Abstract
To determine the seroprevalence of astrovirus MLB1 (MLB1), an indirect enzyme-linked immunosorbent assay (ELISA) was established. MLB1 seropositivity was high in children <6 months old, decreased to a nadir at 12 to 23 months old, and increased to 100% by adulthood. MLB1 infection is common, and primary exposure occurs in childhood.
TEXT
Astroviruses were first discovered in humans in 1975. These “classic astroviruses” consist of eight closely related serotypes that are responsible for about 10% of sporadic nonbacterial diarrhea in children. The extent of human infection by these viruses varies, depending on serotype. For example, 90% of the general population has antibodies to serotype 1, while only 10% has antibodies to serotype 7 (1).
Recently a new clade of astroviruses has been identified. This clade currently consists of astroviruses MLB1, MLB2, and MLB3. All three viruses were initially discovered in stool samples from children with diarrhea (2–4) and have since been detected in additional diarrhea cases as well as control stools (3, 5, 6). MLB1, MLB2, and MLB3 have not yet been associated with any human disease, although we have detected MLB2 in the plasma of a febrile child (7). A recent case control study of MLB1 in a cohort of Indian children found no association of MLB1 with diarrhea (5). Currently it is unclear if the presence of these novel astroviruses in human stool samples represents true human infection. Here we describe the first evidence of human antibody response to MLB1 and demonstrate that infection by MLB1 is common.
An enzyme-linked immunosorbent assay (ELISA) targeting MLB1 open reading frame 2 (ORF2) was established and validated using rabbit antibody raised against an MLB1 peptide as a positive control and preimmune serum as a negative control. Polyclonal MLB1 capsid peptide antibody (DW60) was generated by the immunization of rabbits with TNNQRARSTRPNFTPAPKF (positions 22 to 40) and ARRAMFDALVKTGVSVEEASR (positions 697 to 717) (21st Century Biochemicals). MLB2 capsid peptide (ASNQRTRSAGPGPKC) (positions 22 to 35) and CPLPHRTIEWDESSD (positions 646 to 659) (Genscript) were synthesized and injected into rabbits to produce antibodies DW57 and DW58, respectively. Open reading frames 2 (capsid protein) of MLB1, MLB2, and MLB3 were cloned as follows. MLB1 (nucleotides 3843 to 6113 from GenBank accession no. NC_011400.1) flanked with NotI and PstI restriction sites, MLB2 (nucleotides 3843 to 6080 from GenBank accession no. NC_016155.1) flanked with NotI and XhoI restriction sites, and MLB3 (nucleotides 3843 to 6086 from GenBank accession no. NC_019028.1) flanked with NotI and XhoI were amplified from stool RNA by reverse transcription-PCR with the following primers: SF0118F (5′-ATAGCGGCCGCATGGCTAATGCCAGTAAAGGTGT-3′) and SF0111R (5′-ATAGACGTCTTATTTACAATTGTATGCTTCCTCGT-3′) for MLB1, LG0166 (5′-ATAGCGGCCGCATGGCTAATGCCAATAAGGGTGT-3′) and LG0168 (5′-ATACTCGAGTTATTTGTCTAAAAGACATGCTTCGC-3′) for MLB2, and LG0205 (5′-ATAGCGGCCGCATGGCTAATGCCAATAAGGGTGTG-3′) and LG0206 (5′-ATACTCGAGTTATTTGTCTAAAAGACATGCCTCA-3′) for MLB3. Recombinant baculoviruses were generated by the Bac-to-Bac system (Invitrogen) per the manufacturer's protocol at the St. Jude's Protein Production Core Facility. Human astrovirus serotype 1 capsid protein was produced as previously described (8) with the following modification: an N-terminal His tag was added to the previous construct. Baculovirus-expressed His-tagged RANK protein was kindly provided by Daved Fremont (9).
