Abstract
The complete genome sequence of Nocardia nova SH22a was determined in light of the remarkable ability of rubber and gutta-percha (GP) degradation of this strain. The genome consists of a circular chromosome of 8,348,532 bp with a G+C content of 67.77% and 7,583 predicted protein-encoding genes. Functions were assigned to 72.45% of the coding sequences. Among them, a large number of genes probably involved in the metabolism of xenobiotics and hardly degradable compounds, as well as genes that participate in the synthesis of polyketide- and/or nonribosomal peptide-type secondary metabolites, were detected. Based on in silico analyses and experimental studies, such as transposon mutagenesis and directed gene deletion studies, the pathways of rubber and GP degradation were proposed and the relationship between both pathways was unraveled. The genes involved include, inter alia, genes participating in cell envelope synthesis (long-chain-fatty-acid–AMP ligase and arabinofuranosyltransferase), β-oxidation (α-methylacyl-coenzyme A [α-methylacyl-CoA] racemase), propionate catabolism (acyl-CoA carboxylase), gluconeogenesis (phosphoenolpyruvate carboxykinase), and transmembrane substrate uptake (Mce [mammalian cell entry] transporter). This study not only improves our insights into the mechanism of microbial degradation of rubber and GP but also expands our knowledge of the genus Nocardia regarding metabolic diversity.
INTRODUCTION
Polyisoprene is one of the most important polymeric materials in our society because of its special properties for a wide range of applications. The cis-isomer of polyisoprene [poly(cis-1,4-isoprene)] is known as the main component of natural rubber and can be synthesized by more than 2,500 plants and some fungi (1). Due to its superior elasticity, it is used extensively to produce mobile tires, rubber hoses, latex gloves, conveyers, condoms, etc. The trans-isomer [poly(trans-1,4-isoprene)], which is the main component of gutta-percha (GP), can also be synthesized by a few plants, such as Palaquium gutta, Eucommia ulmoides, and Couma macrocarpa. Unlike rubber, GP is rigid at room temperature. Its enhanced insulation property and excellent resistance against microbial decomposition make it a desirable material for splints, pipes, and golf balls and for filling of tooth canals in dentistry (2). On the other hand, the wide range of applications of polyisoprenes accordingly leads to problems in waste treatment due to the large volume of produced items and their durability. This is particularly the case for rubber. For example, the global annual production of rubber increased to about 26.4 million tons (43% natural rubber and 57% synthetic rubber) in 2012, which is about 8% more than the level in 2010 (3). Incineration is the most widely used method for rubber waste treatment; however, this is not desirable because it causes a secondary pollution (CO, NOx, and SO2) of ambient air (4). A risk of air, water, and soil pollution also occurs during other recycling methods, such as pyrolysis (5). Therefore, it is important to find a more economic and environmentally friendly treatment method, for which microbial degradation may be competitive in the future.
In the past few decades, more than 100 rubber-degrading bacteria have been identified from different habitats (6–9). They are divided into the following two groups according to their degradation behavior (9): (i) the latex-clearing group of bacteria, which produce translucent halos on rubber latex overlay plates (10); and (ii) the adhesive growth group of bacteria, which require direct contact of the cells with rubber substances for growth. Through the identification of degradation intermediates (11), the random mutagenesis of efficient degrading bacteria, and genomic analysis (12, 13), a complete rubber degradation pathway could be depicted (14). In contrast, the knowledge about GP biodegradation is quite limited. In the effort to screen GP-degrading microorganisms, it was interesting to find that GP-degrading ability always occurs in combination with the ability to degrade rubber, but not vice versa. The only six known GP-degrading bacterial isolates (Nocardia nova SH22a, L1b, SEI2b, and SEII5a, Nocardia jiangxiensis SM1, and Nocardia takedensis WE30) were also found to be efficient decomposers of rubber (2). In our previous study (15), the synthetic and natural rubber-degrading strain N. nova SH22a was chosen as a model microorganism to investigate the GP degradation mechanism and the potential relationship between GP and rubber degradation by use of the strategy of transposon mutagenesis. The growth of most degradation-defective mutants was affected on both polyisoprenoids (GP and rubber), indicating that the GP and rubber degradation pathways are quite similar and share many common steps. The determination of transposon insertion loci suggested that enzymes participating in oxidoreduction reactions, β-oxidation, and the synthesis of complex cell envelope lipids are involved in the degradation processes.
In the present study, the identification of enzymes involved in GP and/or rubber degradation was continued, and the complete genome of SH22a was sequenced. Based on the experimental results and an in silico genome analysis, a more detailed polyisoprene degradation pathway (for both the cis-isomer and the trans-isomer) is illustrated. Moreover, the elaboration of the N. nova genome is also quite helpful for a better understanding of the genus Nocardia with respect to the anabolism of secondary intermediates and the catabolism of other organic environmental pollutants (16).
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation conditions.
Bacterial strains and plasmids used in the present study are listed in Table 1. If not stated otherwise, N. nova was grown in CASO broth (Carl Roth, Germany) at 30°C, whereas Escherichia coli was grown in lysogeny broth (LB) at 37°C. In growth experiments, N. nova was grown in mineral salts medium (MSM) (17) at 30°C, and carbon sources were added as indicated in the text. Liquid cultures were grown in Erlenmeyer flasks, which were incubated on a horizontal rotary shaker at 140 rpm. Solid media were prepared by adding 1.6% (wt/vol) agar-agar. Antibiotics were used as described by Sambrook et al. (18) for E. coli strains, whereas 50 μg of kanamycin/ml or 50 μg of apramycin/ml was applied for N. nova strains.
TABLE 1.
Strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Characteristic, description, or sequence (5′–3′)a | Reference, source, or restriction site |
|---|---|---|
| Strains | ||
| N. nova SH22a | GP- and rubber-degrading wild type | 2 |
| N. nova SH22a SE3-4-18 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a SE13-5-5 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a SE16-4-35 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a SE18-1-3 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a SE18-8-1 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a OC11-7-29 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a OC20-2-42 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a SE10-4-21 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a OC14-14-5 | Transposon-induced mutant, GP-degradation defective, Aprr | This study |
| N. nova SH22a SE9-8-16 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a OC19-4-17 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a OC22-3-17 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a OC26-3-28 | Transposon-induced mutant, GP- and rubber-degradation defective, Aprr | This study |
| N. nova SH22a ΔfadD32SH22a | fadD32SH22a deletion mutant, GP- and rubber-degradation defective, Kmr | This study |
| N. nova SH22a Δpck | pck deletion mutant, GP- and rubber-degradation defective, Kmr | This study |
| E. coli XL10-Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔM15 Tn10 (Tetr) Amy Camr] | Agilent Technologies |
| Plasmids | ||
| pMA5096 | Contains transposon Tn5096, Ampr Aprr | 12 |
| pSKΩKm | Kanamycin resistance cassette-containing plasmid | Strain SK07729b |
| pJET1.2/blunt | Blunt-end cloning vector, Ampr | Thermo Fisher Scientific |
| pJET::FAALKN | Used for fadD32SH22a deletion experiment, Ampr | This study |
| pJET::FAALKN::Km | Used for fadD32SH22a deletion experiment, Ampr Kmr | This study |
| pJET::PCKKN | Used for pck deletion experiment, Ampr | This study |
| pJET::PCKKN::Km | Used for pck deletion experiment, Ampr Kmr | This study |
| pNV18.1 | E. coli-Nocardia shuttle vector, Kmr Neor | 96 |
| pNV18.1::fadD32SH22a | Used for complementation experiment, Kmr Neor | This study |
| pNV18.1::pck | Used for complementation experiment, Kmr Neor | This study |
| Oligonucleotides | ||
| FAAL-kn1 | TGAGGGCAACCAGCAGGAG | |
| FAAL-kn2 | CAGAACTCGCCGGGCGCACGATCCCGGGGGCCGCTGATACCTGTGAG | SmaI |
| FAAL-kn3 | CTCACAGGTATCAGCGGCCCCCGGGATCGTGCGCCCGGCGAGTTCTG | SmaI |
| FAAL-kn4 | GATGAAGACGCCGACCGCCTC | |
| PCK-kn1 | AAACATATGGTGGATCTGACGCACGAGG | NdeI |
| PCK-kn2 | CGTCCGAAAAAGCCTATCCCAGGTCGACGAAAACTCTCCTGAGATGAGCG | HincII |
| PCK-kn3 | CGCTCATCTCAGGAGAGTTTTCGTCGACCTGGGATAGGCTTTTTCGGACG | HincII |
| PCK-kn4 | AAACATATGGAACCGCACCCACACCTC | NdeI |
| FAAL-exp1 | GAAGACCATCGAGAAGGTG | |
| FAAL-exp2 | GATGCTGATACACGATGGTC | |
| PCK-exp2 | AGTCCGGTCATGCCCACAC |
Ampr, ampicillin resistance; Aprr, apramycin resistance; Camr, chloramphenicol resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance; Neor, neomycin resistance; Tetr, tetracycline resistance. Underlining in the sequences indicates the restriction endonuclease sites for DNA cloning.
From the strain collection of our lab.
Growth of N. nova on GP and rubber.
Growth experiments with N. nova strains were performed on solid and in liquid MSM with GP or rubber as the sole carbon and energy source. For this purpose, synthetic poly(trans-1,4-isoprene) (99%; Sigma-Aldrich) or poly(cis-1,4-isoprene) (97%; Sigma-Aldrich) was used, respectively. MSM-GP and MSM-rubber sandwich agar plates were used as solid media and were prepared according to a previously described method (12). Nocardia cells were inoculated by pushing toothpicks through the top MSM agar layer into the GP or rubber film to provide cells with direct contact to the polyisoprenes. For growth in liquid MSM, trans- or cis-polyisoprene was cryomilled to a defined grain size (≤500 μm). Strains were cultivated in liquid MSM containing 0.3% (wt/vol; either type) polymer grain.
