Abstract
The immunological consequences of pregnancy-associated malaria (PAM) due to Plasmodium falciparum have been extensively investigated in cross-sectional studies conducted at delivery, but there have been very few longitudinal studies of changes due to PAM during pregnancy. We conducted a prospective study in Benin to investigate the changes associated with PAM in groups of 131 and 111 women at inclusion in the second trimester and at delivery, respectively. Infected women were identified by standard microscopic examinations of blood smears and by quantitative PCR (qPCR) assays and were matched to uninfected control women by age, gestational age, and gravidity. We quantified plasma levels of a panel of soluble immunological mediators and other mediators, as well as the frequencies of peripheral blood mononuclear cell types. Comparisons of these variables in infected and uninfected women used multivariate analyses, and we also assessed the predictive value of variables measured at inclusion for pregnancy outcomes at delivery. In multivariate analyses, peripheral plasma interleukin 10 (IL-10) and gamma interferon-inducible protein 10 (IP-10) levels were associated with PAM at inclusion and at delivery, while higher IL-10 levels distinguished qPCR-detectable submicroscopic infections at inclusion but not at delivery. Maternal anemia at delivery was associated with markers of proinflammatory (increased frequency of monocytes) and anti-inflammatory (increased IL-10 levels and increased activation of regulatory T cells) activity measured at inclusion. Elevated concentrations of IL-10 are associated with the majority of P. falciparum infections during pregnancy, but this marker alone does not identify all submicroscopic infections. Reliably identifying such occult infections will require more sensitive and specific methods.
INTRODUCTION
The concept that pregnancy is associated with immune suppression for fetal allograft tolerance has been well described (1–3). In healthy pregnancies, components of the cellular arm of the immune response have been shown to populate the human decidua during the first trimester and are necessary for placental development (4, 5). In areas where Plasmodium falciparum is endemic, pregnancy (especially the first pregnancy) is associated with increased susceptibility to infection with this parasitic protozoan. Sequestration of P. falciparum-infected erythrocytes (PfiEs) in the placenta, via their adherence to chondroitin sulfate A (CSA) expressed by syncytiotrophoblasts, is the major pathological characteristic of pregnancy-associated malaria (PAM), with potentially multiple detrimental outcomes (6). P. falciparum-infected erythrocytes induce inflammation, characterized particularly by monocytic infiltration of the infected placenta (7–10). One other well-described consequence of PAM is an altered cytokine/chemokine balance in peripheral blood and placental blood (11–17).
We recently showed that P. falciparum infection during pregnancy is associated with modified peripheral blood mononuclear cell (PBMC) profiles, consistently characterized by enhanced B cell activity (18), a finding consistent with the prominent B cell component of the inflammatory response to PfiEs in the placenta reported by others (19). B cells are recognized as having multiple functions, not only as antigen-presenting cells and antibody producers but also as regulators of dendritic cell (DC) function and maturation through their production of the cytokine interleukin 10 (IL-10) (20, 21). The polyclonal activation of B cells is a well-recognized feature of infection with P. falciparum that, in the case of PAM, may be linked to the demonstration of nonspecific binding of IgM antibodies to the parasite-derived ligand for CSA expressed on PfiEs (22, 23).
The concentrations of several molecules, including the cytokines IL-10, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) and the chemokines IFN-γ-inducible protein 10 (IP-10) and monokine induced by IFN-γ (MIG), have been shown in several studies to be increased in placental plasma at delivery in women with PAM (13, 14, 24). Here we used samples collected both during pregnancy and at delivery, including samples from women with submicroscopic infections, to examine in more detail the association between plasma cytokine/chemokine concentrations and PAM. We also wished to determine whether the PBMC profiles determined in the same samples retained their predictive value for infection when analyzed in parallel, following the hypothesis that both cytokines/chemokines and cells are potentially implicated in susceptibility to and protection from PAM.
(This work was presented at the 3rd Symposium of Infectious Diseases in Africa and 4th African Flow Cytometry Workshop: Measurement of Immune Responses, Cape Town, South Africa, 12 to 18 November, 2011.)
MATERIALS AND METHODS
Ethics statement.
The study described here received ethical clearance from the ethics committee of the Health Science Faculty of the University of Abomey-Calavi (Cotonou, Benin).
Study population.