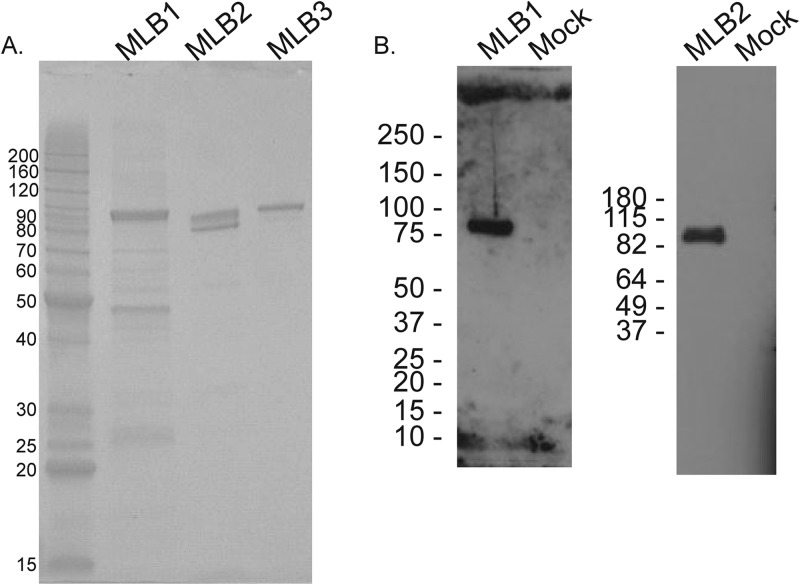
MLB1, MLB2, and MLB3 capsid proteins were expressed in a baculovirus system as N-terminal 6×His-tagged proteins and affinity purified using HiTrap chelating high-performance (HP) columns (GE Healthcare). SDS-polyacrylamide gel electrophoresis and Coomassie staining were used to assess the purity of the protein (Fig. 1A). Also, mass spectrometry was used to confirm the mass of the major product. Additionally, a Western blot assay using the polyclonal rabbit antibodies against MLB1 and MLB2 capsid gave signals of approximately the expected size (88 kDA for MLB1 and 87 kDA for MLB2) (Fig. 1B).
FIG 1.
(A) Coomassie blue staining of a sodium dodecyl sulfate-polyacrylamide gel showing baculovirus-expressed His-tagged MLB1 (expected size, 88 kDa), MLB2 (expected size, 87 kDa), and MLB3 (expected size, 88 kDa) capsid proteins. (B) Western blot using polyclonal MLB1 peptide antibody (DW60) (1:500) against recombinant His-MLB1 capsid (5 ng) and polyclonal MLB2 peptide antibody (DW58) (1:2,500) against recombinant His-MLB2 capsid (100 ng). Mock, protein lysate from uninfected Sf9 cells.
We analyzed 395 deidentified sera from subjects 2 months to 95 years of age, which were obtained from healthy volunteers in North America for previous studies conducted at the Saint Louis University Center for Vaccine Development collected between 1984 and 2009 (examples of studies include references 10 and 11). The ages of the subjects and numbers of sera were as follows: 0 to 6 months, n = 22; 7 to 11 months, n = 23; 12 to 23 months, n = 35; 2 years, n = 13; 3 years, n = 20; 4 to 6 years, n = 47; 7 to 17 years, n = 75; 18 to 32 years, n = 38; 33 to 64 years, n = 42; and >65 years, n = 80. The institutional review boards at Saint Louis University and Washington University School of Medicine approved this study.