Transposon mutagenesis and screening for mutants defective in rubber or GP degradation.
The Tn5096-containing suicide plasmid pMA5096 was employed for the transposon mutagenesis of N. nova SH22a. Details about the transposition procedure were described previously (15). Transposon-induced transformants were screened on MSM-rubber and MSM-GP sandwich plates to identify mutants showing defective phenotypes in rubber or GP utilization. Auxotrophic mutants were excluded by using glucose as the sole carbon and energy source, as described previously (15).
DNA extraction and manipulation.
Total DNA of N. nova was isolated according to the DNeasy Blood & Tissue kit protocol (Qiagen, Germany) or by a previously described method (19). Plasmid DNA was isolated from crude cell lysates by the alkaline extraction method (20). Cells were broken in lysis buffer with additional lysozyme (1 mg/ml) at 37°C for 1 h when plasmids were prepared from N. nova. Other genetic procedures and molecular manipulations were performed according to standard protocols (18). The Pfx DNA polymerase used for DNA amplification by PCR was obtained from Invitrogen. T4 DNA ligase and restriction enzymes were purchased from Thermo Fisher Scientific. Primers used in this study were synthesized by Eurofins MWG (Ebersberg, Germany) and are listed in Table 1.
Construction and complementation of N. nova deletion mutants.
In order to delete the fadD32SH22a gene (NONO_C01500), the flanking regions upstream (flankA; 1.15 kb) and downstream (flankB; 1.0 kb) of fadD32SH22a were amplified by using Pfx polymerase and employing the primer pairs FAAL-kn3/FAAL-kn4 and FAAL-kn3/FAAL-kn4, with total DNA of N. nova SH22a as the PCR template. The resulting fragments (flankA and flankB) were linked to a fused fragment (flankA-B; 2.15 kb) via fusion PCR (21). The flankA-B fragment was subsequently ligated into the cloning vector pJET1.2/blunt (Thermo Fisher Scientific), yielding pJET::FAALKN. A 1.0-kb kanamycin resistance cassette (Km), which was excised from pSKΩKm by using SmaI, was cloned into the single SmaI site between flankA and flankB, yielding pJET::FAALKN::Km. A 3.15-kb linear fragment (flankA-Km-flankB) was excised from pJET::FAALKN::Km by use of Kpn2I and XbaI sites (located in pJET1.2/blunt) and subsequently transferred into competent SH22a cells by electroporation (15) for homologous recombination on kanamycin plates. Genotypes of recombinants were confirmed by PCR, and the positive mutant N. nova SH22a ΔfadD32SH22a was obtained. A similar procedure employing primer pairs PCK-kn1/PCK-kn2 and PCK-kn3/PCK-kn4 was used to construct the pck (NONO_c74450) deletion mutant N. nova SH22a Δpck. The linear fragment used for homologous recombination was excised from plasmid pJET::PCKKN::Km by use of NdeI.
For complementation experiments, a 3.07-kb fragment of fadD32SH22a (primer pair FAAL-exp1/FAAL-exp2) and a 3.1-kb fragment of pck (primer pair PCK-kn1/PCK-exp2), each containing the native promoter, were amplified from total DNA of SH22a. The fragments were cloned into the HincII site of the vector pNV18.1, yielding pNV18.1::fadD32SH22a and pNV18.1::pck. The nucleotide sequences of fadD32SH22a and pck were confirmed by sequencing. The resulting plasmids were then electrotransformed into the ΔfadD32SH22a mutant and the Δpck mutant, respectively, for complementation.
Genome sequencing, assembly, and gap closure.
Genomic DNA of N. nova SH22a was isolated by use of a DNeasy Blood & Tissue kit (Qiagen, Germany). The extracted DNA was used to generate 454 shotgun, paired-end, and Illumina shotgun libraries according to the protocols of the manufacturers (Roche and Illumina). The libraries were sequenced using a 454 GS-FLX system (Titanium GS70 chemistry; Roche Life Sciences, Mannheim, Germany) and a Genome Analyzer II system (Illumina, San Diego, CA). The sequencing of the 454 shotgun libraries resulted in 271,866 reads, 27,749 of which were paired. Sequencing of the Illumina paired-end library resulted in 3,608,918 reads with a length of 112 bp. Assembly of the reads by use of Roche Newbler assembly software 2.6 for scaffolding and MIRA software (22) resulted in 17 scaffolds with 111 contigs, exhibiting an average coverage of 42.23. The remaining gaps were closed with PCR-based techniques and Sanger sequencing of the products, employing the Gap4 (v.4.11) software of the Staden package (23).
Genome annotation and analysis and comparative genomics.
Coding sequences were automatically predicted by YACOP (24), using the open reading frame (ORF) finders Glimmer, Critica, and Z-Curve, while RNAmmer (25) and tRNAscan (26) were used for identification of rRNA and tRNA genes, respectively. Functional annotation of the 7,583 predicted protein-encoding genes was initially carried out with the IMG/ER (Integrated Microbial Genomes/Expert Review, Joint Genome Institute, U.S. Department of Energy) system (27) and then manually judged on the annotation platform Artemis (28) by using criteria such as GC frame plot analysis, the presence of a ribosome binding site, and comparison with sequences in public databases, i.e., Swiss-Prot, TrEMBL, the nonredundant database of the NCBI, KEGG, COG, Pfam, and InterProScan, as well as the sequence analysis servers of SignalP v4.1 (29), TatP v1.0 (30), and TMHMM v2.0. Comparative genomics was done by using the abundance profile search and phylogenetic profiler functions integrated into the IMG/ER system, employing default settings, as well as the BLAST package of NCBI.
Nucleotide sequence accession number.
The sequence of the complete genome of N. nova SH22a is available under NCBI accession number CP006850.
RESULTS AND DISCUSSION
General features of N. nova SH22a genome.
The complete genome of N. nova SH22a comprises a circular chromosome of 8,348,532 bp with a G+C content of 67.77% (Table 2). Unlike the genome of Nocardia farcinica IFM 10152 (31), the SH22a genome does not contain any plasmids. The SH22a genome contains 7,583 predicted protein-encoding genes, among which 5,494 (72.45%) could be assigned a putative function. In addition, 49 tRNA genes and three copies of the 16S-23S-5S rRNA gene were found. Five pseudogenes were detected in the genome of N. nova SH22a.
TABLE 2.
General features of the genome of N. nova SH22a and a comparison of fully sequenced Nocardia genomes
| Characteristic | Value |
|||
|---|---|---|---|---|
| N. nova SH22aa | N. farcinica IFM10152a (31) | N. cyriacigeorgica GUH-2a (97) | N. brasiliensis HUJEG-1b (33) | |
| Genome size (bp) | 8,348,532 | 6,292,344 | 6,194,645 | 9,436,348 |
| No. of circular chromosomes | 1 | 1 | 1 | 1 |
| No. of plasmids | 0 | 2 | 0 | 0 |
| GC content (%) | 67.77 | 70.69 | 68.37 | 68.05 |
| Coding density (%) | 89.5 | 90.4 | 87.1 | 87.7 |
| No. of protein-encoding genes | 7,583 | 5,936 | 5,491 | 8,414 |
| With function prediction | 5,494 | 3,039 | 3,065 | 2,669 |
| Without function prediction | 2,089 | 2,897 | 2,426 | 5,745 |
| No. of rRNA genes | 9 | 9 | 9 | 9 |
| No. of tRNA genes | 49 | 54 | 49 | 51 |
Data were taken from the Integrated Microbial Genomes (IMG) database of the Department of Energy, Joint Genome Institute.
Data are from reference 33.
A comparison of the SH22a genome with the other three fully sequenced genomes of the genus Nocardia (N. farcinica IFM 10152 [31], Nocardia cyriacigeorgica GUH-2 [32], and Nocardia brasiliensis HUJEG-1 [33]) was carried out. These analyses revealed that in comparison to the predicted proteomes of the other Nocardia strains, many protein families of SH22a which might be involved in the degradation of xenobiotics, other hardly degradable compounds, and polymers are represented at higher percentages. These include, inter alia, aldehyde dehydrogenase family proteins and enzymes involved in β-oxidation, which might be a reason for the rubber- and GP-degrading phenotype (as discussed below), but also a large number of transporters, which might be involved in the uptake of the hardly degradable compounds (or degradation intermediates). The latter include, in particular, 14 ATP-dependent transporters of the mammalian cell entry (Mce) type, among which some were already shown to be involved in the transport of lipophilic substances (34, 35) and degradation products of inert polymers (14) in other microorganisms. The number of Mce gene clusters in SH22a is the largest found in a genome so far. Among the four sequenced Nocardia genomes, a unique feature of SH22a is the occurrence of genes for many enzymes that might be responsible for the degradation of aromatic compounds, such as ring-cleavage dioxygenases (e.g., ring-cleavage extradiol dioxygenases [NONO_c21130, NONO_c37930, NONO_c50010, and NONO_c50140] and Rieske [2Fe-2S]/ring-hydroxylating alpha subunit domain-containing proteins [NONO_c42810, NONO_c43140, and NONO_c50820]). Furthermore, the genome of SH22a carries genes for a complete set of enzymes required for the Calvin-Benson-Bassham cycle, which are also present in N. cyriacigeorgica GUH-2 but not in the genomes of N. brasiliensis HUJEG-1 and N. farcinica IFM 10152. Moreover, the genes for two putative [NiFe] hydrogenases (NONO_c30160/NONO_c30170 and NONO_c49680/NONO_c49690) were found exclusively in SH22a. However, growth experiments under an H2-O2-CO2 (8:1:1 [vol/vol/vol]) atmosphere demonstrated that strain SH22a was not able to grow autotrophically, whereas the positive control, Ralstonia eutropha H16, had that capability (data not shown).
GP and rubber catabolism of N. nova SH22a.
