Abstract
Alga-derived biofuels are one of the best alternatives for economically replacing liquid fossil fuels with a fungible renewable energy source. Production of fuel from algae is technically feasible but not yet economically viable. Harvest of dilute algal biomass from the surrounding water remains one of the largest barriers to economic production of algal biofuel. We identified Bacillus sp. strain RP1137 in a previous study and showed that this strain can rapidly aggregate several biofuel-producing algae in a pH- and divalent-cation-dependent manner. In this study, we further characterized the mechanism of algal aggregation by RP1137. We show that aggregation of both algae and bacteria is optimal in the exponential phase of growth and that the density of ionizable residues on the RP1137 cell surface changes with growth stage. Aggregation likely occurs via charge neutralization with calcium ions at the cell surface of both algae and bacteria. We show that charge neutralization occurs at least in part through binding of calcium to negatively charged teichoic acid residues. The addition of calcium also renders both algae and bacteria more able to bind to hydrophobic beads, suggesting that aggregation may occur through hydrophobic interactions. Knowledge of the aggregation mechanism may enable engineering of RP1137 to obtain more efficient algal harvesting.
INTRODUCTION
Energy underlies economies and is the largest single market in the world. However, most energy systems are based on finite nonrenewable resources that increasingly have higher direct and indirect costs. A growing research effort focuses on developing and deploying renewable energy sources to supplement fossil fuels. Research into renewable liquid fuels is of particular interest in the United States, because transportation is almost exclusively powered by petroleum.
Algal biofuels represent one of the best alternatives to sustainably produce fungible liquid fuels. Algae act as self-replicating bioreactors that use light energy to chemically reduce CO2 into useful energy storage molecules. Unlike traditional crops, algae can be grown on land not suitable for agriculture and can be grown in wastewater or saltwater (1, 2). Algae have high growth rates, sometimes doubling their biomass in several hours, and can be harvested multiple times per year (3). Algal biomass is ideally suited for conversion to crude oil via hydrothermal liquefaction, which produces an oil that can be refined in existing refineries and also allows the recovery of limiting nutrients such as nitrogen and phosphorous (4).
While they are technologically feasible, studies have shown algal biofuels are not yet economically viable (5). Furthermore, to our knowledge no company has yet successfully produced algal biofuel at a profit. Only when profitability is achieved will algal biofuels become a self-sustaining venture that can make a significant impact on the production of renewable fuels. Harvest of the algal biomass has been identified as one of the key hurdles to economically producing fuel from algae (5). Algal biomass must be concentrated and most of the water removed before the biomass can be converted to fuel. Mature technologies for algal harvest include filtration, centrifugation, sedimentation, electrocoagulation, dissolved air flotation, chemical flocculation, and bio-aggregation (6). Uduman et al. provide an excellent review of different algal harvest methods, including the advantages and disadvantages of each (6). Bio-aggregation uses biological agents such as extracellular polymeric substances, chitosan, or whole cells to form easily harvestable aggregates (7–11). Several alga-aggregating bacterial strains are known and have been proposed for use in harvesting algae (12–17).
In previous work we described the alga-aggregating bacterium Bacillus sp. strain RP1137 (17). This bacterium can rapidly aggregate multiple algae that are candidates for biofuel production. Aggregation is pH and divalent-cation dependent (17). Fixed cells were also shown to be as effective as live cells at aggregating algae. However, the detailed mechanism of aggregation of algae by RP1137 was unknown. Knowledge of the mechanism may be useful for understanding why it is able to aggregate some algae but not others and for applying the strain to large-scale algal harvest.
In this study, we define the mechanism of algal aggregation by Bacillus sp. strain RP1137. The purpose of this research was to understand the aggregation mechanism of Bacillus sp. strain RP1137 to determine its suitability for harvest of algae and to understand how harvest might be improved.
MATERIALS AND METHODS
Strains and culture conditions.
Liquid cultures of Bacillus sp. strain RP1137 were grown in marine broth 2216 (BD, Franklin Lakes, NJ) at 30°C in 125-ml Erlenmeyer flasks with shaking at 180 rpm. Marine broth 2216 plus 15 g/liter Difco technical agar (BD) was used for solid medium. Nannochloropsis oceanica IMET1 was grown as described before (17). Briefly, N. oceanica was grown in 20-ppt-salinity f/2 medium (18) in 500-ml ported photobioreactors at 25°C with a light/dark photoperiod of 14 h/10 h.
Filtration aggregation assay.
A filtration aggregation assay was used to quantitate the amount of algae that were aggregated under a given condition. This assay has been described in detail (17). Briefly, the assay involves carrying out aggregation reactions with N. oceanica IMET1 and Bacillus sp. strain RP1137 in a 96-well plate. The entire volume of the reaction mixture is then passed through a 50-μm mesh; aggregates that are larger than the mesh are retained, and smaller particles pass through. Chlorophyll fluorescence is measured in the flowthrough and compared to that in control samples without bacteria added to determine the percentage of algae that are aggregated upon addition of the bacteria. Unless noted otherwise, aggregation assays were carried out in deionized water where the pH had been adjusted to 10.5 with NaOH and 10 mM CaCl2 had been added.
Bacterial aggregation efficiency time course.
RP1137 cells were streaked from cryostocks, and a single colony was used to start a 10-ml culture in marine broth medium. The culture was incubated at 30°C in a 125-ml flask with 180-rpm shaking. From the initial culture, three subcultures were started at a calculated optical density (OD) of 0.01 in 200 ml of marine broth. Cultures were grown in 1-liter flasks at 30°C with 180 rpm shaking. Time points were taken every 1 to 2 h for 24 h. At each time point, cells were collected and concentrated by centrifugation at 5,580 × g for 5 min, supernatant was aspirated, and the cell pellet was suspended in 4% paraformaldehyde (PFA) in 1× phosphate-buffered saline (PBS) (pH 7.4) and incubated for 1 h at room temperature. Cells were then concentrated by centrifugation, the supernatant was aspirated, and cells were suspended in 1× PBS to wash the cells. The cells were then again concentrated by centrifugation and suspended in a fresh aliquot of 1× PBS. Fixed cells were used to preserve the surface chemistry of the cell and ensure that chemistry was not altered due to stress responses by the cell. Filtration aggregation assays were carried out using bacteria from each time point with algae from 2 days after subculturing. The samples were normalized by cell surface area to 3 × 108 μm2/ml (described below) so each sample had the same surface area available for interacting with algal cells.
Algal aggregation efficiency time course.
N. oceanica IMET1 cultures were grown as described above. Samples of algae were taken at 2, 5, and 17 days after subculturing, which represent the early exponential, exponential, and stationary phases of growth, respectively. Cells were fixed following the protocol used for the bacterial cells. Samples were normalized by cell surface area per ml (described below) so that each sample had the same available surface area for interacting with bacterial cells. Aggregation assays were carried out using bacterial cells from the exponential phase (OD = 0.7).
Determining cell size and surface area.
