 6.0 | 4.4 ± 1.8 | 64 (32) | |
| PS533, 10 mM l-valine | 7.5 (1st) | 2.3 ± 6.7 | 6.9 ± 7.3 | 4.5 ± 1.6 | 93 (39) |
| 2.5 (2nd) | 9.0 ± 8.7 | 14.5 ± 8.2 | 5.4 ± 2.0 | 110 (40) | |
| PS533, 10 mM l-valine | 10 (1st) | −0.9 ± 6.7 | 3.2 ± 7.3 | 4.1 ± 1.8 | 390 (42.4) |
| 2.5 (2nd) | 2.9 ± 5.5 | 7.8 ± 4.6 | 4.9 ± 2.0 | 491 (46.4) | |
| PS3415, 0.5 mM l-asparagine | 1 (1st) | 2.1 ± 2.9 | 4.6 ± 3.2 | 2.5 ± 0.9 | 59 (11.1) |
| 1 (2nd) | 2.1 ± 1.8 | 5.8 ± 3.0 | 3.7 ± 2.0 | 184 (43.7) |
Heat-activated spores of strains PS533 (wild type) or PS3415 (high GerB* GR levels) were germinated with either l-valine (PS533) or l-asparagine (PS3415) for various times, further commitment was blocked by method A, spores were incubated further for 45 min and reexposed to germinant for 2.5 (PS533) or 1 (PS3415) min, further commitment was blocked again by method A, spores were incubated again for 45 min, and germination parameters were determined as described in Materials and Methods.
The percentage of germinated spores is defined as the ratio of the number of the germinated spores in the 1st or 2nd nutrient exposures to the total number of dormant spores at T0.
Germination of spores with high GerB* GR levels given two exposures to l-asparagine.
The germination of PS533 spores with l-valine is via the activation of the GerA GR, and an obvious issue is that of whether the apparent memory of an initial germinant exposure is unique to the GerA GR or is seen with other GRs as well. The GerB* GR present in high levels in PS3415 spores is the only other B. subtilis GR that responds to a single nutrient germinant, this being l-asparagine (26). Consequently, we chose to examine the effects of two separate short l-asparagine exposures on the commitment and germination of PS3415 spores. Figure 6 shows the germination of PS3415 spores with two 1-min exposures to l-asparagine separated by 45 min, as well as time-lapse DIC image intensities of individual germinated spores and ungerminated spores. The PS3415 spores that germinated due to either the 1st or 2nd nutrient l-asparagine exposures had average ΔTb values of ∼5 min, much longer than the l-asparagine exposure time of 1.0 min (Table 3). Surprisingly, the range of variation in Trelease values of individual germinated spores was relatively narrow and the percentage of initial spores that ultimately germinated due to the 2nd l-asparagine exposure was much larger than the percentage that germinated due to the 1st exposure (Fig. 6B and Table 3). The latter finding suggests that the ungerminated PS3415 spores given the 1st l-asparagine exposure are more able to become committed and ultimately germinate upon a 2nd l-asparagine exposure, even after 45 min, than are naive spores that have never been exposed to l-asparagine.
FIG 6.
Germination of PS3415 spores (high GerB* GR levels) with continuous l-asparagine exposure or with two short l-asparagine exposures. (A) Spores were germinated with 0.5 mM l-asparagine either continuously (◼) or for two 1.0-min exposures separated by 45 min (•), with each exposure followed by removal of l-asparagine by method A using a pipette for germinant removal and rinsing and continued incubation in buffer as described in Materials and Methods. (B) Distribution of Trelease values of spores given two 1.0-min exposures to 0.5 mM l-asparagine, with l-asparagine removed after each exposure as described above and further incubation as described in Materials and Methods. Trelease values were summed over 1-min windows in germination. (C) DIC image intensities of 10 individual PS3415 spores that became committed during the separate 1.0-min exposures to 0.5 mM l-asparagine. (D) DIC image intensities of five individual PS533 spores that had not become committed during the 1-min exposures to 0.5 mM l-asparagine.