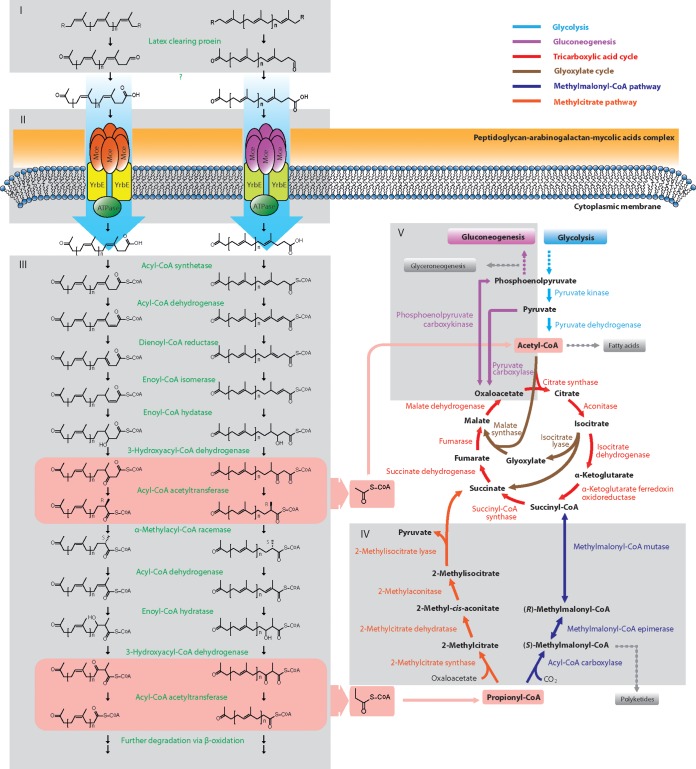
In a previous study, we constructed a Tn5096-based transposon mutagenesis library of N. nova SH22a to investigate the mechanism of microbial GP and rubber degradation. This yielded 76 transposon-induced mutants stably showing defective growth on GP and/or rubber but not on glucose or complex media (15). Further determination of the transposition loci of these mutants and in silico analysis of the SH22a genome led to the characterization of a series of novel enzymes/proteins involved in the degradation of GP and rubber (Fig. 1). The related genes and their possible functions are discussed in detail below.
FIG 1.
Proposed metabolic pathway of polyisoprene degradation in N. nova SH22a. The six gray blocks labeled by roman numbers represent the main parts of the proposed degradation pathways. I, initial oxidative cleavage of polyisoprene chains (indirect evidence from other studies [13, 14, 51]); II, import of oligoisoprenes (direct evidence from transposon-induced mutants of Mce in strain SH22a [this work] and also in the closely related strain G. polyisoprenivorans VH2 [14]); III, β-oxidation (direct evidence from transposon-induced mutants, deletion mutants, and complementation experiments for α-methylacyl-CoA racemase in strain SH22a [15], and also indirect evidence from other studies [62, 98]); IV, propionyl-CoA metabolism (direct evidence from transposon-induced mutants in strain SH22a [this work]); V, gluconeogenesis (direct evidence from transposon-induced mutants, deletion mutants, and complementation experiments for phosphoenolpyruvate carboxykinase [this work]). The two pink bars show the steps alternately releasing degradation products (acetyl-CoA and propionyl-CoA) of β-oxidation.
Adhesion of nocardial cells to GP and rubber substrates.
Some actinobacterial genera belonging to the order Corynebacteriales, such as Nocardia, Rhodococcus, Gordonia, and Mycobacterium, are able to colonize the surfaces of various hydrophobic substances and utilize them as the sole carbon and energy source (36–39). This adhesive growth behavior is thought to be due to the hydrophobic interaction between the hydrophobic cell surface and the substance. Therefore, it is expected that any interruption of the integrity and composition of cell envelopes will significantly affect the adhesion property.
Mycolic acids, which are the major constituent of cell envelopes of the Corynebacteriales (40) and are important for many cell surface properties, such as virulence (41) and low permeability (42), also play a crucial role in the high affinity of cells for numerous hydrophobic substances (43). The mechanism of synthesis, modification, transfer, and assembly of mycolic acids was recently demonstrated in detail, and the related genes were identified in Mycobacterium and Corynebacterium (44–46). fadD32-pks13-accD4 is a well-studied gene cluster that is involved in the terminal steps of mycolic acid synthesis (46). This cluster and its adjacent genes compose a conserved region. It is widely found in many other Corynebacteriales species, including the three fully sequenced Nocardia strains (N. farcinica IFM 10152 [nfa1880 to nfa1900], N. cyriacigeorgica GUH-2 [NOCYR_0144 to NOCYR_0146], and N. brasiliensis HUJEG-1 [O3I_20420 to O3I_20410]). As expected, homologues of fadD32, pks13, and accD4 were detected in the genome of SH22a and were predicted to encode a long-chain-fatty-acid–AMP ligase (FadD32SH22a; NONO_c01500), a polyketide synthase (PKS)-type condensase (NONO_c01510), and an acyl-coenzyme A (acyl-CoA) carboxylase beta subunit (NONO_c01520), respectively.
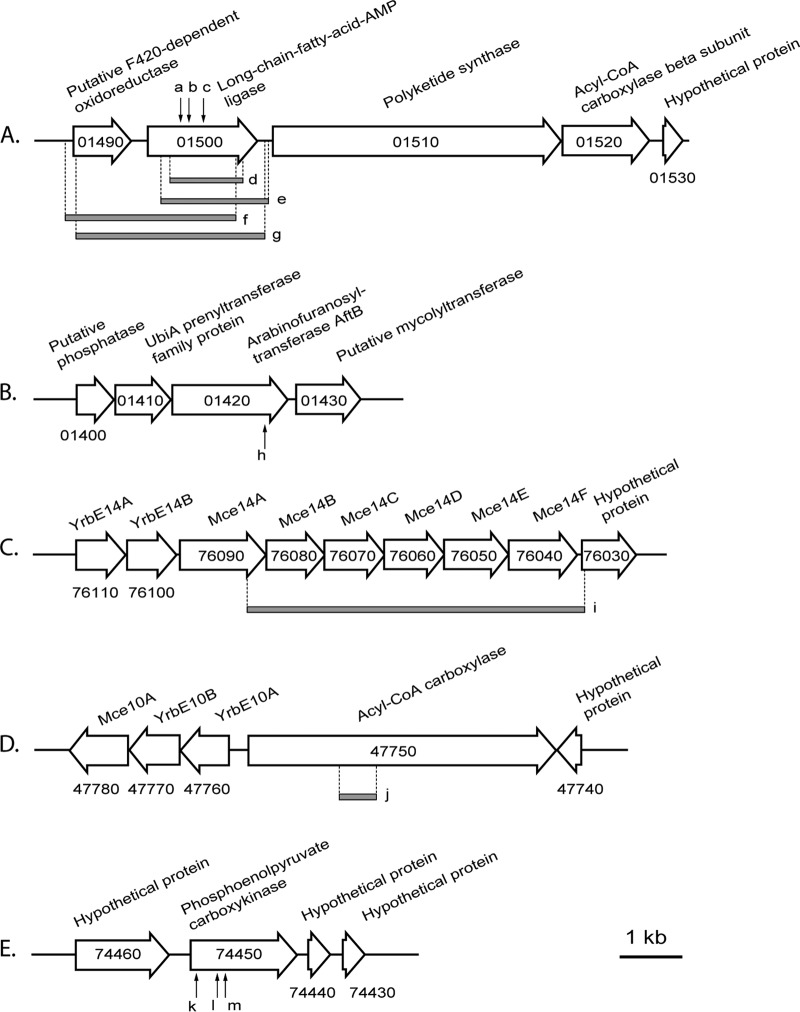
In the genomes of seven transposon-induced mutants (SE3-4-18, SE13-5-5, SE16-4-35, SE18-1-3, SE18-8-1, OC11-7-29, and OC20-2-42) (Table 1) which were defective in both GP and rubber degradation, the Tn5096 transposon-containing plasmid pMA5096 was found to interrupt the same gene, fadD32SH22a, by either inserting into different sites of this gene or replacing a chromosomal region concerning this gene (Fig. 2A). These results indicate a relevancy between mycolic acids and GP and rubber degradation. However, in order to confirm the molecular basis of the phenotypic deficiency in more detail, for example, to exclude the polar effect on the downstream genes pks13SH22a and accD4SH22a or the influence on the upstream gene (NONO_c01490), deletion and complementation experiments were performed for fadD32SH22a. The deletion mutant N. nova SH22a ΔfadD32SH22a, in which fadD32SH22a was replaced by a 1.0-kb kanamycin resistance cassette, was generated by homologous recombination. Like the transposon-induced mutants, this deletion mutant showed a growth deficiency on trans- and cis-polyisoprene, but not glucose, as the sole carbon and energy source (Fig. 3). Moreover, when phytol (0.5% [vol/vol]), a highly hydrophobic, oil-like substance, was used in the liquid MSM for growth, the deletion mutant did not aggregate tightly on the oil drops, whereas the wild type did. When plasmid pNV18.1::fadD32SH22a, harboring the native fadD32SH22a gene, was transferred into the ΔfadD32SH22a strain, the deficiency in GP and rubber degradation was completely eliminated in the resulting recombinant mutant (Fig. 3). In order to observe phenotypes under the same growth conditions, no antibiotics were added to the medium. Accordingly, plasmids were isolated from the complemented mutant after 70 days of cultivation in liquid MSM medium containing cis- or trans-polyisoprene and retransformed into E. coli cells for proliferation. The same restriction patterns of these plasmids confirmed that they were derived from the original parents and could replicate stably in N. nova SH22a even under conditions without selective pressure. These results solidly confirmed that fadD32SH22a is an essential gene for SH22a to degrade both GP and rubber.
FIG 2.