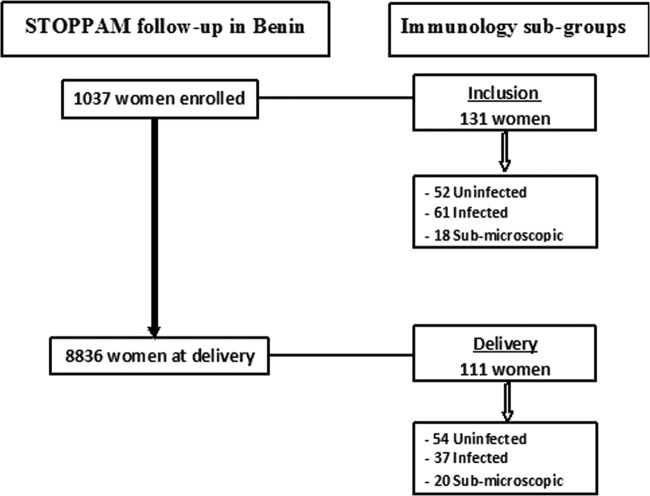
The study populations included subgroups drawn from the cohort of pregnant Beninese women who participated in a longitudinal study known as the Strategies to Prevent Pregnancy-Associated Malaria (STOPPAM) study. The STOPPAM study was conducted in parallel at two study sites in Benin and Tanzania. After giving written informed consent, ∼1,000 pregnant women at gestational ages of ≤24 weeks were included in the district of Comé, located in the Mono province 70 km west of Cotonou, the economic capital of Benin. The STOPPAM study design in Benin has been described in detail elsewhere (25), as has the procedure used to select women for the immunological substudy described here (18). First, at inclusion in the study, we selected a subgroup of 131 women who were identified as being infected with P. falciparum (for methods, see below) and an uninfected control group appropriately matched for age, gravidity, and gestational age. Second, at delivery, we identified a subgroup of 111 women with varying infection histories during pregnancy, as determined from the results of our own active and passive surveillance. The latter subgroup included women with no evidence of infection from inclusion through delivery (“uninfected”) and women with infections at delivery who might or might not have been infected earlier (“infected”). The women selected for inclusion in the subgroups were not the same at the two time points.
Sample collection.
Venous blood samples, collected in Vacutainer tubes with citrate phosphate dextrose adenine (CPDA) anticoagulant, were transported to the research laboratories within 4 h. Plasma samples were separated and stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were isolated using Leucosep tubes (Greiner-Bio), according to the manufacturer's instructions, and were subsequently used for immunophenotyping as described previously (18).
Detection of P. falciparum infections.
Infection status was determined using a rapid diagnostic test (RDT) (Parascreen; Zephyr Biomedical Systems). Thin and thick smears of peripheral and placental intervillous blood, to identify plasmodial parasites, were prepared using standard methods and were double-read retrospectively in routine microscopic examinations performed by two experienced technicians. Quantitative PCR (qPCR) was performed after DNA extraction from blood spots on filter paper using the Chelex method, as described elsewhere (26).
Women identified as infected had positive PCR results and had parasites detected in thin and thick smears of peripheral and/or placental blood, while uninfected women had negative results for both PCR assays and microscopic examinations of thin and thick smears. Women with a positive PCR result but a negative blood smear result constituted a separate group identified as having submicroscopic infections. In only one case was a sample defined as negative by PCR but positive by microscopy.
Cytometric bead arrays for quantitation of cytokines and chemokines.
Plasma levels of IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α cytokines were analyzed using a human inflammatory kit (cytometric bead assay [CBA]; BD Biosciences, San Diego, CA, USA), and levels of regulated on activation normal T cell expressed and secreted (RANTES), monokine induced by IFN-γ (MIG), monocyte chemotactic protein 1 (MCP-1), and IFN-γ-inducible protein 10 (IP-10) were analyzed with a human chemokines kit (CBA; BD Biosciences). The procedure used adhered to the manufacturer's recommendations and has been described in detail elsewhere (17).
ELISAs.
Plasma concentrations of alpha interferon (IFN-α) and gamma interferon (IFN-γ) were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (Mabtech, Stockholm, Sweden), as were those of soluble vascular endothelial growth factor receptor 1 (sVEGF-R1)/Flt1, soluble urokinase plasminogen activator receptor (suPAR), angiopoietin 1 (Ang-1), and Ang-2 (R&D, Minneapolis, MN). The methods used conformed to the manufacturer's recommendations and have been described in detail elsewhere (17).
Peripheral blood mononuclear cell immunophenotyping.