The results seen with the two l-valine or l-asparagine exposures noted above certainly suggested that an initial germinant exposure makes spores that have not become committed be more receptive to becoming committed upon a 2nd germinant exposure. A further issue related to the suggestion presented above is whether the effect of the 1st germinant exposure on spores' responses to a 2nd germinant exposure is stable or is lost upon extended incubation. To test this, we varied the interval between successive 1-min l-asparagine exposures of PS3415 spores from 30 min to 12 h (Fig. 7 and Table 4). When the interval between the two l-asparagine exposures was 30 min, the proportion of spores that germinated due to the 2nd exposure was ∼52%, in comparison to ∼23% with the 1st exposure (Table 4 and Fig. 7B; note that different preparations of PS3415 spores were used in the experiments whose results are shown in Fig. 6 and 7). However, when the interval between the two l-asparagine exposures was increased to 60 min or 120 min, the percentage of spores that germinated due to the 2nd exposure was reduced to 40% or 35%, respectively, and a further increase in the interval between the two exposures to 12 h reduced the proportion of spores that germinated due to the 2nd exposure to 26% (Fig. 7C and D and Table 4). These results suggest that whatever happens in the 1st l-asparagine exposure to make spores more receptive to commitment upon a 2nd exposure is reversible, although it is at least somewhat stable for up to 120 min during incubation in 25 mM Tris-HCl buffer (pH 8.3) at 37°C.
FIG 7.
Germination of PS3415 spores (high GerB* GR levels) with short l-asparagine exposures separated by various intervals. (A) Spores were exposed to 0.8 mM l-asparagine for two 1.0-min periods separated by 30 (□), 60 (○), or 120 (Δ) min, with each pulse followed by germinant removal by method A using a vacuum suction pump and further incubation, and spore germination was followed, all as described in Materials and Methods. (B to D) The distribution of Trelease values of the germinated spores given two 1.0-min exposures to 0.8 mM l-asparagine, separated by 30 (B), 60 (C), and 120 (D) min, respectively, as described for panel A. The distribution of Trelease values in 1-min windows is defined as the ratio of the number of spores that had Trelease values in that 1-min window to the number of dormant spores at T0. A total of ∼460 germinated spores were examined in these experiments. Note that a different preparation of PS3415 spores was used in this experiment than was used in the experiment performed as described for Fig. 6.
TABLE 4.
Parameters of the germination of PS3415 spores (high GerB* GR levels) with two 1-min germinant exposures separated by different intervalsa
| Germinant pulse separation | Exposure | Tlag − Tb (min) | Trelease − Tb (min) | ΔTrelease (min) | No. of germinated spores examined (% germinationb) |
|---|---|---|---|---|---|
| 30 min | 1st | 3.4 ± 2.7 | 7.4 ± 4.1 | 4.0 ± 2.2 | 103 (23) |
| 2nd | 6.0 ± 9.6 | 11.2 ± 9.4 | 5.2 ± 2.4 | 237 (52) | |
| 60 min | 1st | 3.3 ± 5.4 | 8.2 ± 6.7 | 4.8 ± 2.5 | 112 (24) |
| 2nd | 6.0 ± 7.6 | 11.5 ± 8.5 | 5.5 ± 2.3 | 185 (40) | |
| 120 min | 1st | 3.7 ± 6.8 | 9.6 ± 8.0 | 5.9 ± 3.4 | 109 (23) |
| 2nd | 3.5 ± 7.1 | 10.0 ± 8.3 | 6.5 ± 3.8 | 161 (35) | |
| 12 h | 1st | 3.4 ± 4.0 | 7.7 ± 4.8 | 4.4 ± 2.4 | 57 (22) |
| 2nd | 3.6 ± 1.0 | 7.1 ± 1.8 | 3.3 ± 1.4 | 68 (26) |
Heat-activated PS3415 spores (high GerB* GR levels) were exposed for 1 min to 0.8 mM l-asparagine, further commitment was blocked by method A, spores were incubated for various times and given a second 1-min exposure to 0.8 mM l-asparagine, further commitment was again blocked by method A, spores were incubated for an additional 45 min, and germination parameters were determined, all as described in Materials and Methods. Values in the table (except for those corresponding to the 12-h interval) were taken from the results shown in Fig. 7. Note that the preparation of PS3415 spores used in this experiment was different from that used in the experiment shown in Fig. 6.
The percentage of germinated spores is defined as the ratio of the number of the germinated spores due to the 1st or 2nd germinant exposure to the total number of dormant spores at T0.