Visualization of pMA5096 insertions in the genome of N. nova SH22a transposon-induced mutants and identification of genes adjacent to the transposition loci. Arrows indicate the insertion sites of pMA5096, and the gray bars represent the missing chromosomal regions caused by pMA5096 transposition. Mutants: a, OC11-7-29; b, SE3-4-18; c, SE16-4-35; d, SE18-8-1; e, SE13-5-5; f, OC20-2-42; g, SE18-1-3; h, SE10-4-21; i, OC14-14-5; j, SE9-8-16; k, OC26-3-28; l, OC22-3-17; m, OC19-4-17. The prefix “NONO_c” of locus tags is omitted in the diagram. The lengths of open arrows showing the genes indicate the proportional lengths of transcription of the corresponding genes. Annotations of genes are given above the corresponding arrows.
FIG 3.
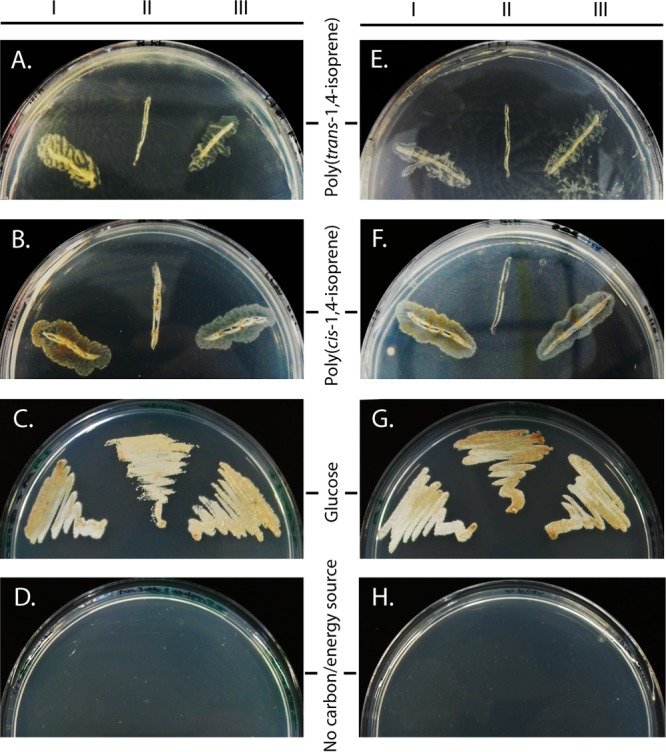
Growth of N. nova SH22a on solid MSM medium in the presence or absence of poly(trans-1,4-isoprene), poly(cis-1,4-isoprene), or glucose as the sole source of carbon and energy. I, N. nova SH22a wild-type strain; II, N. nova SH22a ΔfadD32SH22a (A to D) or N. nova SH22a Δpck (E to H); III, N. nova SH22a ΔfadD32SH22a(pNV18.1::fadD32SH22a) (A to D) or N. nova SH22a Δpck(pNV18.1::pck) (E to H).
The long-chain-fatty-acid–AMP ligase FadD32 is known to catalyze the activation of meromycolic acids, which are precursors of mycolic acids (47). Portevin et al. (48) reported that the ΔfadD32::km disruption mutant of Corynebacterium glutamicum was devoid of mycolic acids. It can be expected that this also happens in the ΔfadD32SH22a mutant, because FadDSH22a exhibits a high similarity toward FadD32 of C. glutamicum (42% identity). In addition, the organization of the whole cluster (fadD32-pks13-accD4) is highly conserved in Corynebacteriales species. Since it is known that mycolic acids are important for the adhesive growth of mycolic acid-containing actinobacteria on hydrophobic substances (43), the prevention of mycolic acid synthesis in SH22a may thereby weaken the affinity of the cell surface for hydrophobic substances, e.g., GP, rubber, and phytol. As a result, a deficiency in the utilization of GP and rubber was exhibited. On the other hand, the modified cell envelopes might also hamper the assembly of certain macromolecule-specific transporters in the cytoplasmic membrane, which in turn would affect the uptake of GP and rubber substrates.
Furthermore, another cell wall synthesis gene was identified in the GP and rubber degradation-defective mutant SE10-4-21. pMA5096 was found to insert into the 3′-terminal region of NONO_c01420 (Table 1; Fig. 2B). This ORF is located in an “ancient conserved region” (NONO_c01400 to NONO_c01520) which is known to be involved in cell envelope synthesis and also includes the fadD32-pks13-accD4 cluster of CMN (Corynebacteria, Mycobacteria, and Nocardia) group actinobacteria (49). NONO_c01420 was annotated as an arabinofuranosyltransferase gene (51% amino acid sequence identity to AftB of Mycobacterium tuberculosis) whose product catalyzes the β(1→2) linkage of terminal arabinofuranosyl residues to the arabinan skeleton in arabinogalactan biosynthesis (50). Since the terminal arabinofuranosyl residues subsequently provide partial sites for mycolic acid esterification, this step is important for the assembly of mycolic acids. Seidel et al. found that deletion of the aftB gene in C. glutamicum directly resulted in a decreased abundance of cell wall-bound mycolic acids (50). Therefore, it is expected that the disruption of NONO_c01420 led to an incomplete assembly of cell walls, which also, in turn, affected the required adhesion between nocardial cells and substrates.
Initial oxidation.
In order to serve as substrates that can be metabolized by bacterial cells, the high-molecular-weight GP or rubber polymers should first be degraded to low-molecular-weight oligomers outside cells (Fig. 1). For cis-isomers, an endocleavage mechanism is adopted by rubber-degrading bacteria for this conversion (6, 51). Previous studies have shown that this is an O2-dependent process. Shortened rubber oligomers with aldehyde and keto groups at their termini were generated after the oxidative cleavage of double bonds of poly(cis-1,4-isoprene) (6, 51). Before now, two enzymes (RoxA [rubber oxygenase] [52] and Lcp [latex-clearing protein] [13]) involved in the initial cleavage step had been identified. In contrast to RoxA, which occurs in a few Gram-negative bacteria, such as Xanthomonas sp. 35Y (rubber-degrading bacterium) and Haliangium ochraceum (non-rubber-degrading bacterium) (53), Lcp is more widely distributed in rubber-degrading actinobacteria, such as Streptomyces sp. K30 (13), N. farcinica (51), Actinoplanes missouriensis (8), Gordonia westfalica, and Gordonia polyisoprenivorans (54). It was observed that this protein is not only required for latex-clear-zone formation but also essential for the growth of adhesive-group bacteria on rubber (14). In the present study, an ORF (NONO_c40940) coding for an LCP protein was identified in the genome of SH22a. The corresponding translation product exhibits high sequence identity to the well-studied LCPs (52% identity to LCP of Streptomyces sp. K30, 64% identity to LCP of N. farcinica E1, and 63% and 72% identity to LCP1 and LCP2 of G. polyisoprenivorans VH2, respectively) (13, 14, 51). Considering that the GP degradation pathway is quite similar to that of rubber degradation, we speculate that LCPs also play an essential role in GP degradation.
Since aldehydes are unstable toward oxidation and usually toxic to bacterial cells, the aldehyde groups of GP and rubber oxidative cleavage products are supposed to be oxidized immediately to the corresponding acids outside cells. Rose et al. speculated that oxiAB, which is located directly downstream of lcp and predicted to encode an oxidoreductase complex, might be responsible for this reaction in Streptomyces sp. K30 (13). However, such a genetic organization pattern of lcp-oxiAB or the deduced proteins showing similarities to those encoded by oxiAB was not observed in most known rubber-degrading bacteria, including SH22a. Therefore, there must be another enzyme catalyzing this reaction. The candidate could be one of the numerous oxygenases encoded by the SH22a genome. However, an insertion of pMA5096 in one of the predicted oxygenase-encoding genes was not detected in the transposon-induced mutants. This might be due to the presence of two (or more) oxygenases catalyzing this step.
Mce transporter.
Although mce clusters, which have identical structures consisting of two yrbE genes (yrbEA and yrbEB), encoding integral membrane proteins, followed by six mce genes (mceA to mceF), encoding exported or membrane-tethered proteins, were first identified due to their critical role in bacterial invasion and survival in mammalian cells (55), not all bacteria possessing mce clusters are found as pathogens (such as Mycobacterium smegmatis and Streptomyces coelicolor). This indicates that the products of mce clusters have physiological functions other than acting as virulence factors, e.g., acting as novel ABC uptake transporters (56). This speculation was soon proved by showing that the Mce4 system of Rhodococcus jostii RHA1 functions as a steroid uptake transporter (34).
Regarding GP and rubber degradation, the cleavage products have to be transported into cells for further metabolism. A preliminary hint, obtained from the rubber-degrading bacterium G. polyisoprenivorans VH2 (14), suggested that these transporters are probably encoded by mce clusters (Fig. 1). During our identification of genes involved in GP and/or rubber degradation, we detected a mutant, OC14-14-5 (Table 1; Fig. 2C), in which five complete mce genes (NONO_c76080 to NONO_c76040) within the mce14 cluster were missing because of the insertion of pMA5096. Interestingly, the disruption of mce14 specifically led to a GP degradation-defective phenotype, while the growth of this mutant on rubber was not affected at all. When the protein sequences encoded by the mce14 cluster were compared with those for mce clusters of G. polyisoprenivorans VH2, the best match was for one (GPOL_c27400 to GPOL_c27470) showing an involvement in rubber degradation (14). However, when this analysis was performed in reverse, this gordonial mce cluster did not show highest similarity exclusively to the mce14 cluster of SH22a. Therefore, we suppose that the Mce14 transporter system of SH22a is specifically involved in GP degradation and that the uptake of rubber substrates is transported by another Mce transporter system(s). An important characteristic of ABC-type transporters is the requirement of an ATPase to provide energy for substrate translocation. Mkl family ATPases, whose coding genes were found to be proximal to certain mce clusters in nonmycobacterial actinomycetes or separated from any mce cluster in Mycobacterium, were thought to be the enzymes involved (34, 56, 57). The genes for three Mkl ATPases (NONO_c31130, NONO_c50930, and NONO_c69480) were detected in the genome of SH22a. NONO_c50930 and NONO_c69480 are located immediately upstream of mce clusters, i.e., mce11 (NONO_c50920 to NONO_c50850) and mce13 (NONO_c69470 to NONO_c69400), respectively, whereas NONO_c31130 is separate from mce clusters and located near a putative transcriptional regulator gene (NONO_c31120).