ELISAs were optimized and performed as described previously (12) with the following changes: horseradish peroxidase-conjugated protein A/G (Thermo-Scientific) (which binds IgG, IgM, and IgA) diluted 1:10,000 was used, the His-MLB1 capsid concentration was 25 ng/well, and the His-MLB2 capsid concentration was 75 ng/well. For the preincubation assays, serum samples were diluted in blocking buffer and preincubated overnight at 4°C with 10× the coating amount (250 ng for MLB1 plates or 750 ng for MLB2 plates) of recombinant His-MLB1 capsid, His-MLB2 capsid, His-MLB3 capsid, His-human astrovirus 1 capsid, or His-RANK (an irrelevant protein). To establish the cutoff value, we calculated the average of the 30 lowest serum samples and set the cutoff 3 standard deviations (SD) above this mean (for MLB1, mean of 30 lowest = 0.283 OD units, SD = 0.06988 OD units, cutoff = 0.492 OD units; for MLB2, mean of 30 lowest = 0.2011 OD units, SD = 0.03677 OD units, cutoff = 0.311 OD units). To assess plate-to-plate variation, preimmune rabbit serum and MLB1 peptide antibody (DW60) were analyzed on every ELISA plate. The coefficient of variance for the preimmune rabbit serum was 14.80% (MLB1), and that for the positive control was 26.17% (MLB1).
In a cohort of healthy vaccine trial participants, the overall seropositivity of MLB1 was 86.6% (342/395). Anti-MLB1 capsid antibodies appear to be maternally transferred based on the relatively high seroprevalence in the 0- to 6-month age group (68.1%) coupled with a lower positivity rate (31.4%) in the 12- to 23-month age group. The seropositivity rate then rapidly increased and reached 100% in the 7- to 17-year age group (Fig. 2) and remained high thereafter. Thus, MLB1 seroepidemiology parallels that of human astrovirus 1 but is in contrast to the low seroprevalence rates of human astrovirus 7 (1).
FIG 2.
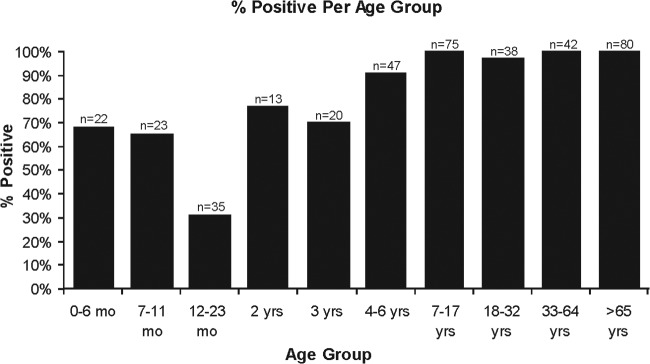
ELISA results for MLB1. A total of 395 serum samples were assayed for antibodies against MLB1 capsid protein.
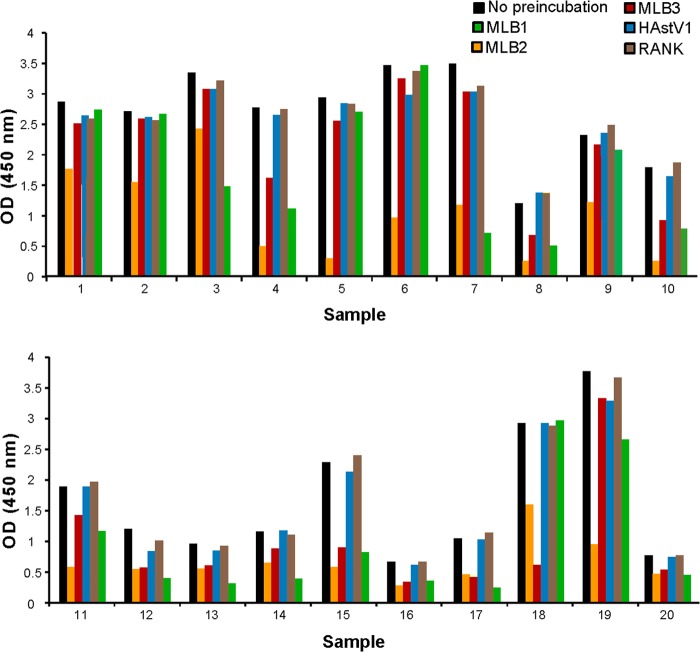
To assess the specificity of the MLB1 assay, preincubation assays were performed on 10 serum samples arbitrarily chosen among samples with high-intensity signal (optical density [OD] of >2.400). Preincubation with His-MLB1 capsid reduced the ELISA signal on average by 70%; in contrast, the signal remained constant when preincubated with His-HAstV-1, His-MLB2, His-MLB3, and His-RANK (Fig. 3). Sample 10 did not compete with any antigen, including His-MLB1, at the standard 1:100 serum dilution; however, competition was observed with His-MLB1 but not the other proteins at a more dilute serum concentration (1:800), suggesting that this sample had high-titer MLB1 antibodies (data not shown).