Cells from the time course were stained in 1× SYBR green I nucleic acid stain for 10 min in the dark. SYBR green staining was used to illuminate the cell body and provide crisp cell margins that were amenable to automated image analysis. Cells were then visualized on a Zeiss Axioplan microscope with excitation from a Zeiss X-Cite 120Q Iris FL light source using a filter cube with a 470/40 BP excitation filter, an FT 495 dichroic mirror, and a 525/50 BP emission filter. Cells were diluted or concentrated as needed to obtain well-separated cells. The volume of each field of view was determined using the known depth of the bacterial hemacytometer and the height and width of the field of view. For each time point, 20 to 30 fields of view were captured and saved as TIFF files. Image processing was done in Cell Profiler (19) with the following series of commands in a custom pipeline: LoadImage, ColorToGrey, IdentifyPrimaryObjects, ReassignObjectNumbers, MeasureObjectSizeShape, and ExportToSpreadsheet. LoadImage imports the images. ColorToGrey converts the image to greyscale to reduce processor time. IdentifyPrimaryObjects was used to find and identify objects using the Otsu global algorithm, a 4- to 40-pixel cutoff, and a 0.02 to 1 threshold cutoff. ReassignObjectNumbers was used to join cells within a filament into one object using a six-pixel cutoff. MeasureObjectSizeShape was used to measure the perimeter and area of the identified objects. ExportToSpreadsheet was used to export the data as a .cvs file for import into Microsoft Excel for further analysis. Data were converted from pixels to micrometers using data gathered from a stage micrometer. Cell length was approximated by dividing cell perimeter by 2; this provides a good estimate of cell length for filamentous bacilli, though it does introduce a slight overestimate of the absolute size of the cells. To obtain cell surface area for normalization, both perimeter and area data are used. The key parameter needed, but unavailable directly in Cell Profiler, for calculating surface area of a cell is the radius of the cell. To derive the radius of individual cells, the two-dimensional (2D) images of cells were used, and the bacilli were modeled as rectangles with half circles on each end. The resulting equation for area is the sum of the area of a circle and the area of a rectangle: A = πr2 + 2r[(P/2) − 2r], where A is the area of the cell, r is the radius of the cell, and P is the perimeter of the cell. Since A and P are measured values, the equation can be solved for r using the quadratic equation, which yields two solutions, one of which is the real radius of the cell. Derived radius values were checked against the manually measured average radius along the length of individual cells. Calculated values are very close to measured values, indicating that the method can be used to accurately calculate cell radius in an automated format. Cell radius was used to calculate surface area of a three-dimensional cell by modeling the cell as two halves of a sphere plus the surface area of a cylinder minus the ends. The surface area of individual cells was calculated for 900 to 1,600 cells per time point. Cell numbers per ml were then used to calculate available surface area per unit volume. Surface area per ml of individual sample was used to normalize available surface area for interaction with algal cells between samples at different time points. The available surface area of N. oceanica IMET1 time points was determined using a pipeline similar to that used for RP1137 cells with the following modifications. Images were captured using chlorophyll autofluorescence. Nannochloropsis cells are spherical, so the measured area of the 2D images could be used to directly derive the radius using the equation for the area of a circle (A = πr2). The radius could then be used to calculate the 3D surface area of a sphere (A = 4πr2). Surface area per unit volume was determined by combining surface area data with cell concentration data. All experiments were normalized by surface area using the cells prepared as described above. The OD of the culture is provided to make clear which growth phase was used in each experiment.
LiCl treatment of RP1137 cells.
Lithium chloride treatment was done according the protocol of Lortal et al. (20). RP1137 cells were concentrated by centrifugation at 20,000 × g for 3 min, and the cell pellet was suspended in either distilled water, 5 M LiCl, 7.5 M LiCl, or 10 M LiCl. Cells were incubated at these conditions for 15, 45, and 120 min at room temperature and then concentrated by centrifugation. The cell pellets were suspended in pH 10.5 deionized water with 10 mM CaCl2 and used for aggregation assays.
Base titration of whole bacterial cells.
Live RP1137 cells were used for base titration experiments. Cells were taken in the exponential phase (OD = 0.7; 3.4 × 106 cells/ml) and stationary phase (OD = 1.6; 7.2 × 106 cells/ml). Culture volumes were normalized by surface area to ensure that the same amount of bacterial cell surface was being titrated in each sample. Cells were concentrated by centrifugation at 15,000 × g for 5 min, and the cell pellet was suspended in pH 5 deionized water. This washing step was repeated twice more to ensure that salts had been removed, and the cells were equilibrated to pH 5. Base in the form of 0.25 M NaOH was added to the cell suspension, and pH was recorded after each addition when the value stabilized.
Calcium binding assay.
Calcium binding was evaluated by measuring the concentration of calcium remaining after 1 ml of cells had been added. Calcium binding assays were performed with fixed RP1137 cells from the exponential phase (OD = 0.7) of growth. Known concentrations of CaCl2 were added to cells in pH 10 deionized water. Cells were then removed by centrifugation at 20,000 × g for 3 min. The calcium concentration in the supernatant was measured using the LaMotte calcium hardness colorimetric kit (LaMotte, Chestertown, MD). The kit was adapted for use in a 96-well format and measurement in a Spectramax M5 plate reader. The readout for the assay was absorbance at 635 nm. Absorbance at this wavelength is linear for calcium concentrations between 0 and 160 μM. Samples were diluted to ensure that they were within the linear range of the assay. Absorbance values were compared to a CaCl2 standard curve to determine the concentration of calcium remaining.
Calcium coordination experiment.
RP1137 and Nannochloropsis cells were separately suspended in pH 10.5 water with 10 mM CaCl2. To ensure that the cells were preloaded with calcium, both bacteria and algae were concentrated by centrifugation at 20,000 × g, and the cell pellets were suspended in the same solution. This preloading step was repeated once. The cells were then used in filtration aggregation assays, in contrast to controls, where only the algal cells were preloaded with calcium.
C18 binding assay.
Binding of cells to C18 resin was performed with fixed RP1137 cells from the exponential phase of growth (OD = 0.7) and with fixed Nannochloropsis cells from 5 days postinoculation. Dry C18 beads with a 10-μm diameter were purchased from Hamilton (Reno, NV). Beads were reconstituted in methanol overnight. The beads were concentrated by centrifugation at 20,000 × g for 1 min. The beads were then suspended in pH 10.5 deionized water with 10 mM CaCl2. This process of concentration and suspension in pH 10.5 deionized water with 10 mM CaCl2 was repeated twice more to ensure that methanol was removed, and the beads were equilibrated in the test solution. The equilibration process was repeated without CaCl2 for a separate aliquot of beads to obtain beads for the no-calcium samples. For each experiment, 200 beads were used per cell, as this was found to give maximal binding with the minimum number of beads. Equal numbers of algal or bacterial cells were incubated with C18 beads (200:1 bead-to-cell ratio) in the presence or absence of 10 mM CaCl2. The mixtures were analyzed with an Accuri C6 flow cytometer to count the number of unbound algal or bacterial cells. The beads were distinguished from cells by their larger forward scatter area with an upper forward scatter area cutoff of 1,270,000. A lower cutoff of 44,000 was used to remove background particles found within the medium. RP1137 cells fell between these two cutoffs. Algal chlorophyll autofluorescence was used to distinguish Nannochloropsis cells from their associated bacterial cells using the FL3 channel (LP filter; excitation, 488 nm; emission, 670 nm) on the flow cytometer. Only particles that were between the two forward-scatter cutoffs and had a chlorophyll autofluorescence greater than 10,000 were counted as algal cells. These settings counted the unbound bacterial or algal cells, which allowed comparison of the number of bound cells in the presence and absence of calcium.
Zeta potential.
Measurement of cell surface charge or zeta potential was done on a dynamic light scattering instrument (Malvern Instruments Ltd., Worcestershire, United Kingdom). Measurements were done on fixed algal cells from 2, 5, and 17 days after being subcultured and on fixed RP1137 cells that were taken in the exponential phase (OD = 0.7) and stationary phase (OD = 1.6). Cells were concentrated by centrifugation at 20,000 × g for 1 min. The supernatant was aspirated, and the cells were suspended in pH 10.5 deionized water. To ensure removal of trace salts, the cells were again concentrated by centrifugation, supernatant was aspirated, and the cells were suspended in pH 10.5 deionized water. For each sample, zeta potential was measured at 0, 0.156, 0.313, 0.625, 1.25, 2.5, 5, 10, 20, and 40 mM CaCl2.
SDS inhibition of aggregation.
Inhibition of aggregation by sodium dodecyl sulfate (SDS) was tested using the filtration aggregation assay. Fixed algae from 2 days after subculture were used. Fixed RP1137 cells from the exponential phase (OD = 0.7) were used. Both algae and bacteria were concentrated by centrifugation at 20,000 × g for 1 min. The supernatant was aspirated and the cells were suspended in pH 10.5 deionized water with 10 mM CaCl2. This washing step was repeated once. In the treatment samples, SDS was added to algae to a final concentration of 1%. Filtration aggregation assays were carried out as described for quantitation of the percentage of algae aggregated in the SDS-treated cells compared to the untreated controls.
Calcium displacement of pinacyanol.