β-Oxidation.
β-Oxidation is known as a basic and iterative biological process catalyzing the cleavage of two carbon units each cycle from long-chain hydrocarbons. It is not only required for the breakdown of fatty acids in lipid catabolism of prokaryotic and eukaryotic organisms but also important for the complete decomposition of a variety of aliphatic and aromatic hydrocarbons in many bacteria (39, 58–61). In recent studies, an involvement of β-oxidation enzymes (e.g., acyl-CoA synthetase, thiolase, and α-methylacyl-CoA racemase [MCR]) in the degradation of rubber and/or GP was observed in strain SH22a (15) and another rubber-degrading bacterium, G. polyisoprenivorans VH2 (14, 62), which strongly indicates that β-oxidation is also employed by these bacteria for the further metabolism of polyisoprenes (Fig. 1). In the present study, we identified 79 putative acyl-CoA synthetases, 81 putative acyl-CoA dehydrogenases, 1 putative 2,4-dienoyl-CoA reductase, 64 putative enoyl-CoA hydratases/isomerases, 10 putative 3-hydroxyacyl-CoA dehydrogenases, 24 putative thiolases, and 5 putative MCRs that may participate in β-oxidation encoded in the genome of strain SH22a (see the supplemental material). The large number of enzyme candidates for each step may explain why only one β-oxidation-related enzyme (MCR) was identified from transposon-induced mutants of SH22a (15), since two or more homologues may commonly catalyze the same degradation step.
Central metabolism related to GP and rubber degradation.
Although GP and rubber are macromolecular compounds, their degradation products will undoubtedly enter the central metabolism for generating energy and providing a carbon skeleton for synthesizing other essential molecules. Genes for central metabolism, such as those for the Embden-Meyerhof-Parnas pathway (glycolysis), the pentose phosphate pathway, the tricarboxylic acid (TCA) cycle, the glyoxylate cycle, and the gluconeogenesis pathway, were detected in the genome of SH22a.
Results of our previous study (15) as well as the present study support the hypothesis that GP degradation undergoes a pathway similar to that of rubber degradation. This process is quite similar to that of polyunsaturated fatty acid degradation and generates acetyl-CoA and propionyl-CoA (Fig. 1) as degradation products. Whereas acetyl-CoA directly enters the TCA cycle and/or the glyoxylate bypass, propionyl-CoA is coupled with the central metabolism via two pathways probably occurring in SH22a, the methylcitrate pathway and the methylmalonyl-CoA pathway. The former pathway mediates the oxidation of propionyl-CoA to pyruvate (63, 64). Two prpDBC clusters (NONO_c35960 to NONO_c35980 and NONO_c62610 to NONO_c62590), encoding the main enzymes (2-methylcitrate dehydratase, 2-methylisocitrate lyase, and 2-methylcitrate synthase) of this pathway, were deduced from the SH22a genome. The methylmalonyl-CoA pathway catalyzes the conversion of propionyl-CoA to succinyl-CoA by the enzymes acyl-CoA carboxylase (ACCase), methylmalonyl-CoA epimerase, and methylmalonyl-CoA mutase and is known as an important route for propionate metabolism in some microorganisms (65, 66). More importantly, this pathway enables the production of (S)-methylmalonyl-CoA, which serves as a key precursor for the synthesis of many polyketides (67, 68). While only one copy each of the genes encoding methylmalonyl-CoA epimerase (NONO_c12700) and methylmalonyl-CoA mutase (NONO_c41040/NONO_c41050) was detected in the SH22a genome, ACCase was found to be encoded at different genetic loci. These include five carboxylase systems, where the α-subunit (NONO_c10710, NONO_c11500, NONO_c50500, NONO_c68560, and NONO_c71920) and β-subunit (NONO_c10730, NONO_c11450, NONO_c50510, NONO_c68570, and NONO_c71930) genes are located next (or close) to each other and four separate β-subunits (NONO_c01520, NONO_c07340, NONO_c20810, and NONO_c33050). Besides these typical prokaryotic ACCases, which comprise multiple subunits, we also detected genes for two eukaryotic-type ACCases (NONO_c40170 and NONO_c47750), which are large, multidomain proteins containing a biotin carboxylase domain, biotin carboxyl carrier protein domain, and carboxyltransferase domain. Due to the fact that mycobacterial ACCases utilize both acetyl-CoA and propionyl-CoA as substrates (69), we speculated that some of these nocardial ACCases also catalyze the conversion of propionyl-CoA to methylmalonyl-CoA and are involved in GP and rubber degradation. In line with this speculation, we identified an ACCase disruption mutant, SE9-8-16, showing impaired growth on both GP and rubber. The transposon was mapped in the eukaryotic-type ACCase gene NONO_c47750 (Fig. 2D). The defective phenotype can be explained either by the catalysis of the carboxylation of propionyl-CoA by other ACCase systems or by participation of the methylcitrate pathway in propionyl-CoA metabolism. Further investigations need to be performed in the future.
In the absence of sugars as the carbon and energy source, organisms convert intermediates derived from the TCA cycle or the glyoxylate bypass to phosphoenolpyruvate (PEP), which subsequently serves as a substrate participating in many important cell processes, such as gluconeogenesis, glyceroneogenesis, amino acid synthesis, and anaplerosis of the TCA or glyoxylate cycle (70). Phosphoenolpyruvate carboxykinase (PEPCK) is the enzyme responsible for this step. It catalyzes the guanosine or adenosine mononucleotide-dependent reversible conversion of oxaloacetate and PEP. In many bacteria, the inactivation of this enzyme significantly affects the growth or survival of cells when organic acids (e.g., acetate, lactate, succinate, and malate) are used as the sole carbon and energy sources (71–74). In the present study, we observed that the PEPCK gene (pck; NONO_c74450) is also essential for SH22a to grow on GP and rubber. Three mutants, OC19-4-17, OC22-3-17, and OC26-3-28 (Table 1; Fig. 2E), in which pck was disrupted by insertions of pMA5096, completely lost the ability to grow on GP and rubber. As expected, the Δpck deletion mutant exhibited the same growth deficiency on polyisoprenes and failed to grow on acetate and pyruvate as well. This defective phenotype was restored when the Δpck mutant was transformed by a plasmid expressing native pck (Fig. 3), indicating that no polar effects were caused by the transposon insertions. Although a second PEPCK gene (NONO_c31000; encodes a sequence with 68% amino acid sequence identity to the former one) was detected in the genome, it seemed that the second enzyme was not active under the employed conditions, since it did not permit growth in the Δpck mutant. The physiological role of this PEPCK remains to be studied further. In the closely related species C. glutamicum, PEP synthesis is catalyzed exclusively by PEPCK, and the enzyme cannot be replaced functionally by an alternative route catalyzed by malic enzyme or oxaloacetate decarboxylase/PEP synthase (72, 75). The impaired growth of the Δpck mutant on GP, rubber, acetate, and pyruvate suggests that the conversion from oxaloacetate to PEP catalyzed by PEPCK is also the only route for PEP synthesis in SH22a, although genetic loci probably coding for malic enzyme (NONO_c64080) and PEP synthases (NONO_c21150, NONO_c48870, and NONO_c59210) synthases were detected.
Genes involved in metabolism of alkanes.
Members of the genus Nocardia exhibit potent metabolic capabilities on a wide range of hydrocarbons (16). They are found to grow on linear and/or branched alkanes with different chain lengths as the sole carbon and energy source (60, 76, 77). The first and key step in alkane metabolism is the terminal or subterminal hydroxylation of alkanes to alkanols. In bacteria, this reaction is catalyzed by three categories of alkane hydroxylases: methane monooxygenase (MMO)-like enzymes, membrane-bound nonheme iron alkane hydroxylase (AlkB or AlkM) complexes or cytochrome P450 (CYP450) family members, and other oxygenases, which mainly act on short-, middle-, and long-chain alkanes, respectively (58, 78). In some cases, these enzymes coexist in the same microorganism and cooperate to contribute a broad range of substrate chain lengths. In the genome of SH22a, no genes for MMOs or MMO-like enzymes (e.g., propane monooxygenase and butane monooxygenase) were detected, indicating that SH22a may not metabolize gaseous alkanes. The AlkB alkane hydroxylase system is a three-component complex composed of a hydroxylase (AlkB) and two electron carriers, namely, rubredoxin and rubredoxin reductase (79, 80). The exploration of the genome revealed that SH22a contains four putative AlkB-encoding genes (NONO_c08910, NONO_c46180, NONO_c63170, and NONO_c63220), located at three chromosomal loci. Two of them (NONO_c46180 and NONO_c63220) are found in possible operons encoding rubredoxin reductases (NONO_c46210) and/or rubredoxins (NONO_c46190, NONO_c46200, NONO_c63200, and NONO_c63210). Although these AlkBs share high amino acid sequence similarities (>70%), their substrate specificity may be heterogeneous. Whereas some catalyze the oxidation of middle-chain-length or long-chain n-alkanes (81), the others may also function on branched alkanes (82). In recent studies, CYP450 members were shown to represent another group of middle-chain alkane hydroxylases in both Gram-negative and Gram-positive bacteria (83). The N. nova SH22a genome encodes 48 CYP450 proteins (PF00067), some of which exhibit similarities to already characterized alkane hydroxylases. These include, inter alia, the CYP450 encoded by NONO_c15420. The corresponding protein has 33% amino acid sequence identity to CYP153A1 of Acinetobacter sp. EB104 and 31% amino acid sequence identity to CYP150A6 of Mycobacterium sp. HXN-1500; these enzymes catalyze the initial step in alkane oxidation (83). Deduced proteins showing similarity to LadA (NONO_c02520) (84, 85) or AlmA (NONO_c13310, NONO_c18230, NONO_c36810, NONO_c40500, and NONO_c51810) (86, 87), which were characterized as catalyzing the terminal oxidation of long- and/or very-long-chain alkanes (up to at least C36) to the corresponding primary alcohols, were also found to be encoded in the genome of SH22a, indicating that this strain may metabolize very-long-chain alkanes as well.