FIG 3.
Effects of preincubation with MLB1, MLB2, MLB3, HAstV1, and RANK on MLB1 ELISA. Human serum samples were preincubated with each protein individually prior to the ELISA. The positive controls were polyclonal MLB1 peptide antibody (DW60) from each plate.
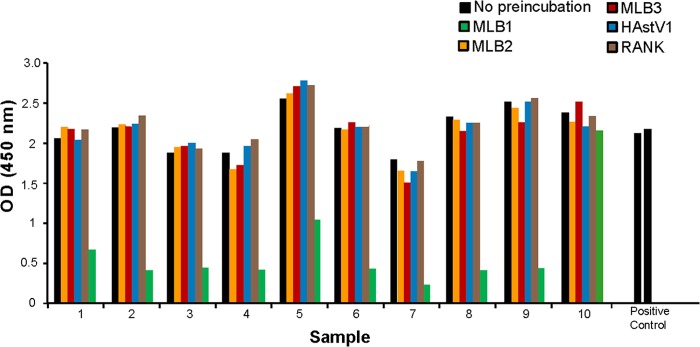
We also attempted to define the seroprevalence of MLB2 by using a parallel ELISA approach with MLB2 capsid as the target antigen. A total of 89.4% of the cohort was positive in this assay. However, preincubation experiments done on 20 samples (10 arbitrarily chosen among samples with high-intensity signal [OD of >2.400] and 10 randomly selected from the cohort) demonstrated significant levels of cross-reaction with MLB1 (50% of samples) and MLB3 (20% of samples), thus precluding definitive interpretation of these results (Fig. 4). This type of one-way cross-reactivity has been described for other viruses (13). We did not evaluate MLB3 seropositivity in an ELISA since MLB3 and MLB2 share 89% amino acid identity in the capsid region. In the future, the development of more specific serologic assays, such as a neutralization assay or the use of monoclonal antibodies will be needed to explicitly define the levels of MLB2 and MLB3 seropositivity and to determine whether these are distinct serotypes.
FIG 4.
Effects of preincubation with MLB1, MLB2, MLB3, HAstV1, and RANK on MLB2 ELISA. Samples were preincubated with each protein individually prior to the ELISA.
The potential role that the MLB clade astroviruses may play in human disease is currently unknown. These viruses might cause diarrhea like the canonical human astrovirus serotypes 1 to 8, although we cannot discount the possibility that they may have other pathogenic potential in the gastrointestinal (GI) tract or elsewhere. We recently described a febrile patient viremic with MLB2 (7), raising the possibility that MLB2 may be involved in fever; another implication of that study is that MLB2, and thus possibly other astroviruses as well, can clearly access cellular compartments outside the GI tract via the circulatory system. Indeed, a distinct novel astrovirus has been reported in brain tissue of an immunocompromised patient with encephalitis (14), and serologic analysis demonstrates that infection with this astrovirus is relatively common (15). Thus, the high rates of seropositivity against MLB1 observed in this study underscore the need to define potential disease states that may be linked to this common human-infecting virus.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health/National Center for Research Resources Washington University-ICTS (KL2 RR024994), National Institutes of Health (R21 AI090199), Children's Discovery Institute (MD-FR-2013-292) and Biomedical Foundation (S.S.-C.), and ALSAC (S.S.-C.). D.W. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
The authors have no conflicts of interest to report.