PBMCs were washed and resuspended at a concentration of 10 million cells/ml. Cells were then labeled using a panel of surface and intracellular marker-specific monoclonal antibodies, as described elsewhere (18). Human anti-blood dendritic cell antigen 1 (BDCA-1)-phycoerythrin (PE) was used for myeloid dendritic cell (mDC) detection and anti-BDCA-2-PE for plasmacytoid DC (pDC) detection (all from Miltenyi Biotec). Anti-CD14-fluorescein isothiocyanate (FITC) was used for detection of monocytes, anti-CD19-FITC for detection of B cells, and the combination of anti-HLA-DR-peridinin chlorophyll protein (PerCP) and anti-CD86-allophycocyanin (APC) (all from BD Pharmingen, San Diego, CA) for assessment of the activation status of cells. Human anti-CD3-APC, anti-CD8-FITC, anti-CD4-PerCP, and anti-CD56-PE (BD Pharmingen) were used for T lymphocyte and natural killer (NK) cell labeling, while anti-CD25-FITC and anti-CD127-PE (BD Pharmingen) were used for regulatory T (Treg) cell labeling. Human anti-FoxP3-APC (BD Pharmingen) was added for Treg cell labeling, according to the manufacturer's recommendations, after permeabilization and fixation with PermFix (BD Pharmingen). Events were recorded using a BD FACSCalibur system, and data were analyzed using CellQuest Pro or FlowJo 7.6 software (TreeStar, Ashland, OR, USA).
Data analysis.
Data analysis was performed using Stata/MP 12.0 (StataCorp, College Station, TX, USA) and GraphPad Prism version 5.00 for Windows (GraphPad, San Diego, CA, USA). Data are expressed as medians with interquartile ranges (IQRs). At both time points, data from study participants with missing values were excluded, with consequent reductions in the numbers of matched pairs remaining, and the use of qPCR assays for parasite detection resulted in data for purportedly uninfected individuals being moved to the infected group, again disrupting the originally defined pair matching. Initial univariate comparisons between groups were therefore conducted using nonparametric Kruskal-Wallis and Mann-Whitney tests, rather than pairwise analyses. Categorical variables were compared with χ2 tests. Multiple logistic regressions were performed using logit commands in Stata, in order to identify factors associated with the risk of P. falciparum infections in pregnant women at inclusion and at delivery. Segregation of values into low and high categories used the median values of the corresponding variables for the uninfected groups as references. In order to assess whether any of the cytokines, chemokines, or other factors measured were independently associated with the risk of anemia (defined as a concentration of hemoglobin below 11 g/dl) or with particular PBMC profiles during pregnancy, multiple logistic regressions were used. A stepwise procedure was performed to select a model including factors associated with infection. Prospective analysis of the associations between factors measured at inclusion and maternal anemia identified at delivery was also conducted using multiple logistic regressions with data collected at inclusion. Two-tailed P values of <0.05 were considered significant.
RESULTS
Detection of P. falciparum infections.
The study described here included subgroups of Beninese women participating in the STOPPAM study. These subgroups were shown previously not to differ in demographic characteristics from the whole cohort (18). Here we wished to determine specifically whether and how circulating plasma levels of cytokines and chemokines during pregnancy might be associated with peripheral blood cell profiles in the same women and/or with the presence or absence of infection with P. falciparum. Since such infections may remain below the level of detection of the routinely used microscopic method of diagnosis, here we performed additional analyses using a sensitive, species-specific, PCR-based method to identify “occult” infections. The latter were detected in a total of 38/242 women. In a subgroup of 131 women assessed at inclusion, the majority of whom were in the second trimester of pregnancy, 52 were uninfected, 61 had infections identified by microscopy, and 18 had submicroscopic infections identified by PCR; among 111 women assessed at delivery, 54 were uninfected, 37 had infections detected by microscopy, and 20 had submicroscopic infections (Table 1 and Fig. 1). In the subgroup of women assessed at inclusion, those with infections detectable by microscopy were significantly younger and significantly less likely to possess a bednet, and a significantly greater proportion were anemic, compared with women who were uninfected (Table 1). Of note, the same variables in those harboring submicroscopic infections at inclusion did not differ significantly with respect to either of the other two groups, while there were no differences in any variable among all of the groups in the subgroup assessed at delivery.
TABLE 1.