Secondary metabolites.
PKSs, nonribosomal peptide synthetases (NRPSs), or their hybrid complexes (PKS-NRPSs) are large multimodular enzymes synthesizing a fascinating class of natural products referred to as polyketides, nonribosomal peptides, or hybrids thereof in bacteria and fungi. Many of these compounds were found to possess significant biological activities of clinical or biotechnological importance (e.g., as virulence factors, novel antibiotics, or immunosuppressive or antitumor agents) (67, 88). The biosynthesis pathways of certain metabolites have been elucidated, particularly in actinobacterial species (89–92). As with many other actinobacteria, strains of Nocardia species exhibit potent capabilities in the synthesis of secondary metabolites (16). In the present study, the genetic basis for the synthesis of these metabolites was examined in N. nova. The SH22a genome possesses, in total, 22 PKS- or NRPS-related gene clusters, among which 6 clusters contain type I PKS (PKS-I) genes, 1 cluster contains type II PKS (PKS-II) genes, 11 clusters contain NRPS genes, and 4 clusters contain PKS-NRPS genes (see the supplemental material). The genus Streptomyces is known to possess extensive secondary metabolic capabilities for producing novel bioactive compounds of clinical and industrial importance. The larger number of PKS and NRPS gene clusters in N. nova than in some Streptomyces species, such as Streptomyces turgidiscabies, Streptomyces scabiei, S. coelicolor, Streptomyces griseus subsp. griseus, and Streptomyces avermitilis (93), was therefore somewhat unexpected. This result provides clues that the genus Nocardia, which was previously known for its pathogenic and potent catabolic properties (16), could also be a valuable source for the production of useful secondary metabolism products.
Research concerning the biosynthesis pathways of polyketides and nonribosomal peptides in the genus Nocardia has been quite limited until now. By comparing the PKS and NRPS gene clusters of SH22a to the functionally elucidated polyketide and nonribosomal peptide biosynthesis pathways, products of most of these clusters cannot be predicted due to the relatively low amino acid sequence similarities and the distinct organization pattern of genes. However, three gene clusters are the exceptions. The gene cluster NONO_c01500 to -c01520 exhibits very high similarity to the fadD32-pks13-accD4 operon of M. tuberculosis (see above) and is supposed to be involved in mycolic acid synthesis. NONO_c01510 encodes a PKS-I condensase catalyzing the last condensation step. Only one PKS-II gene cluster was detected in SH22a. This cluster contains the genes for a complete pathway for type II polyketide backbone synthesis and is therefore predicted to participate in type II polyketide product biosynthesis. The two β-ketoacyl synthase subunits (KSα and KSβ) and the acyl carrier protein (ACP) of the minimal PKS-II are encoded by NONO_c10810, NONO_c10800, and NONO_c10790, respectively. Genes coding for a ketoreductase (NONO_c10780) and an aromatase (NONO_c10770), which are required for the subsequent cyclization of polyketide intermediates, are located directly downstream of the PKS-II genes. In addition, genes coding for proteins exhibiting similarities to ActIV (NONO_c10760), ActVIA (NONO_c10820), ActVI3 (NONO_c18340), ActVI4 (NONO_c57970), ActVA5 (NONO_c37860), and ActVB (NONO_c51280), which are involved in the biosynthesis of a well-studied antibiotic, actinorhodin, in S. coelicolor A3(2) (94, 95), were also found in the SH22a genome, indicating that this strain might synthesize an actinorhodin-like bioactive compound. The virulence-conferring siderophore antibiotics mycobactin and nocobactin NA are secondary metabolites produced by M. tuberculosis and N. farcinica, respectively (91, 92). The assembly of their identical core structure is accomplished by a large PKS-NRPS gene cluster: the mbt (M. tuberculosis) or nbt (N. farcinica) gene cluster. Interestingly, a similar gene cluster that contains nine PKS-NRPS genes (NONO_c08770 to NONO_c08850) was detected in SH22a. Although the gene organization is different from that of the mbt or nbt cluster, the high levels of similarity (46 to 71% amino acid sequence identity) exhibited by these genes and the existence of a siderophore-interacting protein gene (NONO_c08760) upstream of this cluster still strongly suggest its involvement in the synthesis of a siderophore product. This siderophore may play a role in iron acquisition from host cells in an infective event.
Conclusions.
The elaboration of the complete genome of N. nova SH22a, the first bacterium capable of degrading GP (and also rubber), and the generation of transposon and deletion mutants contribute to a deeper understanding of the degradation of both polyisoprene isomers. The importance of the cell envelope synthesis genes fadD32SH22a and aftB for the degradation of the two isomers was clearly shown. Furthermore, genes that are (or may be) responsible for initial oxidative cleavage, uptake of the resulting degradation intermediates, metabolism of these products via β-oxidation, and central metabolism were detected. Several of the corresponding genes were confirmed by the generation of mutants. The previously described assumption that GP and rubber degradation pathways are quite similar and share common steps (15) has been confirmed. The sequencing of the SH22a genome shows that this isolate not only represents a strain of the genus Nocardia possessing manifold potential catabolic capabilities but also may be an interesting candidate for the synthesis of novel bioactive compounds.
Supplementary Material
ACKNOWLEDGMENTS
We greatly acknowledge financial support by the Deutsche Forschungsgemeinschaft (grant STE-386/10-1) for this study.
Footnotes
Published ahead of print 18 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00473-14.
REFERENCES
- 1.Mooibroek H, Cornish K. 2000. Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 53:355–365. 10.1007/s002530051627 [DOI] [PubMed] [Google Scholar]
- 2.Warneke S, Arenskötter M, Tenberge KB, Steinbüchel A. 2007. Bacterial degradation of poly(trans-1,4-isoprene) (gutta percha). Microbiology 153:347–356. 10.1099/mic.0.2006/000109-0 [DOI] [PubMed] [Google Scholar]
- 3.International Rubber Study Group. Apr-Jun 2013. Rubber statistical bulletin. International Rubber Study Group, Singapore, Singapore [Google Scholar]
- 4.Kizinievič O, Mačiulaitis R, Kizinievič V. 2006. Use of rubber waste in the ceramic. Mater. Sci. 12:237–242 [Google Scholar]
- 5.Adhikari B, De D, Maiti S. 2000. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 25:909–948. 10.1016/S0079-6700(00)00020-4 [DOI] [Google Scholar]
- 6.Tsuchii A, Suzuki T, Takeda K. 1985. Microbial degradation of natural rubber vulcanizates. Appl. Environ. Microbiol. 50:965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heisey RM, Papadatos S. 1995. Isolation of microorganisms able to metabolize purified natural rubber. Appl. Environ. Microbiol. 61:3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jendrossek D, Tomasi G, Kroppenstedt RM. 1997. Bacterial degradation of natural rubber: a privilege of actinomycetes? FEMS Microbiol. Lett. 150:179–188. 10.1016/S0378-1097(97)00072-4 [DOI] [PubMed] [Google Scholar]
- 9.Linos A, Berekaa MM, Reichelt R, Keller U, Schmitt J, Flemming HC, Kroppenstedt RM, Steinbüchel A. 2000. Biodegradation of cis-1,4-polyisoprene rubbers by distinct actinomycetes: microbial strategies and detailed surface analysis. Appl. Environ. Microbiol. 66:1639–1645. 10.1128/AEM.66.4.1639-1645.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spence D, Van Niel CB. 1936. Bacterial decomposition of the rubber in Hevea latex. Ind. Eng. Chem. 28:847–850. 10.1021/ie50319a023 [DOI] [Google Scholar]
- 11.Bode HB, Zeeck A, Plückhahn K, Jendrossek D. 2000. Physiological and chemical investigations into microbial degradation of synthetic poly(cis-1,4-isoprene). Appl. Environ. Microbiol. 66:3680–3685. 10.1128/AEM.66.9.3680-3685.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banh Q, Arenskötter M, Steinbüchel A. 2005. Establishment of Tn5096-based transposon mutagenesis in Gordonia polyisoprenivorans. Appl. Environ. Microbiol. 71:5077–5084. 10.1128/AEM.71.9.5077-5084.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose K, Tenberge KB, Steinbüchel A. 2005. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6:180–188. 10.1021/bm0496110 [DOI] [PubMed] [Google Scholar]
- 14.Hiessl S, Schuldes J, Thürmer A, Halbsguth T, Bröker D, Angelov A, Liebl W, Daniel R, Steinbüchel A. 2012. Involvement of two latex clearing proteins during rubber degradation and insights into the subsequent degradation pathway revealed by the genome sequence of Gordonia polyisoprenivorans strain VH2. Appl. Environ. Microbiol. 78:2874–2887. 10.1128/AEM.07969-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Q, Hiessl S, Poehlein A, Steinbüchel A. 2013. Microbial gutta-percha degradation shares common steps with rubber degradation by Nocardia nova SH22a. Appl. Environ. Microbiol. 79:1140–1149. 10.1128/AEM.03016-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Q, Hiessl S, Steinbüchel A. 2014. Functional diversity of Nocardia in metabolism. Environ. Microbiol. 16:29–48. 10.1111/1462-2920.12221 [DOI] [PubMed] [Google Scholar]
- 17.Schlegel HG, Kaltwasser H, Gottschalk G. 1961. Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch. Mikrobiol. 38:209–222. 10.1007/BF00422356 [DOI] [PubMed] [Google Scholar]