DISCUSSION
The analysis of the dynamic germination of multiple individual spores of B. subtilis given one or two short germinant exposures has led to a better understanding of commitment, blocking, and continuation of nutrient germination of these spores, and a number of new conclusions have been obtained. First, when spores were exposed to the germinant l-valine (PS533 spores) or l-asparagine (PS3415 spores), all spores that became committed to germinate then germinated with an average CaDPA release time (Trelease − Tb) of ∼7 or ∼5 min, respectively, after germinant removal, much longer than the actual germinant exposure times (Tb). This finding indicates that after GRs bound their germinants during short germinant exposures, the committed spores had average lag times (Tlag − Tb) of up to 5 min prior to initiating rapid CaDPA release. Note that since the time at which an individual spore became committed to germinate was within the short germinant exposure time Tb, the variation in ΔTb indicates the heterogeneity in CaDPA release times of the committed spores.
The second new conclusion from the current work concerns the time between commitment and Trelease as a function of time in germination. Previous work with spore populations suggested that spores that become committed early have shorter times between commitment and CaDPA release than do spores that become committed late in germination (15). However, precise estimation of this value late in germination was difficult as was noted at the time that these measurements were made. This analysis was simplified a bit when commitment and germination of individual spores were examined in the current work. In particular, if the wild-type spores becoming committed are taken as those with values of Trelease > Tb, then spores becoming committed latest in germination, those given a 10-min 10 mM l-valine exposure that had Trelease values > 10 min, had average Trelease times ∼50% higher than those with Trelease values > 2.5 min after a 2.5-min l-valine exposure. This difference is generally similar to those determined from population measurements (15) and provides further support of the idea that this difference is real.
The third new conclusion from this work is that the factors that affect the time of commitment of individual B. subtilis spores include the length of germinant exposure, the germinant concentration, the sporulation conditions, and heat activation. Long germinant exposure times not only increased the percentage of spores germinated during the exposure but also increased the percentage of the committed spores that germinated after germinant removal. Long germinant exposures also decreased the average Tlag − Tb values while the average Trelease − Tb values were nearly unchanged, indicating that the committed spores tended to initiate CaDPA release during the germinant exposure. For a given germinant exposure time, higher germinant concentrations increased the percentage of committed spores, while average values of Tlag − Tb, Trelease − Tb, and ΔTrelease were nearly unchanged for PS533 spores made in rich liquid medium. Sporulation in rich liquid medium with heat activation also led to higher percentages of committed spores. Presumably, long germinant exposure times, high germinant concentrations, rich sporulation medium, and heat activation resulted in more GRs being activated such that more spores became committed. Overall, these results from examination of the commitment of individual spores are in agreement with the results from examination of the commitment of spore populations (15).
Other new results in this work were surprising, in particular, the finding that B. subtilis spores given a 1st exposure to a nutrient germinant, whether l-valine for PS533 spores or l-asparagine for PS3415 spores, exhibited even more commitment upon a 2nd germinant exposure, especially for the PS3415 spores. The higher level of commitment seen with the 2nd exposure than with the 1st indicated that commitment of spores upon the second exposure was not independent of the 1st exposure, even with intervals between the two exposures of as long as 120 min. It is possible that GRs, in particular, GerB* GRs and, to a lesser extent, GerA GRs, in spores given brief exposures to germinants can interact with their cognate germinant and undergo either of two fates: (i) the GRs can become sufficiently activated such that this exceeds the threshold for commitment, giving spores that germinate 5 to 7 min later even after germinants are removed, or (ii) the GRs can become partially activated such that the threshold for commitment is not reached, either because sufficient numbers of GRs are not activated or because there is an intermediate partially active state of all GRs. We further suggest that the partial GR activation indicated above is at least somewhat stable, such that when these partially activated spores are given a 2nd germinant exposure, the activation of sufficient GRs to reach the threshold for commitment in these spores is more rapid than during the 1st exposure, such that a higher percentage of spores become committed with the 2nd exposure. It is of course possible that it is not partially activated GRs that are responsible for the increased responsiveness of spores to become committed after a 1st germinant exposure but is rather some downstream member of the germination signaling cascade. However, given that the overall signal transduction pathway in spore germination is not clear, we have considered only GRs as the target for partial activation.