Characteristics of study subjects
| Variable | At inclusion |
At delivery |
||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected (n = 52) | Submicroscopic (n = 18) | Infected (n = 61) | Pa | Uninfected (n = 54) | Submicroscopic (n = 20) | Infected (n = 37) | Pa | |
| Age in yrs (median [IQR]) | 25.5 (8.0)b | 23.5 (7.0) | 22.0 (8.0)b | 0.047 | 27 (9) | 25 (5.5) | 25 (10) | 0.41 |
| Primigravid (%) | 30.7 | 27.8 | 26.2 | 0.86 | 12.9 | 10.0 | 24.32 | 0.24 |
| Gestational age in wks (median [IQR]) | 18.5 (4.6) | 16.9 (4.8) | 17.4 (5.1) | 0.12 | 39.6 (1.3) | 40.1 (1.4) | 39.4 (2.3) | 0.36 |
| Possessing bednet (%) | 36.5c | 33.3 | 19.6c | 0.12 | 38.9 | 30.0 | 29.7 | 0.60 |
| Hemoglobin level of <11 g/dl (%) | 50.0d | 66.6 | 81.6d | 0.002 | 31.5 | 35.0 | 45.9 | 0.36 |
| No. of parasites/μl by qPCR (median [IQR]) | 0 | 52.0 (210.7) | 714.6 (2,981.4) | 0 | 5.3 (105.8) | 4,119.8 (24,088.9) | ||
Kruskal-Wallis and χ2 tests.
Mann-Whitney test, P = 0.015.
χ2 test, P = 0.045.
χ2 test, P < 0.001.
FIG 1.
Study profile.
Circulating levels of cytokines and chemokines associated with P. falciparum infections at inclusion and at delivery.
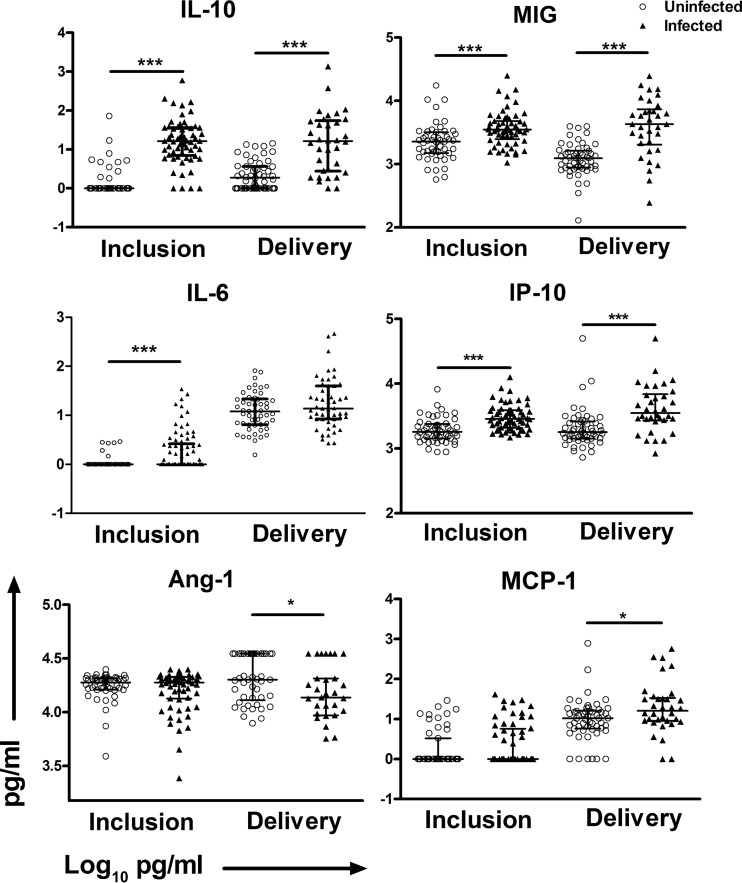
There are few published studies concerning the effects of submicroscopic infections during pregnancy and their potential association with poor pregnancy outcomes (27, 28). Therefore, we first addressed the changes in the levels of circulating cytokines and chemokines as a function of the women's infection status. At inclusion, there were significantly higher levels of IL-10 in women with submicroscopic infections than in uninfected women, but otherwise there were no differences (see Table S1 in the supplemental material). This evidence, albeit limited, of modified immune status in women with submicroscopic infections led us to combine them with the group of women harboring microscopically detectable infections, as one group, in all subsequent analyses. Univariate analyses revealed significantly increased levels of IL-6, IL-10, MIG, and IP-10 at inclusion among the infected women, in comparison with the uninfected women, while significantly higher levels of IL-10, MIG, MCP-1, and IP-10 and lower levels of Ang-1 were observed in infected versus uninfected women at delivery (Table 2 and Fig. 2).
TABLE 2.