To determine if calcium displaces pinacyanol bound to the bacterial cell surface, fixed RP1137 cells from the exponential phase were concentrated by centrifugation at 20,000 × g for 1 min, supernatant was aspirated, and the cells were suspended in pH 10.5 deionized water. This washing step was repeated once. RP1137 cells were then stained with 20 μM pinacyanol chloride (Sigma). The stained cells were added to an equal volume of water or water with CaCl2, resulting in a final pinacyanol concentration of 10 μM. The final concentrations of CaCl2 solutions tested were 0, 0.15, 0.6, 2.5, and 10 mM. The samples were mixed by vortexing and then centrifuged at 20,000 × g for 3 min. The supernatant was aspirated to remove unbound dye, and the cells were suspended in a fresh aliquot of water with the same calcium concentration. Absorbance of the dyed cell solutions was measured at 485 nm in an M5 Spectromax plate reader. Absorbance spectra from 450 to 650 nm were gathered at 5-nm increments for unstained RP1137 cells, stained cells, water plus pinacyanol, and water plus pinacyanol plus 10 mM CaCl2.
Algal aggregation by different Bacillus strains.
To determine if other Bacillus strains share the aggregation phenotype found in RP1137, we tested the aggregation ability of Bacillus megaterium QM B1551 and Bacillus subtilis SMY. Cells were grown in LB medium (BD, Franklin Lakes, NJ) at 37°C with 180-rpm shaking. The cells were harvested in the exponential phase and fixed using the protocol listed above. Cell density-normalized filtration aggregation assays were then carried out.
Effect of higher pH and calcium concentrations on algal self-aggregation.
To determine if Nannochloropsis cells self-aggregate under the conditions tested and at higher pHs and calcium concentrations, we performed filtration aggregation assays with only the algae (no bacteria added). The pH of the algae culture was adjusted with NaOH. Calcium concentrations were adjusted with CaCl2. Once the algal culture was at the desired condition, the filtration aggregation assay was performed to quantitate the amount of algae in aggregates.
RESULTS AND DISCUSSION
Characterization of cell length over a growth period.
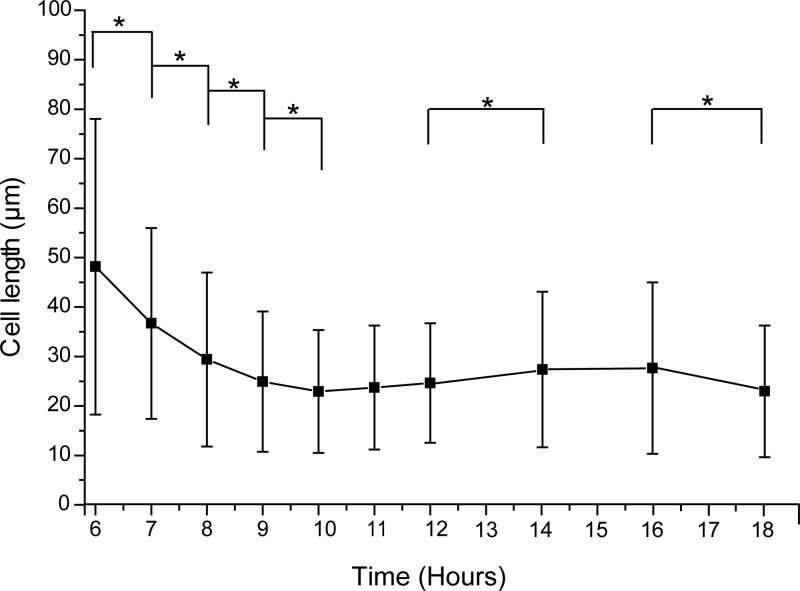
In this study, we aimed to characterize the mechanism by which Bacillus sp. strain RP1137 aggregates algae. A better understanding of the mechanism may help to improve the efficiency of the system. In previous work, we showed that divalent cations are important for aggregation and that fixed cells are just as effective at aggregating algae as live cells, pointing to the cell surface as the important cell structure for investigation of the aggregation mechanism (17). Our previous work also ruled out filament length as an important factor for aggregation, but initial observations suggested that aggregation ability changes over the growth cycle of a culture. Since the cell morphology changes over the growth period, we cannot accurately normalize by optical density or cell counts. To accurately normalize between time points, we first needed to characterize this change in morphology. Single cells and filaments of cells are present in RP1137 cultures. In this paper, we define cell length as the total length of either a lone single cell or the length of a chain of cells in a filament. Changes in cell length are shown in Fig. 1, for which cell length was measured over time through a combination of fluorescence microscopy and automated image analysis of thousands of cells. The results show that cell length changed over a growth period.
FIG 1.
Bacillus sp. strain RP1137 cell length changes over a growth period. Time points match the growth curve shown in Fig. 2. Data are means and standard errors of cell length measurements of 900 to 1,600 individual cells per time point. *, P < 0.0005.
Change in aggregation ability of RP1137 over a growth period.
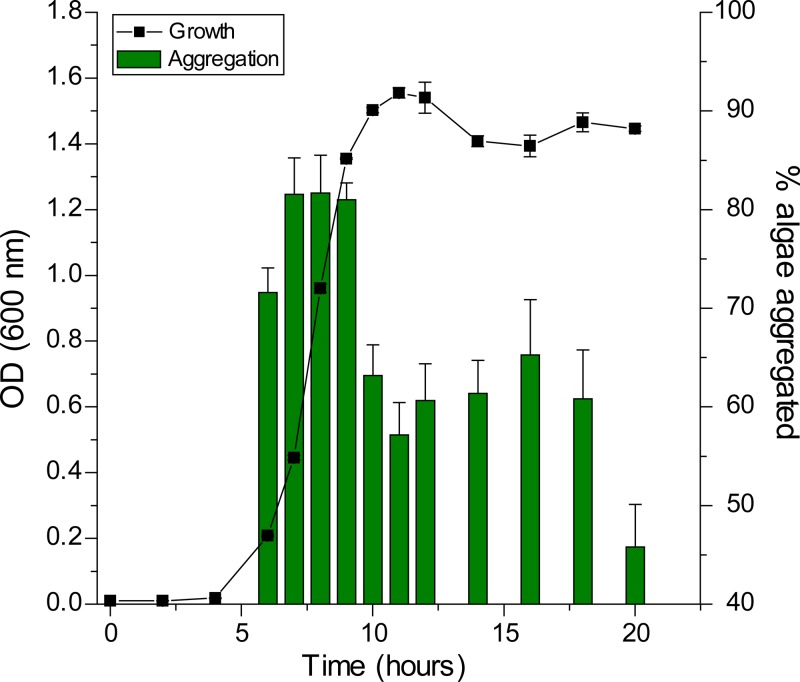
With the cell length data, we were able to calculate cell surface area data, which were used to normalize the cell surface area between time points. Normalization of cell surface area allowed us to isolate changes in the cell surface composition from changes in the amount of cells or amount of cell surface area in a sample. Using normalization, aggregation potential changes over a growth cycle were determined. In Fig. 2, the percentage of algae aggregated is plotted with the growth curve of the bacterium. Aggregation is most effective in the exponential phase of bacterial growth, where 80% of the algae are found in the aggregates. As the cells enter stationary phase, the aggregation potential per normalized cell surface area decreases and reaches a minimum value of 40% aggregated algae at 20 h. These data show that the aggregation potential of the bacterial cell surface decreases when the cells enter stationary phase, indicating that the surface chemistry of the cell likely changes. From the perspective of applying RP1137 for algal harvest, these results show that it is important to use bacteria from the exponential phase to obtain the best aggregation ability.
FIG 2.
Normalized aggregation efficiency of RP1137 cells is highest in the exponential phase. Samples are normalized by cell surface area so that the same surface area is available for aggregating algae at each time point. Data are means and standard errors for eight independent aggregation reactions. A value of 100% indicates aggregation of all algal cells.
Change in aggregation ability of N. oceanica IMET1 over time.