Another potential explanation for the higher level of commitment in a 2nd germinant exposure is that this is due to the known heterogeneous distribution of GRs between individual spores in populations, with some spores having high GR levels and other spores low levels (22, 32). However, this factor seems unlikely to be important since (i) spores with GR levels far from the population mean are present at rather low levels and (ii) the spores that have the highest GR levels would tend to become committed during the 1st germinant exposure, although the gamma distribution of GR levels in individuals in spore populations indicates that spores with the highest GR levels are less abundant than spores with average GR levels. More importantly, if the activation of spores with average GR levels was not high enough to trigger germination in the 1st germinant exposure and the activation of these GRs by the 2nd exposure was independent of the 1st exposure, then those spores with average GR levels would not be committed in the 2nd exposure. This would lead to even lower levels of commitment in the 2nd exposure, which is the opposite of what was observed.
The finding that increasing the interval between the two l-asparagine exposures from 30 min to 12 h led to a decrease in the commitment of spores carrying the GerB* GR with the 2nd germinant exposure from 52% to 26% suggested that the partially activated state of spores' GRs can relax to an unactivated ground state over time. One might expect that increasing the intervals between two germinant exposures, changing the temperature of the incubation between exposures, or blocking GR binding sites prior to the 2nd exposure could deactivate spores' GRs, which would further decease commitment with the 2nd germinant exposure; this was certainly the case for longer intervals between the exposures. In addition, in experiments with PS533 spores we found that replacement of a 1st short 10 mM l-alanine exposure with 10 mM d-alanine caused any uncommitted spores to become refractory to further activation (data not shown). Thus, even when the d-alanine was removed and spores were rinsed and reexposed to l-alanine, no individual spores germinated with a 2nd exposure to l-alanine (data not shown).
Overall, the findings reported in this work have provided new insight into the germination behavior of bacterial spores with nutrient germinants. In particular, that initial germinant exposures can potentiate spores' responses to subsequent germinant exposures indicates that spores have memory, with this memory possibly being stored in the activation state of spores' GRs.
ACKNOWLEDGMENTS
We are grateful to Guiwen Wang and David Pan for participation in collecting some germination data.
This work was supported by a Department of Defense Multi-disciplinary University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911F-09-1-0286 (P.S. and Y.Q.L.) and by a grant from the Army Research Office under contract number W911NF-12-1-0325.
Footnotes
Published ahead of print 25 April 2014
REFERENCES
- 1.Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556. 10.1016/j.mib.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Setlow P, Johnson EA. 2007. Spores and their significance, p 45–79 In Doyle MP, Buchanan R. (ed), Food microbiology: fundamentals and frontiers, 4th ed. ASM Press, Washington, DC [Google Scholar]
- 3.Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94. 10.1016/j.tim.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525. 10.1111/j.1365-2672.2005.02736.x [DOI] [PubMed] [Google Scholar]
- 5.Moir A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526–530. 10.1111/j.1365-2672.2006.02885.x [DOI] [PubMed] [Google Scholar]
- 6.Mongkolthanaruk W, Cooper GR, Mawer JS, Allan RN, Moir A. 2011. Effect of amino acid substitutions in the GerAA protein on the function of the alanine-responsive germinant receptor of Bacillus subtilis spores. J. Bacteriol. 193:2268–2275. 10.1128/JB.01398-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moir A, Kemp EH, Robinson C, Corfe BM. 1994. The genetic analysis of bacterial spore germination. J. Appl. Bacteriol. 77:9S–16S. 10.1111/j.1365-2672.1994.tb03037.x [DOI] [PubMed] [Google Scholar]
- 8.Zuberi AR, Moir A, Feavers IM. 1987. The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene 51:1–11. 10.1016/0378-1119(87)90468-9 [DOI] [PubMed] [Google Scholar]
- 9.Corfe BM, Sammons RL, Smith DA, Mauël C. 1994. The gerB region of the Bacillus subtilis 168 chromosome encodes a homologue of the gerA spore germination operon. Microbiology 140:471–478. 10.1099/00221287-140-3-471 [DOI] [PubMed] [Google Scholar]
- 10.Irie R, Fujita Y, Kobayashi M. 1996. Nucleotide sequence and gene organisation of the gerK spore germination locus of Bacillus subtilis 168. J. Gen. Appl. Microbiol. 42:141–153. 10.2323/jgam.42.141 [DOI] [Google Scholar]