Plasma cytokine and chemokine concentrations at inclusion and at delivery
| Variable | Concn in pg/ml (median [IQR]) at inclusion |
Concn in pg/ml (median [IQR]) at delivery |
||||
|---|---|---|---|---|---|---|
| Uninfected (n = 52) | Infected (n = 79) | Pa | Uninfected (n = 54) | Infected (n = 57) | Pa | |
| Il-1β | 1.0 (0.0) | 1.0 (0.0) | 0.91 | 1.0 (0.0) | 1.0 (0.5) | 0.32 |
| IL-6 | 1.0 (0.0) | 1.0 (1.5) | 0.002 | 12.1 (15.0) | 13.8 (31.5) | 0.15 |
| IL-8 | 9.6 (12.2) | 11.8 (17.0) | 0.24 | 114.0 (423.2) | 99.73 (40.7) | 0.81 |
| IL-10 | 1.0 (0.0) | 12.3 (19.9) | <0.0001 | 1.8 (2.6) | 6.0 (26.3) | <0.0001 |
| IL-12p70 | 1.0 (0.0) | 1.0 (0.0) | 0.59 | 1.0 (0.0) | 1.0 (0.0) | 0.11 |
| TNF-α | 1.0 (0.0) | 1.0 (0.0) | 0.71 | 1.0 (0.0) | 1.0 (0.0) | 0.27 |
| RANTES | 58,383 (61,130) | 63,393 (67,573) | 0.84 | 64,620 (63,760) | 51,957 (55,947) | 0.19 |
| MIG | 2,272 (1,701) | 3,494 (2,618) | 0.0001 | 124 (746) | 2,649 (4,813) | 0.0001 |
| MCP-1 | 1 (2.3) | 1 (3.8) | 0.38 | 10.4 (10.0) | 13.1 (24.3) | 0.029 |
| IP-10 | 1,806 (969) | 2,634 (1,803) | <0.0001 | 1,790 (1,165) | 2,871 (3,423) | 0.0010 |
| Ang-1 | 18,844 (4,459) | 19,190 (6,183) | 0.77 | 20,005 (21,454) | 14,853 (25,661) | 0.050 |
| suPAR | 2,047 (754) | 2,150 (937) | 0.36 | 2,014 (1,215) | 2,562 (1,829) | 0.17 |
| IFN-α | 3.5 (0.0) | 3.5 (0.0) | 0.50 | 3.5 (5.5) | 3.5 (5.8) | 0.69 |
| IFN-γ | 1.0 (0.0) | 1.0 (0.0) | 0.51 | 1.0 (6.5) | 1.0 (0.0) | 0.18 |
| sVEGF-R1/Flt1 | 748 (1,244) | 747 (1,347) | 0.80 | 4,547 (4,759) | 4,222 (5,851) | 0.91 |
Comparisons between groups used the nonparametric Mann-Whitney test.
FIG 2.
Plasma levels of IL-10, IL-6, Ang-1, MIG, IP-10, and MCP-1 with P. falciparum infections at inclusion and at delivery. The scatter plots include bars depicting the medians with interquartile ranges of plasma levels of IL-10, IL-6, Ang-1, MIG, IP-10, and MCP-1 from 52 uninfected women and 79 infected women at inclusion and from 54 uninfected and 57 infected women at delivery. The statistical significance of differences between groups was determined using the nonparametric Mann-Whitney U test. *, P < 0.05; ***, P < 0.001.
P. falciparum infection-altered cellular profiles during pregnancy.
As we showed previously, P. falciparum infection is associated with altered peripheral blood mononuclear cell (PBMC) profiles at inclusion and at delivery in our study population (18). Here, PCR-based methods that identified women who were previously classified as uninfected but who actually harbored submicroscopic P. falciparum infections were used to determine the effects of such infections, if any, on PBMC profiles at inclusion and at delivery, as for the cytokines/chemokines described above. For the full range of variables analyzed, we found no significant differences at either time point between women with submicroscopic infections and either of the other two groups (see Table S2 in the supplemental material).
In univariate analyses at inclusion, the frequency of mDCs expressing HLA-DR in infected versus uninfected women was significantly lower, while there were nonsignificant trends for (i) more cells with a high expression level of CD86 (CD86hi B cells), (ii) more effector T (Teff) cells (CD4+ CD25+ CD127+) (and consequently a lower Treg cell [CD4+ CD25+ CD127−]/Teff cell ratio), and (iii) fewer NK cells (CD56+) in infected women (Table 3). Similar analyses at delivery revealed significantly higher frequencies of both Teff cells and CD86hi B cells in infected women but significantly lower frequencies of pDCs (BDCA-1+) and NK T cells (CD3+ CD56+) than in uninfected women, as described previously (Table 3).
TABLE 3.