- 18.Sambrook JE, Fritsch F, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 19.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struni K. 1987. Current protocols in molecular biology. John Wiley & Sons, Inc, New York, NY [Google Scholar]
- 20.Birnboim HC, Doly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513–1523. 10.1093/nar/7.6.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yon J, Fried M. 1989. Precise gene fusion by PCR. Nucleic Acids Res. 17:4895. 10.1093/nar/17.12.4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevreux B, Wetter T, Suhai S. 1999. Genome sequence assembly using trace signals and additional sequence information, p 45–56 In Computer science and biology: proceedings of the German Conference on Bioinformatics (GCB). GCB, Hannover, Germany [Google Scholar]
- 23.Staden R, Beal KF, Bonfield JK. 2000. The Staden package, 1998. Methods Mol. Biol. 132:115–130 [DOI] [PubMed] [Google Scholar]
- 24.Tech M, Merkl R. 2003. YACOP: enhanced gene prediction obtained by a combination of existing methods. In Silico Biol. 3:441–451 [PubMed] [Google Scholar]
- 25.Lagesen K, Hallin P, Rødland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35:3100–3108. 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. 2009. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 25:2271–2278. 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]
- 28.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- 29.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 30.Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. 2005. Prediction of twin-arginine signal peptides. BMC Bioinformatics 6:167. 10.1186/1471-2105-6-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa J, Yamashita A, Mikami Y, Hoshino Y, Kurita H, Hotta K, Shiba T, Hattori M. 2004. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. U. S. A. 101:14925–14930. 10.1073/pnas.0406410101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoropogui A, Pujic P, Normand P, Barbe V, Belli P, Graindorge A, Roche D, Vallenet D, Mangenot S, Boiron P, Rodriguez-Nava V, Ribun S, Richard Y, Cournoyer B, Blaha D. 2013. The Nocardia cyriacigeorgica GUH-2 genome shows ongoing adaptation of an environmental Actinobacteria to a pathogen's lifestyle. BMC Genomics 14:286. 10.1186/1471-2164-14-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vera-Cabrera L, Ortiz-Lopez R, Elizondo-Gonzalez R, Ocampo-Candiani J. 2013. Complete genome sequence analysis of Nocardia brasiliensis HUJEG-1 reveals a saprobic lifestyle and the genes needed for human pathogenesis. PLoS One 8:e65425. 10.1371/journal.pone.0065425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohn WW, van der Geize R, Stewart GR, Okamoto S, Liu J, Dijkhuizen L, Eltis LD. 2008. The actinobacterial mce4 locus encodes a steroid transporter. J. Biol. Chem. 283:35368–35374. 10.1074/jbc.M805496200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrellad MA, McNeil M, Santangelo ML, Blanco FC, Garcia E, Klepp LI, Huff J, Niederweis M, Jackson M, Bigi F. 2014. Role of the Mce1 transporter in the lipid homeostasis of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 94:170–177. 10.1016/j.tube.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchii A, Takeda K, Suzuki T, Tokiwa Y. 1996. Colonization and degradation of rubber pieces by Nocardia sp. Biodegradation 7:41–48. 10.1007/BF00056557 [DOI] [PubMed] [Google Scholar]
- 37.Orr IG, Hadar Y, Sivan A. 2004. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 65:97–104. 10.1007/s00253-004-1584-8 [DOI] [PubMed] [Google Scholar]
- 38.Linos A, Steinbüchel A, Spröer C, Kroppenstedt RM. 1999. Gordonia polyisoprenivorans sp. nov., a rubber-degrading actinomycete isolated from an automobile tyre. Int. J. Syst. Bacteriol. 49:1785–1791. 10.1099/00207713-49-4-1785 [DOI] [PubMed] [Google Scholar]
- 39.Berekaa MM, Steinbüchel A. 2000. Microbial degradation of the multiply branched alkane 2,6,10,15,19,23-hexamethyltetracosane (squalane) by Mycobacterium fortuitum and Mycobacterium ratisbonense. Appl. Environ. Microbiol. 66:4462–4467. 10.1128/AEM.66.10.4462-4467.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitman WB, Goodfellow M, Kämpfer P, Busse H-J, Trujillo ME, Ludwig W, Suzuki K-I. (ed). 2012. Bergey's manual of systematic bacteriology, 2nd ed, vol 5 Springer-Verlag, New York, NY [Google Scholar]
- 41.Vander Beken S, Al Dulayymi JR, Naessens T, Koza G, Maza-Iglesias M, Rowles R, Theunissen C, De Medts J, Lanckacker E, Baird MS, Grooten J. 2011. Molecular structure of the Mycobacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur. J. Immunol. 41:450–460. 10.1002/eji.201040719 [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Rosenberg EY, Nikaido H. 1995. Fluidity of the lipid domain of cell wall from Mycobacterium chelonae. Proc. Natl. Acad. Sci. U. S. A. 92:11254–11258. 10.1073/pnas.92.24.11254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bendinger B, Rijnaarts HH, Altendorf K, Zehnder AJ. 1993. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids. Appl. Environ. Microbiol. 59:3973–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takayama K, Wang C, Besra GS. 2005. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18:81–101. 10.1128/CMR.18.1.81-101.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420–1422. 10.1126/science.276.5317.1420 [DOI] [PubMed] [Google Scholar]
- 46.Portevin D, De Sousa-D'Auria C, Houssin C, Grimaldi C, Chami M, Daffé M, Guilhot C. 2004. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc. Natl. Acad. Sci. U. S. A. 101:314–319. 10.1073/pnas.0305439101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trivedi OA, Arora P, Sridharan V, Tickoo R, Mohanty D, Gokhale RS. 2004. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature 428:441–445. 10.1038/nature02384 [DOI] [PubMed] [Google Scholar]
- 48.Portevin D, de Sousa-D'Auria C, Montrozier H, Houssin C, Stella A, Lanéelle MA, Bardou F, Guilhot C, Daffé M. 2005. The acyl-AMP ligase FadD32 and AccD4-containing acyl-CoA carboxylase are required for the synthesis of mycolic acids and essential for mycobacterial growth: identification of the carboxylation product and determination of the acyl-CoA carboxylase components. J. Biol. Chem. 280:8862–8874. 10.1074/jbc.M408578200 [DOI] [PubMed] [Google Scholar]
- 49.Ramulu HG, Swathi A, Guruprasad L. 2006. The Rv3799-Rv3807 gene cluster in Mycobacterium tuberculosis genome corresponds to the ‘ancient conserved region' in CMN mycolyltransferases. Evol. Bioinform. Online 2:377–385 [PMC free article] [PubMed] [Google Scholar]
- 50.Seidel M, Alderwick LJ, Birch HL, Sahm H, Eggeling L, Besra GS. 2007. Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 282:14729–14740. 10.1074/jbc.M700271200 [DOI] [PubMed] [Google Scholar]
- 51.Ibrahim EM, Arenskötter M, Luftmann H, Steinbüchel A. 2006. Identification of poly(cis-1,4-isoprene) degradation intermediates during growth of moderately thermophilic actinomycetes on rubber and cloning of a functional lcp homologue from Nocardia farcinica strain E1. Appl. Environ. Microbiol. 72:3375–3382. 10.1128/AEM.72.5.3375-3382.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braaz R, Fischer P, Jendrossek D. 2004. Novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Appl. Environ. Microbiol. 70:7388–7395. 10.1128/AEM.70.12.7388-7395.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birke J, Röther W, Schmitt G, Jendrossek D. 2013. Functional identification of rubber oxygenase (RoxA) in soil and marine myxobacteria. Appl. Environ. Microbiol. 79:6391–6399. 10.1128/AEM.02194-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bröker D, Dietz D, Arenskötter M, Steinbüchel A. 2008. The genomes of the non-clearing-zone-forming and natural-rubber-degrading species Gordonia polyisoprenivorans and Gordonia westfalica harbor genes expressing Lcp activity in Streptomyces strains. Appl. Environ. Microbiol. 74:2288–2297. 10.1128/AEM.02145-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley LW. 1993. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. Science 261:1454–1457. 10.1126/science.8367727 [DOI] [PubMed] [Google Scholar]
- 56.Casali N, Riley LW. 2007. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8:60. 10.1186/1471-2164-8-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joshi SM, Pandey AK, Capite N, Fortune SM, Rubin EJ, Sassetti CM. 2006. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc. Natl. Acad. Sci. U. S. A. 103:11760–11765. 10.1073/pnas.0603179103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rojo F. 2009. Degradation of alkanes by bacteria. Environ. Microbiol. 11:2477–2490. 10.1111/j.1462-2920.2009.01948.x [DOI] [PubMed] [Google Scholar]
- 59.Annweiler E, Michaelis W, Meckenstock RU. 2002. Identical ring cleavage products during anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin indicate a new metabolic pathway. Appl. Environ. Microbiol. 68:852–858. 10.1128/AEM.68.2.852-858.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le TN, Mikolasch A, Awe S, Sheikhany H, Klenk HP, Schauer F. 2010. Oxidation of aliphatic, branched chain, and aromatic hydrocarbons by Nocardia cyriacigeorgica isolated from oil-polluted sand samples collected in the Saudi Arabian Desert. J. Basic Microbiol. 50:241–253. 10.1002/jobm.200900358 [DOI] [PubMed] [Google Scholar]