Next, we measured the aggregation potential of N. oceanica IMET1 cultures at different growth stages. The same bacterial sample was used for the experiments, and the algal cells were normalized between samples using the cell surface area. Unlike bacterial cell morphology, algal cell morphology does not change significantly over a growth cycle (data not shown). Algal aggregation was tested at 2, 5, and 17 days after subculturing. Cells in the 2-day samples were just beginning to grow, while the algae were fully into the exponential phase by day 5. At 17 days, the algae were in stationary phase. Aggregation was most efficient with cells at the 5-day time point (93.4% ± 0.7%) and significantly more efficient than with cells at 2 days (88.4% ± 2.3%; P = 0.0005) and 17 days (68.3% ± 10.2%, P = 0.0003). The data show that the algae are most effectively aggregated during the exponential phase and have decreased aggregation ability when they enter stationary phase. Little is known about the cell surface of Nannochloropsis; however, these results suggest that the surface chemistry of the cell changes with the growth phase of the alga. Changes in surface chemistry are discussed below. The change in aggregation efficiency indicates that algae are harvested with RP1137 cells, it is best to harvest the algae when the algae are in the exponential growth phase. However, the harvest time must be balanced according to the production system that is being used. For example, if high-lipid algae are desired, then it may not be advantageous to harvest in the exponential phase. However, there are several reasons why harvesting in the exponential phase would be attractive. First, while oil content can be higher in the starved stationary-phase cells, the increased oil content is typically counterbalanced by a loss in total biomass yield over the same time period. A second consideration is that continuously harvesting in the exponential phase would keep the cells in a rapidly growing state. This removes the nonproductive lag period at the beginning of growth as well as the decrease or cessation of growth that occurs in stationary phase. Thus, harvesting in the exponential phase may produce more total biomass and more fuel per unit area in a given time period. For some conversion methods, such as hydrothermal liquefaction, large amounts of high-protein biomass are desirable, and therefore harvesting within the exponential phase is optimal.
LiCl treatment and S-layer proteins.
Our previous work showed that proteinase K treatment of the cell surface did not significantly decrease aggregation, suggesting that surface proteins were unlikely to be involved in aggregation (17). While proteinase K can cleave surface proteins (21), it may not cleave proteins that do not have an exposed cleavage site. S-layer proteins are often involved in adhesion of Gram-positive bacteria to either biotic or abiotic surfaces and can be involved in aggregation (22). S-layer proteins are also typically attached to peptidoglycan via electrostatic interactions and can be removed by LiCl treatment (20). To test if the S-layer is involved in aggregation, RP1137 cells were treated with 5, 7.5, and 10 M LiCl for 15, 45, and 120 min. The treated cells did not have a significant decrease in their aggregation ability under any of the conditions tested (see Fig. S1 in the supplemental material), suggesting that the S-layer proteins are not involved in the aggregation phenotype.
Base titration of the RP1137 cell surface.
The data we have collected show that specific or nonspecific protein-protein interactions at the cell surface are inconsistent with available data. We next hypothesized that perhaps a more general property of the cell surface is involved in the aggregation phenotype of RP1137. Since the bacterial cells show a significant difference in aggregation ability between the exponential and stationary phase, we decided to test if the surface chemistry of the cells at these growth stages is different and, specifically, if the density of deprotonatable residues at the cell surface is different. To test this, we used base titration of whole live cells. The results of base titration of RP1137 cells (Fig. 3) show that cells in exponential phase have a lower number of deprotonatable residues than cells in stationary phase, because pH increases more quickly as base is added in exponential-phase cells than in stationary-phase cells. Since these experiments were normalized by cell surface area, this translates to more positive or neutral residues per unit area of cell surface. The data show there is a measurable difference in surface chemistry between these two populations of cells.
FIG 3.
Base titration curves of live RP1137 cells. Curves represent individual trials from RP1137 cells from either exponential phase (Exp) or stationary phase (Stat).
Binding of calcium to the cell surface.
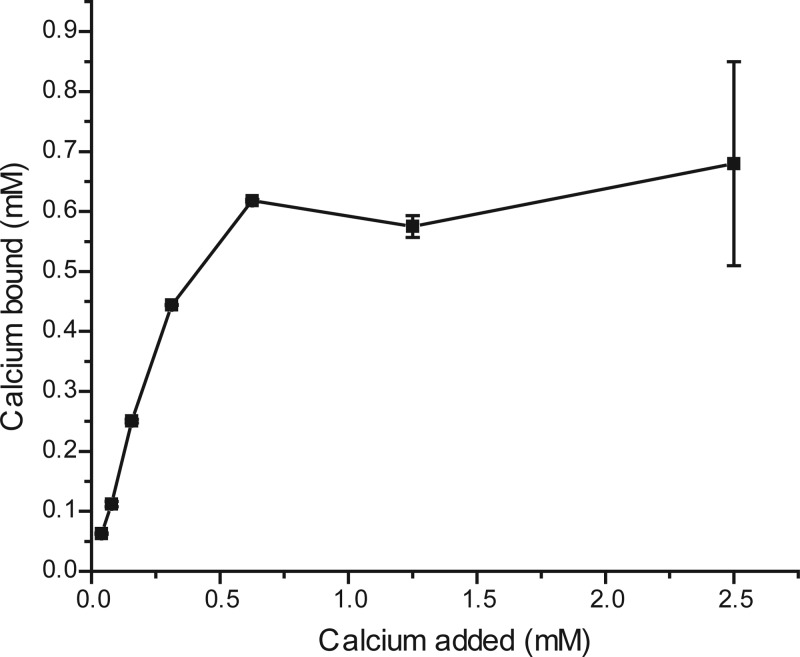
Cell surface chemistry can also be affected by the ions that are present. Previously, we showed that aggregation is dependent on divalent cations, and we hypothesized that these ions reduce or neutralize negative charge at the cell surface (17). To determine if calcium ions bind to the surface of the bacterium, we measured the amount of calcium removed by the cells at increasing calcium concentrations. Figure 4 shows that calcium binds to the bacteria and that bound calcium increases with increasing calcium concentration up to 0.625 mM. A saturating concentration of surface-bound calcium ions could allow cells to aggregate in at least two ways. Charge neutralization eliminates electrostatic repulsion between cells and is commonly cited as a method in which cells can get close enough to adhere via other attractive forces such as hydrophobic or Van der Waals type interactions (23). Another method of interaction cited in the literature is coordination of ions bound to one cell by another cell (24). In this method of aggregation, two cells interact via bridged ions. This is similar to the commonly used nickel-NTA affinity chromatography system, which binds proteins via the common coordination of a nickel ion by the 6×His tag on a protein and a nitrilotriacetic acid residue attached to a solid substrate. The ion coordination model of aggregation predicts that algal and bacterial cells that have been preloaded with calcium separately before combination will not interact efficiently because they both already bind ions at their cell surface and thus are less likely to bind via common ions. However, the charge neutralization model predicts a different outcome. It predicts that cells preloaded with calcium before combination will interact, which will lead to aggregation. We tested the hypothesis that aggregation occurs by coordination of common ions by preloading algal and bacterial cells with calcium separately before mixing them. The results showed that the cells still aggregated, with an average value of 74.6% ± 1.1% algae aggregated. These results point toward the charge neutralization model of aggregation rather than the coordination model. This result is further supported by the finding that, unlike RP1137 cells, the Nannochloropsis cells do not tightly bind calcium at their cell surface.
FIG 4.
Binding of calcium to RP1137 cells increases with increasing calcium concentrations. Data are means and standard errors from three independent calcium binding reactions.
Measurement of zeta potential.