Peripheral blood cellular profiles at inclusion and at delivery
| Variable | Median (IQR) at inclusion |
Median (IQR) at delivery |
||||
|---|---|---|---|---|---|---|
| Uninfected (n = 52) | Infected (n = 79) | Pa | Uninfected (n = 54) | Infected (n = 57) | Pa | |
| Monocytes (%) | 7.6 (7.7) | 8.9 (5.5) | 0.47 | 9.4 (7.4) | 9.8 (7.4) | 0.70 |
| B cells (%) | 12.5 (6.5) | 13.2 (6.3) | 0.13 | 11.8 (4.9) | 12.0 (7.0) | 0.53 |
| mDCs (%) | 0.3 (0.2) | 0.3 (0.2) | 0.88 | 0.28 (0.1) | 0.2 (0.2) | 0.26 |
| pDCs (%) | 0.41 (0.3) | 0.4 (0.3) | 0.49 | 0.3 (0.3) | 0.2 (0.3) | 0.040 |
| CD86+ monocytes (%) | 7.0 (3.8) | 6.1 (4.1) | 0.58 | 5.05 (3.2) | 5.4 (4.4) | 0.89 |
| CD86+ B cells (%) | 0.9 (0.9) | 1.1 (1.0) | 0.072 | 0.93 (0.8) | 1.3 (1.5) | 0.025 |
| CD86+ mDCs (%) | 0.8 (1.3) | 0.8 (1.4) | 0.69 | 1.3 (2.2) | 1.2 (2.2) | 0.58 |
| CD86+ pDCs (%) | 0.8 (1.3) | 1.1 (1.1) | 0.27 | 1.1 (1.2) | 1.3 (1.6) | 0.45 |
| HLA-DR+ monocytes (%) | 39.2 (20.3) | 34.0 (20.7) | 0.20 | 32. 8 (24.1) | 35.2 (32.5) | 0.42 |
| HLA-DR+ B cells (%) | 56.2 (20.3) | 56.2 (26.1) | 0.82 | 62.6 (30.7) | 54.2 (39.8) | 0.42 |
| HLA-DR+ mDCs (%) | 148.6 (49.1) | 125.2 (67.4) | 0.004 | 139.2 (74.3) | 121.8 (94.5) | 0.40 |
| HLA-DR+ pDCs (%) | 54.2 (31.7) | 52.3 (31.6) | 0.75 | 40.6 (31.5) | 48.6 (30.9) | 0.23 |
| Treg cells (%) | 3.8 (2.05) | 3.6 (2.1) | 0.30 | 3.6 (2.4) | 3.6 (2.1) | 0.92 |
| Teff cells (%) | 4.0 (5.2) | 5.1 (6.5) | 0.07 | 1.6 (2.0) | 2.6 (4.02) | 0.002 |
| Treg cell/Teff cell ratio | 0.9 (1.8) | 0.7 (1.1) | 0.06 | 2.3 (2.3) | 1.4 (1.4) | 0.001 |
| Activated Treg cells (%) | 2.3 (2.7) | 2.1 (3.0) | 0.54 | 1.6 (0.6) | 1.7 (0.8) | 0.12 |
| Activated Teff cells (%) | 1.2 (0.2) | 1.2 (0.41) | 0.57 | 1.3 (0.23) | 1.3 (0.4) | 0.78 |
| NK cells (%) | 4.8 (4.3) | 3.9 (3.4) | 0.052 | 5.3 (5.3) | 4.8 (5.4) | 0.14 |
| CD3+ cells (%) | 67.9 (14.6) | 68.1 (12.1) | 0.38 | 65.0 (11.7) | 62.2 (17.4) | 0.55 |
| NK T cells (%) | 1.4 (1.6) | 1.6 (1.6) | 0.77 | 2.4 (1.5) | 1.88 (2.0) | 0.034 |
| CD4+ cells (%) | 63.6 (10.4) | 63.6 (8.8) | 0.42 | 56.7 (12.7) | 58.9 (12.1) | 0.42 |
| CD8+ cells (%) | 27.4 (9.1) | 27.1 (8.3) | 0.88 | 31.8 (13.5) | 31.0 (9.2) | 0.40 |
| CD4+ cell/CD8+ cell ratio | 2.3 (1.2) | 2.3 (1.0) | 0.69 | 1.8 (1.2) | 1.9 (1.1) | 0.38 |
Comparisons between groups used the nonparametric Mann-Whitney test.
Multiple regression analyses of associations of cytokine levels, chemokine levels, and peripheral blood cell profiles with P. falciparum infections during pregnancy.