- 11.Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Co-operativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this co-operativity by alterations in the GerB receptor. J. Bacteriol. 188:28–36. 10.1128/JB.188.1.28-36.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster SJ, Johnstone K. 1986. The use of inhibitors to identify early events during Bacillus megaterium KM spore germination. Biochem. J. 237:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart GSAB, Johnstone K, Hagelberg E, Ellar DJ. 1981. Commitment of bacterial spores to germinate: a measure of the trigger reaction. Biochem. J. 198:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatasubramanian P, Johnstone K. 1989. Biochemical analysis of the Bacillus subtilis 1604 spore germination response. J. Gen. Microbiol. 135:2723–2733 [DOI] [PubMed] [Google Scholar]
- 15.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433. 10.1128/JB.00326-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Huang SS, Li YQ. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936–6941. 10.1021/ac061090e [DOI] [PubMed] [Google Scholar]
- 17.Stringer SC, Webb MD, George SM, Pin C, Peck MW. 2005. Heterogeneity of times required for germination and outgrowth from single spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 71:4998–5003. 10.1128/AEM.71.9.4998-5003.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Kong L, Wang G, Setlow P, Li YQ. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J. Biomed. Opt. 15:056010. 10.1117/1.3494567 [DOI] [PubMed] [Google Scholar]
- 19.Zhang PF, Garner W, Yi X, Yu J, Li YQ, Setlow P. 2010. Factors affecting the variability in the time between addition of nutrient germinants and rapid DPA release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619. 10.1128/JB.00345-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringer SC, Webb MD, Peck MW. 2011. Lag time variability in individual spores of Clostridium botulinum. Food Microbiol. 28:228–235. 10.1016/j.fm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 21.Setlow P, Liu J, Faeder JR. 2012. Heterogeneity in bacterial spore populations, p 201–216 In Abel-Santos E. (ed), Bacterial spores: current research and applications. Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 22.Ghosh S, Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787–1797. 10.1128/JB.01668-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Kong L, Wang G, Scotland M, Ghosh S, Setlow B, Setlow P, Li YQ. 2012. Analysis of the slow germination of multiple individual superdormant Bacillus subtilis spores using multifocus Raman microspectroscopy and differential interference contrast microscopy. J. Appl. Microbiol. 112:526–536. 10.1111/j.1365-2672.2011.05230.x [DOI] [PubMed] [Google Scholar]
- 24.Kong L, Doona CJ, Setlow P, Li YQ. 2014. Monitoring rates and heterogeneity of high-pressure germination of Bacillus spores by phase-contrast microscopy of individual spores. Appl. Environ. Microbiol. 80:345–353. 10.1128/AEM.03043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabrera-Martinez R-M, Tovar-Rojo F, Vepachedu VR, Setlow P. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457–2464. 10.1128/JB.185.8.2457-2464.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart K-AV, Yi X, Ghosh S, Setlow P. 2012. Germination protein levels and rates of germination of spores of Bacillus subtilis with overexpressed or deleted genes encoding germination proteins. J. Bacteriol. 194:3156–3164. 10.1128/JB.00405-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450 In Harwood CR, Cutting AM. (ed), Molecular biological methods for Bacillus. John Wiley, Chichester, United Kingdom [Google Scholar]
- 29.Ramirez-Peralta A, Zhang P, Li Y-q, Setlow P. 2012. Effects of sporulation conditions on germination and germination protein levels of spores of Bacillus subtilis. Appl. Environ. Microbiol. 78:2689–2697. 10.1128/AEM.07908-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong LB, Zhang PF, Wang GW, Setlow P, Li YQ. 2011. Characterization of bacterial spore germination using phase contrast microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers. Nat. Protoc. 6:625–639. 10.1038/nprot.2011.307 [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Yi X, Li YQ, Setlow P. 2011. Germination of individual Bacillus subtilis spores with alterations in the GerD and SpoVA proteins important in spore germination. J. Bacteriol. 193:2301–2311. 10.1128/JB.00122-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffiths KK, Zhang J, Cowan AE, Yu JJ, Setlow P. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol. Microbiol. 81:1061–1077. 10.1111/j.1365-2958.2011.07753.x [DOI] [PMC free article] [PubMed] [Google Scholar]