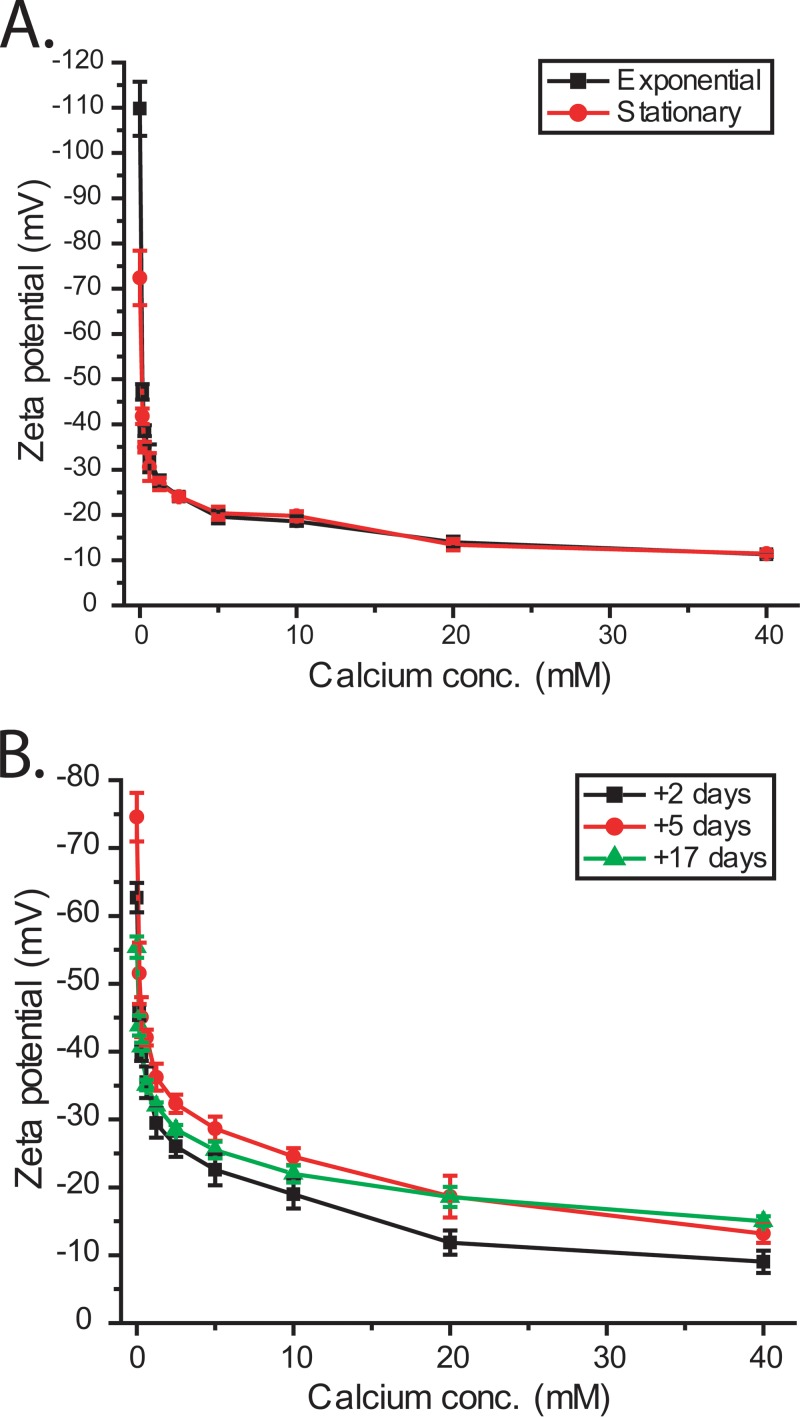
To test the hypothesis that calcium ions cause charge neutralization, we used dynamic light scattering to measure apparent cell surface charge or zeta potential at different calcium concentrations. Zeta potential was measured for both bacterial and algal cells at different stages of growth. The data shown in Fig. 5 demonstrate that surface charge in both bacteria and algae decreases with increasing calcium concentration. In the absence of salt, the exponential-phase bacterial cells had a more negative charge, −110 ± 6 mV, than the stationary-phase cells, which had a zeta potential of −72 ± 6 mV. As calcium concentration increased, the zeta potential of both exponential- and stationary-phase bacterial cells became similar, with the charge curves overlapping at 10 mM calcium, which is the optimal concentration for aggregation. At this concentration, the charge was −18.6 ± 1.05 mV for exponential-phase cells and −19.8 ± 1.05 mV for stationary-phase cells, both of which are at or below −20 mV, which is often considered the threshold where charge is no longer strong enough to separate cells by electrostatic repulsion. The surface charge of algal cells also becomes less negative as calcium concentrations increase, but compared to the bacterial cells, the algae require a higher calcium concentration to get below the −20 mV threshold. This result suggests that the bacterial cells have a higher affinity for calcium ions than the algal cells. The calcium binding and zeta potential measurements show that bacterial cells bind calcium, which results in charge neutralization. Next, we aimed to determine what forces likely mediated the binding of the RP1137 and Nannochloropsis cells.
FIG 5.
Surface charge of RP1137 cells (A) and Nannochloropsis cells (B) at different stages of growth and different calcium concentrations. Charge decreases with increasing calcium concentration. Data are means and standard errors for three biological replicates each with three technical replicates.
RP1137 binding to C18 resin.
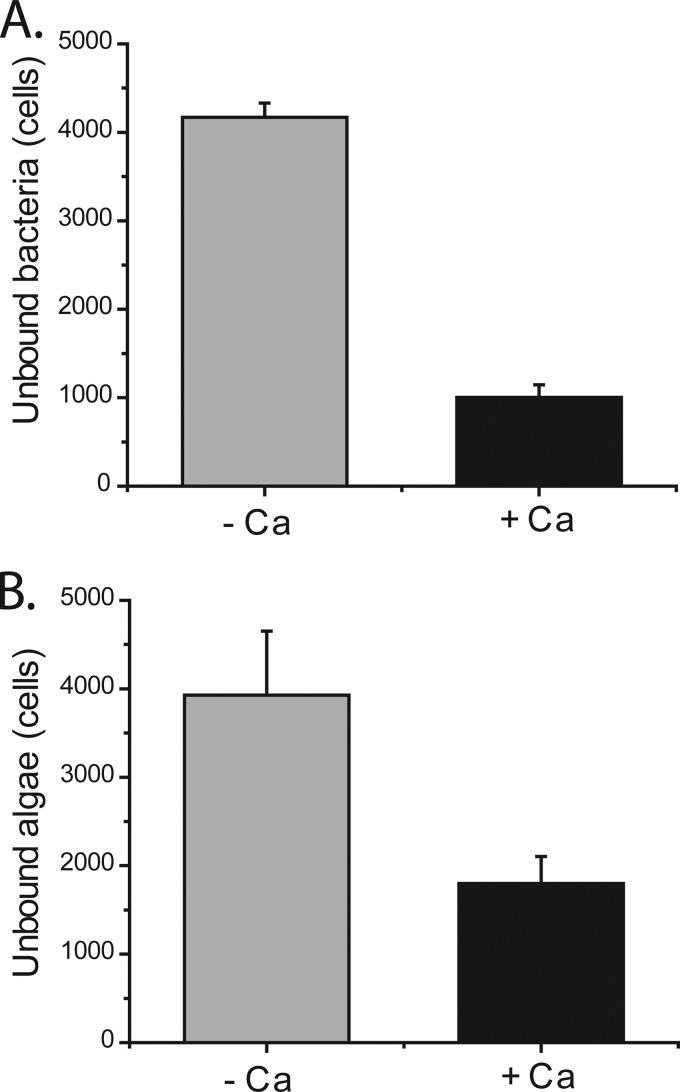
From previous work we knew that surface proteins, filament length, and lectin-carbohydrate type interactions were not involved in the underlying mechanism of aggregation (17). Aggregation of multiple and distinct algal species by RP1137 also pointed toward a general instead of a specific mechanism of aggregation (17). Since the cell surface charge decreased upon addition of calcium, we hypothesized that bacterial and algal cells interact via hydrophobic type interactions and thus should become more able to interact with a hydrophobic surface under these conditions. We tested this by measuring the number of individual cells that were not bound to hydrophobic C18 beads in the presence or absence of calcium. Unbound cells were measured because it is easier to get accurate data on free cells than on cells bound to the beads. Figure 6A shows that there were fewer unbound bacterial cells in the presence of calcium. This shows that the bacterial cells are more able to bind to a hydrophobic surface when calcium is added. The algal cells show a similar trend, with fewer unbound cells present in the presence of calcium (Fig. 6B). These data demonstrate that the algal cells are also more able to interact with a hydrophobic surface in the presence of calcium. Together these data support the hypothesis that bacterial and algal cells interact at least in part through hydrophobic interactions. Hydrophobic interactions are often involved in aggregation (23), so this result fits with what others have found. If both bacterial and algal cells can interact with a defined hydrophobic surface, then we propose they could interact with each other. This mechanism of interaction suggests that we should be able to inhibit the interaction by coating the cells with an anionic detergent prior to the aggregation process.