In order to determine the independent associations between PAM and the different cytokines, chemokines, and peripheral blood cell populations identified by univariate analyses, we next included all of the variables in multiple logistic regression analyses. The models tested also included the variables anemia, bednet possession, gravidity, and maternal ages at inclusion and at delivery. The model including cytokine and chemokine levels at inclusion showed an increased risk of infection with P. falciparum at delivery to be associated with elevated levels of IL-6, IL-10, and IP-10. The same model with variables at delivery showed that the risk of PAM increased with higher IL-10 and IP-10 levels, primigravidity, and younger age (data not shown). In the same model with cytokines replaced with PBMCs, anemia, primigravidity, maternal age below the median, and a higher frequency of CD86hi B cells were associated with PAM at inclusion. For PAM at delivery, the model showed that the risk was increased with a higher frequency of CD86hi B cells but also with fewer pDCs and NK cells and with a lower Treg cell/Teff cell ratio (data not shown).
The final model, which combined cytokines, chemokines, and PBMCs, showed that the risk of infection increased with higher levels of IL-6, IL-10, and IP-10 at inclusion (Table 4) and with higher levels of IL-10 and IP-10 and a lower Treg cell/Teff cell ratio at delivery (Table 5). The levels of the cytokines and chemokines included in the final model are correlated (see Tables S4 and S5 in the supplemental material). A discussion of the relevance of these correlations to the interpretation of the results of the analyses is presented below.
TABLE 4.
Multiple logistic regression analysis of independent associations between selected parameters and P. falciparum infections at inclusion
| Factor at inclusiona | Odds ratio (95% confidence interval) | Pb |
|---|---|---|
| IL-6 level above median | 3.72 (1.09–12.64) | 0.035 |
| IL-10 level above median | 17.29 (6.23–47.96) | <0.0001 |
| IP-10 level above median | 3.03 (1.01–9.12) | 0.048 |
| Anemia | 2.92 (0.98–8.73) | 0.054 |
The median value for the uninfected group was used for dichotomization.
Hosmer-Lemeshow test (4 degrees of freedom [df]), χ2 = 0.61; P = 0.96. The model included bednet possession, gravidity, and age.
TABLE 5.
Multiple logistic regression analysis of independent associations between selected parameters and P. falciparum infections at delivery
| Factor at deliverya | Odds ratio (95% confidence interval) | Pb |
|---|---|---|
| IL-10 level above median | 7.82 (2.38–25.67) | 0.001 |
| IP-10 level above median | 3.37 (1.11–10.22) | 0.032 |
| Treg cell/Teff cell ratio above median | 2.60 (0.87–7.80) | 0.086 |
The median value for the uninfected group was used for dichotomization.
Hosmer-Lemeshow test (6 df), χ2 = 9.75; P = 0.13. The model included bednet possession, gravidity, and age.
Factors predicting maternal anemia at delivery.
Various cytokines and chemokines have been proposed to have predictive values for the identification of infection with P. falciparum during pregnancy. Therefore, we were interested to know to what extent the different variables we measured at inclusion, including the PBMC profiles, might be predictive of maternal anemia, as one of the primary clinical outcomes identifiable at delivery. For this purpose, we included a range of factors measured at inclusion in multiple logistic regression analyses. Table 6 shows that higher levels of circulating IL-10, lower levels of IL-1β, and increased frequencies of monocytes and of activated Treg cells (FoxP3hi) detectable at inclusion were all associated with an increased risk of maternal anemia at delivery.
TABLE 6.
Predictive value for maternal anemia at delivery of factors measured at inclusion
| Factor at inclusion | Odds ratio (95% confidence interval) | Pa |
|---|---|---|
| IL-10 level above median | 5.3 (1.49–19.3) | 0.010 |
| IL-1β level above median | 0.05 (0.004–0.59) | 0.017 |
| HLA-DR+ B cell proportion above median | 3.0 (0.85–10.6) | 0.088 |
| Activated Treg cell proportion above median | 4.5 (1.19–16.7) | 0.026 |
| Monocyte proportion above median | 7.0 (1.86–26.0) | 0.004 |
Hosmer-Lemeshow test (7 df), χ2 = 4.35; P = 0.74.
DISCUSSION
Defining alterations in the profiles of soluble factors and cells in peripheral blood associated with P. falciparum infections in pregnant women serves multiple purposes. From a fundamental biological point of view, such information contributes to our understanding of the pathophysiological effects of infection, particularly with respect to the host's response to it. Studies with a longitudinal design, as was the case in our STOPPAM project, are beginning to broaden our knowledge of the immunological components of that response. From a translational viewpoint, such studies can reveal potentially novel ways of detecting PAM events that might otherwise go unidentified, thus helping to clarify issues such as the timing of occurrence of infections and the need to adjust or to improve existing preventive measures.