Footnotes
Published ahead of print 30 April 2014
REFERENCES
- 1.Koopmans MP, Bijen MH, Monroe SS, Vinje J. 1998. Age-stratified seroprevalence of neutralizing antibodies to astrovirus types 1 to 7 in humans in The Netherlands. Clin. Diagn. Lab. Immunol. 5:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkbeiner SR, Kirkwood CD, Wang D. 2008. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virol. J. 5:117. 10.1186/1743-422X-5-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkbeiner SR, Holtz LR, Jiang Y, Rajendran P, Franz CJ, Zhao G, Kang G, Wang D. 2009. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol. J. 6:161. 10.1186/1743-422X-6-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Holtz LR, Bauer I, Franz CJ, Zhao G, Bodhidatta L, Shrestha SK, Kang G, Wang D. 2013. Comparison of novel MLB-clade, VA-clade and classic human astroviruses highlights constrained evolution of the classic human astrovirus nonstructural genes. Virology 436:8–14. 10.1016/j.virol.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtz LR, Bauer IK, Rajendran P, Kang G, Wang D. 2011. Astrovirus MLB1 is not associated with diarrhea in a cohort of Indian children. PLoS One 6:e28647. 10.1371/journal.pone.0028647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Li Y, Jin Y, Li DD, Li X, Duan ZJ. 2013. Recently identified novel human astroviruses in children with diarrhea, china. Emerg. Infect. Dis. 19:1333–1335. 10.3201/eid1908.121863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtz LR, Wylie KM, Sodergren E, Jiang Y, Franz CJ, Weinstock GM, Storch GA, Wang D. 2011. Astrovirus MLB2 viremia in febrile child. Emerg. Infect. Dis. 17:2050–2052. 10.3201/eid1711.110496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser LA, Carter M, Schultz-Cherry S. 2007. Astrovirus increases epithelial barrier permeability independently of viral replication. J. Virol. 81:11937–11945. 10.1128/JVI.00942-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson CA, Warren JT, Wang MW, Teitelbaum SL, Fremont DH. 2012. RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor. Structure 20:1971–1982. 10.1016/j.str.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treanor JJ, Betts RF, Smith GE, Anderson EL, Hackett CS, Wilkinson BE, Belshe RB, Powers DC. 1996. Evaluation of a recombinant hemagglutinin expressed in insect cells as an influenza vaccine in young and elderly adults. J. Infect. Dis. 173:1467–1470. 10.1093/infdis/173.6.1467 [DOI] [PubMed] [Google Scholar]
- 11.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, Iacuzio D, Wolff M. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N. Engl. J. Med. 338:1405–1412. 10.1056/NEJM199805143382002 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NL, Le BM, Wang D. 2009. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg. Infect. Dis. 15:1199–1205. 10.3201/eid1508.090270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Che XY, Qiu LW, Liao ZY, Wang YD, Wen K, Pan YX, Hao W, Mei YB, Cheng VC, Yuen KY. 2005. Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J. Infect. Dis. 191:2033–2037. 10.1086/430355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan PL, Wagner TA, Briese T, Torgerson TR, Hornig M, Tashmukhamedova A, Firth C, Palacios G, Baisre-De-Leon A, Paddock CD, Hutchison SK, Egholm M, Zaki SR, Goldman JE, Ochs HD, Lipkin WI. 2010. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 16:918–925. 10.3201/eid1606.091536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burbelo PD, Ching KH, Esper F, Iadarola MJ, Delwart E, Lipkin WI, Kapoor A. 2011. Serological studies confirm the novel astrovirus HMOAstV-C as a highly prevalent human infectious agent. PLoS One 6:e22576. 10.1371/journal.pone.0022576 [DOI] [PMC free article] [PubMed] [Google Scholar]