FIG 6.
Binding of RP1137 cells (A) and Nannochloropsis cells (B) to hydrophobic C18 beads in the presence or absence of 10 mM CaCl2. Both RP1137 and Nannochloropsis cells bind more effectively to the beads in the presence of calcium. Data are means and standard errors for three biological replicates with three technical replicates each.
SDS inhibition of aggregation.
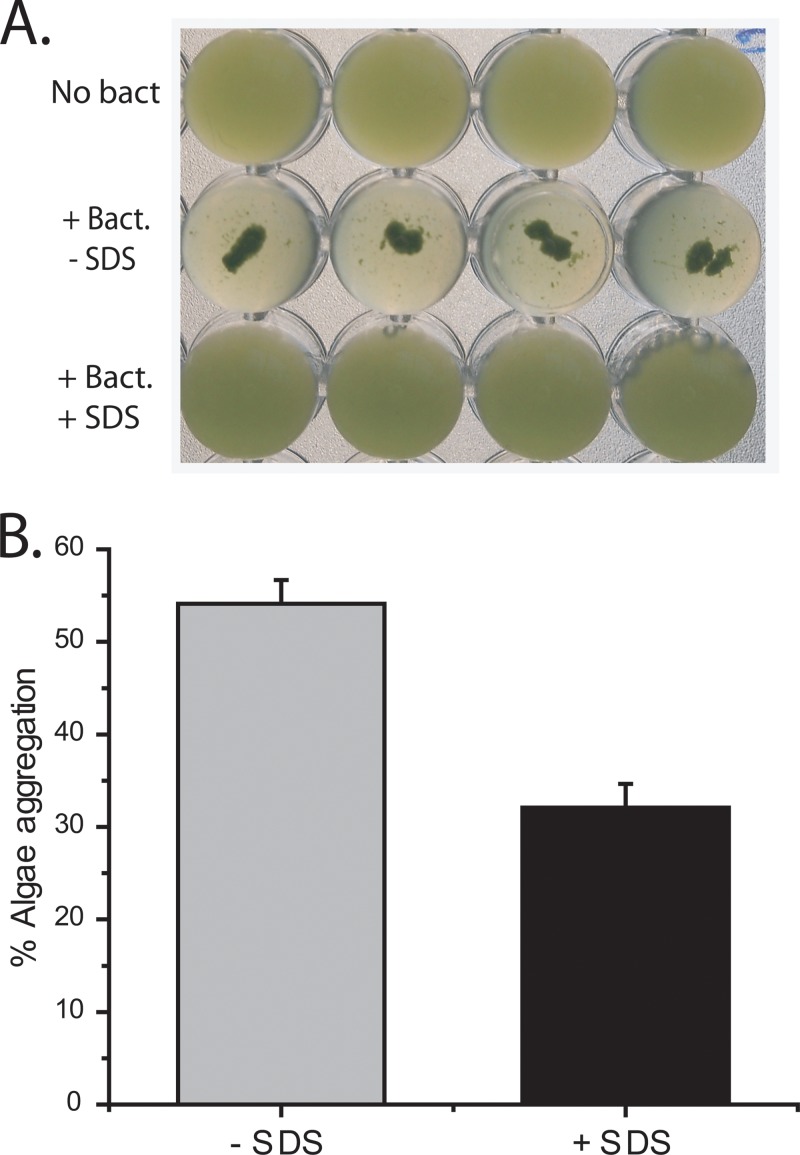
To test the hypothesis that an anionic detergent would disrupt an aggregation process that occurs via hydrophobic interactions, we precoated the bacterial and algal cells with the anionic detergent sodium dodecyl sulfate (SDS). SDS has a hydrophobic tail attached to an anionic sulfate group. If the bacterial and algal surfaces are more hydrophobic, then the SDS should be oriented with the hydrophobic tail toward the cell and the anionic sulfate residue facing the solvent (water). The cells in this setup now have an external negative charge, which should inhibit aggregation. We observed that addition of SDS resulted in a significant decrease in aggregation (P = 5.19E-05) (Fig. 7). Visually, aggregation appeared to be completely inhibited (Fig. 7A); however, quantitation using the aggregation assay showed that small aggregates were still formed (Fig. 7B). The aggregation assay works by removing aggregates that do not pass through a mesh with 50-μm by 50-μm square holes. Individual Nannochloropsis cells are spherical and have a diameter of about 3 μm (17). The results indicate that the formation of large aggregates is inhibited but that smaller aggregates (>50 μm) are still formed. These smaller aggregates incorporated less of the algae. These results indicate that the interaction between bacteria and algal cells was not completely disrupted under the conditions tested. It is possible that other forms of bonding are important in the interaction between these cell types. Finally, the cells may have gained sufficient momentum when mixed by vortexing to overcome the electrostatic repulsion and allow the some of them to get close enough to interact. The idea that the bacterial and algal cells bind each other via hydrophobic interactions implies that the cells should also self-aggregate in the absence of the other cell type; this was observed for RP1137 cells at high pH in the presence of divalent cations. Nannochloropsis cells do form small self-aggregates of 5 to 10 cells, but they do not form large aggregates. The reason Nannochloropsis cells do not form large aggregates is unknown; however, in the C18 bead assays, more of the bacterial population was bound to the beads than the algal population, suggesting that there are fewer algal cells whose cell surface is hydrophobic enough to bind the beads within the population. We speculate that the smaller the population of hydrophobic cells, the lower the chance of finding other hydrophobic cells with which they can interact. This explanation must be tempered with the knowledge that up to 95% of the algal population can be harvested with RP1137, which may imply that the interaction between algae and bacteria is different than the interaction of algae with other algae. Further study is needed to clarify what other forces besides hydrophobicity may be at play.
FIG 7.
Aggregation is inhibited by the presence of SDS. (A) Visual result showing the no-bacteria controls, algae with bacteria, and algae with bacteria and SDS. (B) Quantitation of the percentage of algae aggregated with and without SDS using the filtration aggregation assay. The percent alga aggregation is calculated relative to the value for the no-bacteria control. Data are means and standard errors for four independent aggregation reactions. A value of 100% indicates aggregation of all algal cells.
Determining a binding site for calcium at the cell surface.