- 61.Leuthner B, Heider J. 2000. Anaerobic toluene catabolism of Thauera aromatica: the bbs operon codes for enzymes of β oxidation of the intermediate benzylsuccinate. J. Bacteriol. 182:272–277. 10.1128/JB.182.2.272-277.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arenskötter Q, Heller J, Dietz D, Arenskötter M, Steinbüchel A. 2008. Cloning and characterization of α-methylacyl coenzyme A racemase from Gordonia polyisoprenivorans VH2. Appl. Environ. Microbiol. 74:7085–7089. 10.1128/AEM.01491-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Textor S, Wendisch VF, De Graaf AA, Müller U, Linder MI, Linder D, Buckel W. 1997. Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch. Microbiol. 168:428–436. 10.1007/s002030050518 [DOI] [PubMed] [Google Scholar]
- 64.Horswill AR, Escalante-Semerena JC. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. J. Bacteriol. 181:5615–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wegener WS, Reeves HC, Rabin R, Ajl SJ. 1968. Alternate pathways of metabolism of short-chain fatty acids. Bacteriol. Rev. 32:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans CT, Sumegi B, Srere PA, Sherry AD, Malloy CR. 1993. [13C]propionate oxidation in wild-type and citrate synthase mutant Escherichia coli: evidence for multiple pathways of propionate utilization. Biochem. J. 291:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischbach MA, Walsh CT. 2006. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106:3468–3496. 10.1021/cr0503097 [DOI] [PubMed] [Google Scholar]
- 68.Gaisser S, Reather J, Wirtz G, Kellenberger L, Staunton J, Leadlay PF. 2000. A defined system for hybrid macrolide biosynthesis in Saccharopolyspora erythraea. Mol. Microbiol. 36:391–401. 10.1046/j.1365-2958.2000.01856.x [DOI] [PubMed] [Google Scholar]
- 69.Rainwater DL, Kolattukudy PE. 1982. Isolation and characterization of acyl coenzyme A carboxylases from Mycobacterium tuberculosis and Mycobacterium bovis, which produce multiple methyl-branched mycocerosic acids. J. Bacteriol. 151:905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang JQ, Kalhan SC, Hanson RW. 2009. What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 284:27025–27029. 10.1074/jbc.R109.040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Østerås M, Finan TM, Stanley J. 1991. Site-directed mutagenesis and DNA sequence of pckA of Rhizobium NGR234, encoding phosphoenolpyruvate carboxykinase: gluconeogenesis and host-dependent symbiotic phenotype. Mol. Gen. Genet. 230:257–269. 10.1007/BF00290676 [DOI] [PubMed] [Google Scholar]
- 72.Riedel C, Rittmann D, Dangel P, Möckel B, Petersen S, Sahm H, Eikmanns BJ. 2001. Characterization of the phosphoenolpyruvate carboxykinase gene from Corynebacterium glutamicum and significance of the enzyme for growth and amino acid production. J. Mol. Microbiol. Biotechnol. 3:573–583 [PubMed] [Google Scholar]
- 73.Marrero J, Rhee KY, Schnappinger D, Pethe K, Ehrt S. 2010. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. U. S. A. 107:9819–9824. 10.1073/pnas.1000715107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schreier HJ, Dejtisakdi W, Escalante JO, Brailo M. 2012. Transposon mutagenesis of Planctomyces limnophilus and analysis of a pckA mutant. Appl. Environ. Microbiol. 78:7120–7123. 10.1128/AEM.01794-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hyeon JE, Kang DH, Kim YI, You SK, Han SO. 2012. GntR-type transcriptional regulator PckR negatively regulates the expression of phosphoenolpyruvate carboxykinase in Corynebacterium glutamicum. J. Bacteriol. 194:2181–2188. 10.1128/JB.06562-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhatia M, Singh HD. 1996. Biodegradation of commercial linear alkyl benzenes by Nocardia amarae. J. Biosci. 21:487–496. 10.1007/BF02703213 [DOI] [Google Scholar]
- 77.Alvarez HM, Souto MF, Viale A, Pucci OH. 2001. Biosynthesis of fatty acids and triacylglycerols by 2,6,10,14-tetramethyl pentadecane-grown cells of Nocardia globerula 432. FEMS Microbiol. Lett. 200:195–200. 10.1111/j.1574-6968.2001.tb10715.x [DOI] [PubMed] [Google Scholar]
- 78.van Beilen JB, Funhoff EG. 2007. Alkane hydroxylases involved in microbial alkane degradation. Appl. Microbiol. Biotechnol. 74:13–21. 10.1007/s00253-006-0748-0 [DOI] [PubMed] [Google Scholar]
- 79.van Beilen JB, Panke S, Lucchini S, Franchini AG, Röthlisberger M, Witholt B. 2001. Analysis of Pseudomonas putida alkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk genes. Microbiology 147:1621–1630 [DOI] [PubMed] [Google Scholar]
- 80.Lo Piccolo L, De Pasquale C, Fodale R, Puglia AM, Quatrini P. 2011. Involvement of an alkane hydroxylase system of Gordonia sp. strain SoCg in degradation of solid n-alkanes. Appl. Environ. Microbiol. 77:1204–1213. 10.1128/AEM.02180-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marín MM, Smits TH, van Beilen JB, Rojo F. 2001. The alkane hydroxylase gene of Burkholderia cepacia RR10 is under catabolite repression control. J. Bacteriol. 183:4202–4209. 10.1128/JB.183.14.4202-4209.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takei D, Washio K, Morikawa M. 2008. Identification of alkane hydroxylase genes in Rhodococcus sp. strain TMP2 that degrades a branched alkane. Biotechnol. Lett. 30:1447–1452. 10.1007/s10529-008-9710-9 [DOI] [PubMed] [Google Scholar]
- 83.van Beilen JB, Funhoff EG, van Loon A, Just A, Kaysser L, Bouza M, Holtackers R, Röthlisberger M, Li Z, Witholt B. 2006. Cytochrome P450 alkane hydroxylases of the CYP153 family are common in alkane-degrading eubacteria lacking integral membrane alkane hydroxylases. Appl. Environ. Microbiol. 72:59–65. 10.1128/AEM.72.1.59-65.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng L, Wang W, Cheng J, Ren Y, Zhao G, Gao C, Tang Y, Liu X, Han W, Peng X, Liu R, Wang L. 2007. Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc. Natl. Acad. Sci. U. S. A. 104:5602–5607. 10.1073/pnas.0609650104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L, Liu X, Yang W, Xu F, Wang W, Feng L, Bartlam M, Wang L, Rao Z. 2008. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: unveiling the long-chain alkane hydroxylase. J. Mol. Biol. 376:453–465. 10.1016/j.jmb.2007.11.069 [DOI] [PubMed] [Google Scholar]
- 86.Throne-Holst M, Wentzel A, Ellingsen TE, Kotlar HK, Zotchev SB. 2007. Identification of novel genes involved in long-chain n-alkane degradation by Acinetobacter sp. strain DSM 17874. Appl. Environ. Microbiol. 73:3327–3332. 10.1128/AEM.00064-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang W, Shao Z. 2012. Genes involved in alkane degradation in the Alcanivorax hongdengensis strain A-11-3. Appl. Microbiol. Biotechnol. 94:437–448. 10.1007/s00253-011-3818-x [DOI] [PubMed] [Google Scholar]
- 88.Hertweck C. 2009. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 48:4688–4716. 10.1002/anie.200806121 [DOI] [PubMed] [Google Scholar]
- 89.Zhang H, Wang Y, Wu J, Skalina K, Pfeifer BA. 2010. Complete biosynthesis of erythromycin A and designed analogs using E. coli as a heterologous host. Chem. Biol. 17:1232–1240. 10.1016/j.chembiol.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 90.Gokhale RS, Saxena P, Chopra T, Mohanty D. 2007. Versatile polyketide enzymatic machinery for the biosynthesis of complex mycobacterial lipids. Nat. Prod. Rep. 24:267–277. 10.1039/b616817p [DOI] [PubMed] [Google Scholar]
- 91.Quadri LE, Sello J, Keating TA, Weinreb PH, Walsh CT. 1998. Identification of a Mycobacterium tuberculosis gene cluster encoding the biosynthetic enzymes for assembly of the virulence-conferring siderophore mycobactin. Chem. Biol. 5:631–645. 10.1016/S1074-5521(98)90291-5 [DOI] [PubMed] [Google Scholar]
- 92.Hoshino Y, Chiba K, Ishino K, Fukai T, Igarashi Y, Yazawa K, Mikami Y, Ishikawa J. 2011. Identification of nocobactin NA biosynthetic gene clusters in Nocardia farcinica. J. Bacteriol. 193:441–448. 10.1128/JB.00897-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komaki H, Ichikawa N, Oguchi A, Hanamaki T, Fujita N. 2012. Genome-wide survey of polyketide synthase and nonribosomal peptide synthetase gene clusters in Streptomyces turgidiscabies NBRC 16081. J. Gen. Appl. Microbiol. 58:363–372. 10.2323/jgam.58.363 [DOI] [PubMed] [Google Scholar]
- 94.Fernández-Moreno MA, Martínez E, Boto L, Hopwood DA, Malpartida F. 1992. Nucleotide sequence and deduced functions of a set of cotranscribed genes of Streptomyces coelicolor A3(2) including the polyketide synthase for the antibiotic actinorhodin. J. Biol. Chem. 267:19278–19290 [PubMed] [Google Scholar]
- 95.Fernández-Moreno MA, Martínez E, Caballero JL, Ichinose K, Hopwood DA, Malpartida F. 1994. DNA sequence and functions of the actVI region of the actinorhodin biosynthetic gene cluster of Streptomyces coelicolor A3(2). J. Biol. Chem. 269:24854–24863 [PubMed] [Google Scholar]
- 96.Chiba K, Hoshino Y, Ishino K, Kogure T, Mikami Y, Uehara Y, Ishikawa J. 2007. Construction of a pair of practical Nocardia-Escherichia coli shuttle vectors. Jpn. J. Infect. Dis. 60:45–47 [PubMed] [Google Scholar]
- 97.Zoropogui A, Pujic P, Normand P, Barbe V, Beaman B, Beaman L, Boiron P, Colinon C, Deredjian A, Graindorge A, Mangenot S, Nazaret S, Neto M, Petit S, Roche D, Vallenet D, Rodríguez-Nava V, Richard Y, Cournoyer B, Blaha D. 2012. Genome sequence of the human- and animal-pathogenic strain Nocardia cyriacigeorgica GUH-2. J. Bacteriol. 194:2098–2099. 10.1128/JB.00161-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bode HB, Kerkhoff K, Jendrossek D. 2001. Bacterial degradation of natural and synthetic rubber. Biomacromolecules 2:295–303. 10.1021/bm005638h [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.