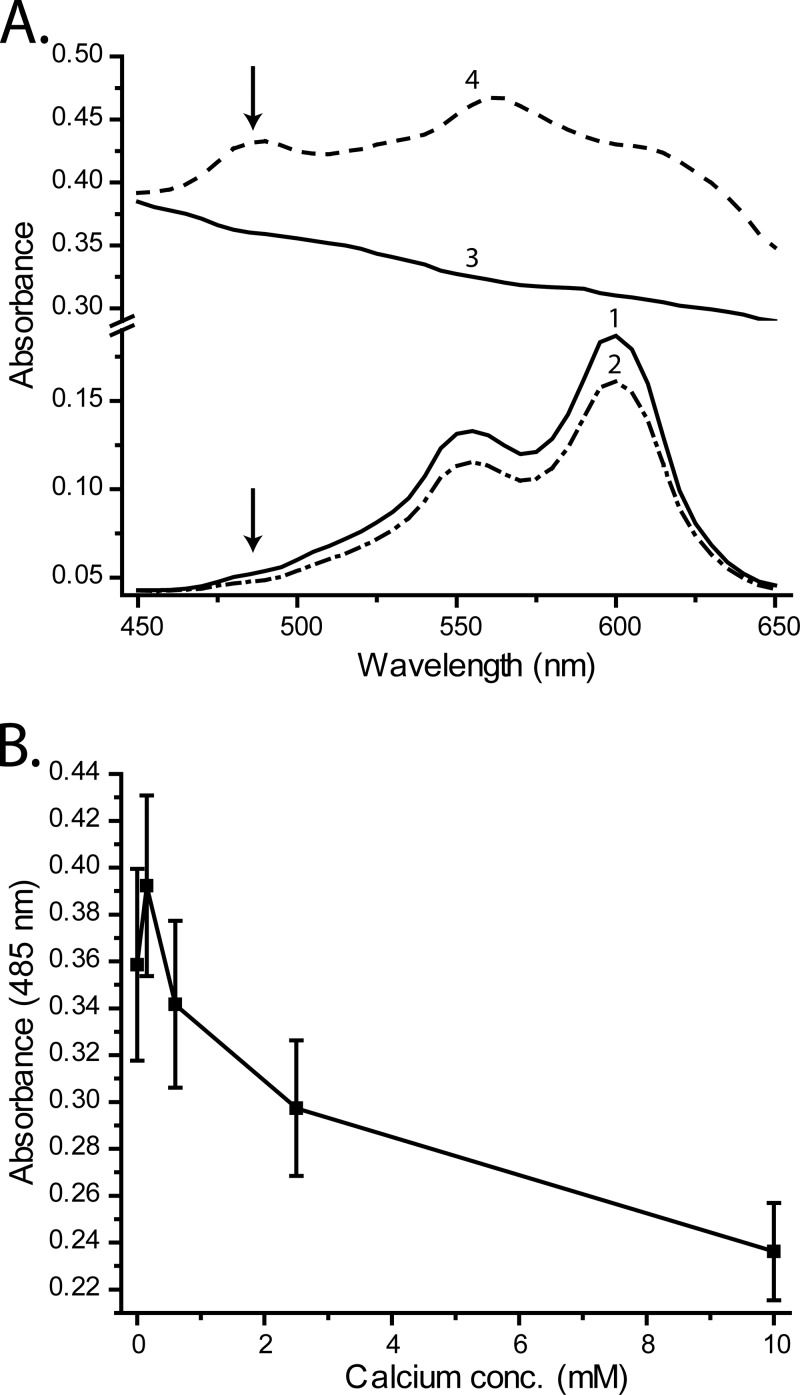
The calcium binding data indicated that the ions bind to the bacterial cells, which results in charge neutralization. Teichoic acids are negatively charged and bind various cations, including calcium (25), making them a plausible target for charge neutralization by calcium ions. Previous studies have shown that teichoic acids have a higher affinity for calcium than magnesium (25), matching data we gathered in a previous study that showed that RP1137 has a higher affinity for calcium than magnesium (17). We tested whether calcium binds the teichoic acid residues of RP1137 using the dye pinacyanol chloride. Pinacyanol chloride binds purified teichoic acids and upon binding undergoes an absorbance shift which results in a new absorbance band centered at 485 nm (26). Interestingly, calcium competes for binding of teichoic acid with the dye. When calcium is present, the dye is removed from teichoic acid and the absorbance band at 485 nm is no longer present (26). Here we used this property to determine if calcium binds teichoic acids on RP1137 cells. The absorbance spectra of the dye alone, dye with calcium, cells, and cells with dye are shown in Fig. 8A. The cells in the presence of the dye showed the characteristic peak in absorbance at 485 nm, as was observed for purified teichoic acid. The peak is not present in the cells alone, dye alone, or dye with calcium. Next, the cells were stained with pinacyanol and then exposed to different concentrations of calcium. The cells were then washed to remove unbound dye, and the absorbance of the cells at 485 nm was recorded. If calcium binds teichoic acids, then it should displace the dye and result in a decreased absorbance at 485 nm with increasing calcium concentration, which is what we observed (Fig. 8B). We must be cautious with our interpretation, as the original work cited was with purified and not cell-bound teichoic acids. However, this result supports the hypothesis that calcium binds to teichoic acids of RP1137 cells. The result does not rule out the possibility that calcium also binds to other parts of the cell. Interestingly, when the pinacyanol displacement data are compared to the zeta potential and calcium binding data, we see a discrepancy in calcium binding kinetics. Both zeta potential and calcium binding data suggest that the RP11137 cells saturate below 1 mM calcium, while the pinacyanol displacement data suggest that saturation does not occur until higher concentrations. This seeming contradiction can be explained by the positive shift in the calcium dissociation that would be predicted when both calcium and pinacyanol compete for the same substrate.
FIG 8.
Pinacyanol dye binding to RP1137 cells is disrupted by the addition of calcium. (A) Absorbance spectra of water plus dye (curve 1), water plus dye plus 10 mM CaCl2 (curve 2), RP1137 cells alone (curve 3), and RP1137 cells plus dye (curve 4). Arrows indicate the position of the 485-nm absorbance band indicative of pinacyanol binding of teichoic acid. (B) Absorbance of pinacyanol-stained cells at 485 nm with increasing calcium concentration. Data are means and standard errors for three biological replicates with two technical replicates each.
Similar mechanisms of aggregation have been proposed for other aggregating bacteria, where charge neutralization with divalent cations is implicated in the mechanism of aggregation (27). Divalent cations have also been implicated in the autoflocculation of some algae (28). The mechanism described here is similar to algal aggregation by Paenibacillus kribbensis, where both high pH and calcium ions were implicated in the aggregation mechanism (14). However, in the study on Paenibacillus kribbensis, only pH and calcium were investigated in terms of the mechanism of aggregation, whereas here we conducted a more detailed investigation. If the mechanism of aggregation in RP1137 is based on charge neutralization via calcium binding to teichoic acids, then it would be expected that other related Gram-positive bacteria might demonstrate a similar aggregation phenotype. RP1137 is most closely related to Bacillus megaterium, so to test this hypothesis, we compared the aggregation ability of B. megaterium QM B1551 to that of RP1137. We also compared the aggregation ability of the more distantly related Bacillus subtilis strain SMY to that of RP1137. The results show that RP1137 and B. megaterium QM B1551 have very similar algal aggregation efficiencies, while Bacillus subtilis SMY does not aggregate algae (see Fig. S2 in the supplemental material). These results suggest that not all Gram-positive organisms have the algal aggregation phenotype but that it is likely that bacteria closely related to RP1137 share the phenotype. A final question that arises from this research is whether the bacterium is needed for aggregation or whether aggregation of the algae alone can occur, particularly at higher calcium concentrations and higher pHs. To test this, we performed aggregation assays with only the Nannochloropsis cells at different combinations of pH and calcium concentration. The results shown in Fig. S3 in the supplemental material demonstrate that at pH 10 and 10 mM calcium, the optimal conditions for algal aggregation by RP1137, 5.3% ± 3.3% of aggregation can be attributed to algal self-aggregation. Self-aggregation increases with increasing pH and calcium concentration to a maximum of 34.6% ± 2.3% at pH 12 and 25 mM calcium. The conditions needed for aggregation of algal by RP1137 can be achieved in most algal production systems simply by stopping bubbling of air or CO2 through the culture. The pH of the dense cultures used for biofuel production can easily achieve pHs greater than the minimum of pH 9 that is required for aggregation. Calcium concentrations are also usually high enough, though in cases of low salinity the bacterial cells can be charged with calcium prior to use. Higher pH and calcium concentrations can cause self-aggregation; however, to achieve the 35% harvest efficiency would require addition of calcium to concentrations 2.5-fold higher than those in seawater and increasing pH beyond what the algae alone can achieve.
It is important to point out that the current system for harvesting algae is not economically feasible without further development. However, we speculate that the chemical characteristics of the RP1137 cell surface could be used to design means of attaching the cells to solid substrates, such as hydrophobic or magnetic beads, to aid in recovering the cells after aggregation. In a previous study, we showed that aggregation is pH dependent and reversible. If fixed RP1137 cells can be attached to beads and maintain their aggregation phenotype, then there is the possibility of reusing the cells multiple times. In this scheme, the fixed cells would be attached to beads and then used to aggregate algae. The aggregates could be recovered, and then pH would be lowered either by adding acid or by allowing the concentrated, and thus light-limited, algae to lower pH via respiration. Lowering pH reverses the aggregation process and separates the algae from the bacterium-bead complex. The bacterium-bead complex can then be separated from the algae and reused for another round of harvest. A final implication of the results presented in this study is that if the interaction between the algae and the bacteria is a hydrophobic interaction, then it may be possible to simply use hydrophobic beads in place of the bacteria for harvest of algae.
Conclusion.
Information about the mechanism of aggregation is useful primarily for predicting which algae RP1137 can harvest. The mechanistic data presented here suggest that RP1137 is able to harvest algae where charge neutralization occurs in either seawater or fresh water with 2 to 20 mM calcium ions at a pH greater than or equal to 9. RP1137 will not likely aggregate algae that have significant negative charge (>−30 mV) under these conditions. The data also point to charge neutralization of teichoic acids as the site where calcium binds to RP1137 cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Maryland Industrial Partnerships grant “Mass cultivation of algae on wastewaters for fuels and products” (PI, Feng Chen). This work was supported by IMET contribution number 14-125 and UMCES contribution number 4907.
We thank Leah Blasiak for critical reading of the manuscript. We thank Jacques Ravel for the gift of the B. megaterium QM B1551 strain and Harold Schreier for the B. subtilis SMY strain.
Footnotes
Published ahead of print 25 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00887-14.
REFERENCES
- 1.Waltz E. 2009. Biotech's green gold? Nat. Biotechnol. 27:15–18. 10.1038/nbt0109-15 [DOI] [PubMed] [Google Scholar]
- 2.Chisti Y. 2008. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 26:126–131. 10.1016/j.tibtech.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B. 2008. Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg. Res. 1:20–43. 10.1007/s12155-008-9008-8 [DOI] [Google Scholar]
- 4.Biddy M, Davis R, Jones S, Zhu Y. 2013. Whole algae hydrothermal liquefaction technology pathway. Pacific Northwest National Laboratory (PNNL), Richland, WA: http://www.nrel.gov/docs/fy13osti/58051.pdf [Google Scholar]
- 5.Richardson JW, Johnson MD, Outlaw JL. 2012. Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. 1:93–100. 10.1016/j.algal.2012.04.001 [DOI] [Google Scholar]
- 6.Uduman N, Qi Y, Danquah MK, Forde GM, Hoadley A. 2010. Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2:017201. 10.1063/1.3294127 [DOI] [Google Scholar]
- 7.Sirin S, Trobajo R, Ibanez C, Salvado J. 2012. Harvesting the microalgae Phaeodactylum tricornutum with polyaluminum chloride, aluminium sulphate, chitosan and alkalinity-induced flocculation. J. Appl. Phycol. 24:1067–1080. 10.1007/s10811-011-9736-6 [DOI] [Google Scholar]
- 8.Lavoie A, Noüe J. 1983. Harvesting microalgae with chitosan. J. World Maricult. Soc. 14:685–694 [Google Scholar]
- 9.Divakaran R, Sivasankara Pillai V. 2002. Flocculation of algae using chitosan. J. Appl. Phycol. 14:419–422. 10.1023/A:1022137023257 [DOI] [Google Scholar]
- 10.Pavoni JL, Echelber Wf, Tenney MW. 1972. Bacterial exocellular polymers and biological flocculation. J. Water Pollut. Control Fed. 44:414–429 [PubMed] [Google Scholar]
- 11.Yuan SJ, Sun M, Sheng GP, Li Y, Li WW, Yao RS, Yu HQ. 2011. Identification of key constituents and structure of the extracellular polymeric substances excreted by Bacillus megaterium TF10 for their flocculation capacity. Environ. Sci. Technol. 45:1152–1157. 10.1021/es1030905 [DOI] [PubMed] [Google Scholar]
- 12.Nontembiso P, Sekelwa C, Leonard MV, Anthony OI. 2011. Assessment of bioflocculant production by Bacillus sp. Gilbert, a marine bacterium isolated from the bottom sediment of Algoa Bay. Mar. Drugs 9:1232–1242. 10.3390/md9071232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardes A, Iversen MH, Grossart HP, Passow U, Ullrich MS. 2011. Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME J. 5:436–445. 10.1038/ismej.2010.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh HM, Lee SJ, Park MH, Kim HS, Kim HC, Yoon JH, Kwon GS, Yoon BD. 2001. Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp AM49. Biotechnol. Lett. 23:1229–1234. 10.1023/A:1010577319771 [DOI] [Google Scholar]
- 15.Wang H, Laughinghouse HDT, Anderson MA, Chen F, Willliams E, Place AR, Zmora O, Zohar Y, Zheng T, Hill RT. 2012. Novel bacterial isolate from Permian groundwater, capable of aggregating potential biofuel-producing microalga Nannochloropsis oceanica IMET1. Appl. Environ. Microbiol. 78:1445–1453. 10.1128/AEM.06474-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon JH, Oh HM, Yoon BD, Kang KH, Park YH. 2003. Paenibacillus kribbensis sp. nov. and Paenibacillus terrae sp. nov., bioflocculants for efficient harvesting of algal cells. Int. J. Syst. Evol. Microbiol. 53:295–301. 10.1099/ijs.0.02108-0 [DOI] [PubMed] [Google Scholar]
- 17.Powell RJ, Hill RT. 2013. Rapid aggregation of biofuel-producing algae by the bacterium Bacillus sp. strain RP1137. Appl. Environ. Microbiol. 79:6093–6101. 10.1128/AEM.01496-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillard RRL. 1975. Culture of phytoplankton for feeding marine invertebrates, p 29–60 In Smith WL, Chanley MH.Culture of marine invertebrate animals. Plenum Press, New York, NY [Google Scholar]
- 19.Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE. 2011. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics 27:1179–1180. 10.1093/bioinformatics/btr095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lortal S, Vanheijenoort J, Gruber K, Sleytr UB. 1992. S-layer of Lactobacillus helveticus ATCC 12046—isolation, chemical characterization and re-formation after extraction with lithium-chloride. J. Gen. Microbiol. 138:611–618. 10.1099/00221287-138-3-611 [DOI] [Google Scholar]
- 21.Sabarth N, Hurvitz R, Schmidt M, Zimny-Arndt U, Jungblut PR, Meyer TF, Bumann D. 2005. Identification of Helicobacter pylori surface proteins by selective proteinase K digestion and antibody phage display. J. Microbiol. Methods 62:345–349. 10.1016/j.mimet.2005.04.030 [DOI] [PubMed] [Google Scholar]
- 22.Garrote GL, Delfederico L, Bibiloni R, Abraham AG, Perez PF, Semorile L, De Antoni GL. 2004. Lactobacilli isolated from kefir grains: evidence of the presence of S-layer proteins. J. Dairy Res. 71:222–230. 10.1017/S0022029904000160 [DOI] [PubMed] [Google Scholar]
- 23.Hermansson M. 1999. The DLVO theory in microbial adhesion. Colloid Surface B 14:105–119. 10.1016/S0927-7765(99)00029-6 [DOI] [Google Scholar]
- 24.Sobeck DC, Higgins MJ. 2002. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 36:527–538. 10.1016/S0043-1354(01)00254-8 [DOI] [PubMed] [Google Scholar]
- 25.Marquis RE, Mayzel K, Carstensen EL. 1976. Cation exchange in cell walls of gram-positive bacteria. Can. J. Microbiol. 22:975–982. 10.1139/m76-142 [DOI] [PubMed] [Google Scholar]
- 26.Pal MK, Ghosh TC, Ghosh JK. 1990. Studies on the conformation of and metal ion binding by teichoic acid of Staphylococcus aureus. Biopolymers 30:273–277. 10.1002/bip.360300305 [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Cho DH, Ramanan R, Kim BH, Oh HM, Kim HS. 2013. Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour. Technol. 131:195–201. 10.1016/j.biortech.2012.11.130 [DOI] [PubMed] [Google Scholar]
- 28.Schlesinger A, Eisenstadt D, Bar-Gil A, Carmely H, Einbinder S, Gressel J. 2012. Inexpensive non-toxic flocculation of microalgae contradicts theories; overcoming a major hurdle to bulk algal production. Biotechnol. Adv. 30:1023–1030. 10.1016/j.biotechadv.2012.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.