In a previously published study of women in the Tanzanian cohort of the STOPPAM project, we revealed that the levels of most of the plasma-derived factors we studied here were stable during pregnancy, regardless of the women's P. falciparum-specific infection status (17). In the Beninese cohort assessed here, we focused on assessments of samples from only two time points, i.e., inclusion and delivery, for a larger number of women, rather than samples from multiple consecutive time points for a comparatively smaller number of women, as in the study of the Tanzanian cohort. Importantly, in this regard, we know from our own parasite genotypic data (N. Tuikue Ndam, unpublished observations) that the overwhelming majority of P. falciparum infections detected at delivery in the Beninese cohort were acute, i.e., they had been acquired within 4 weeks of delivery. Our own work, as well as others' work, has shown that infections with P. falciparum during pregnancy are associated with increased levels of cytokines and chemokines such as TNF-α, IFN-γ, IL-10, MCP-1, and IP-10 in peripheral or placental plasma (11, 13, 15, 17, 29, 30). Studies of the placenta have revealed inflammatory immune infiltrates that are likely a direct reflection of the altered cytokine/chemokine profiles, although causality in this respect is difficult to prove (7–10, 19). The study we present here represents an attempt to address this issue by combining assessments of soluble peripheral blood plasma-borne factors with assessments of peripheral blood cellular profiles for infected and uninfected pregnant women. One potential limitation of the study concerns the fact that the women in the different subgroups were not the same at inclusion and at delivery, which precludes the possibility of pairwise comparisons between the two time points. It should be noted in this context that, in such a study and in such a setting, optimal study design and conduct are not always realistic practical possibilities, and compromises often must be sought.
In one important respect, our analyses here differ from those we conducted with data from the Tanzanian arm of the STOPPAM study (18). That difference concerns the identification of submicroscopic P. falciparum infections that we performed here. We and others have consistently shown that IL-10 is present at higher concentrations in the plasma of infected women (31). In this context, it is perhaps not surprising that we found significantly increased levels of IL-10 in women with submicroscopic infections, in comparison with uninfected women, but this finding held only for comparisons between the groups at inclusion and not between those at delivery. We conjecture that this is a reflection of the fact that the infections present at delivery, as outlined above, were recently acquired and therefore pathological inflammatory changes in the placenta were not as well established as would be expected in what we assume to be the persistent (chronic) infections detected at inclusion. For delivery samples, this conjecture is supported by the lack of malarial pigment (hemozoin)-containing cells in the placental blood of women with placental infections in the current study (N. Fievet, unpublished observations). We speculate that the presence of submicroscopic parasitemia at delivery may thus reflect the most recently acquired infections, with the least detectable changes in terms of soluble blood-borne factors. In the same context, the significantly elevated levels of IL-10 in women with submicroscopic infections at inclusion, in the absence of changes in any other parameter we measured, could be an indication of the fact that such infections were persistent in nature, with a more chronic evolution. Separately, the lack of any significant increase in the levels of TNF-α in the peripheral plasma of women with P. falciparum infections of any density and at either time point is consistent with our own data from Tanzania (17) and with data in a study from Ghana (31) but not with data in a study from Tanzania (24), although it should be noted that the latter study assessed only samples taken at delivery.
The pattern observed with submicroscopic infections aside, the combination of increased levels of IL-10 and IP-10 associated with infections during pregnancy that we report here after multivariate analyses is again consistent with our data from Tanzania (17). Notably, all of these data are derived from samples taken from women without any symptoms of malaria, and the findings thus serve to emphasize the fact that infections with P. falciparum that, for the most part, are subclinical do have measurable effects on some recognized markers of PAM, although their specificity requires further verification. The increased peripheral blood plasma concentrations of IL-6 associated with P. falciparum infections at inclusion we report here also echo our findings for the Tanzanian cohort of the STOPPAM project (17), although in the latter case this association did not survive multivariate analyses. With respect to our findings concerning altered cellular profiles associated with PAM, our earlier report of increased frequencies of activated B cells (CD86hi), regardless of the timing of the infection, was confirmed here. It is important to note the fact that, in the multivariate analyses we performed, none of the cellular profiles was shown to have independent associations with infection when combined with cytokine/chemokine profiles, suggesting that the latter likely represent the most robust markers among the broad panel that we measured here. It is also important to note that the levels of IL-6, IL-10, and IP-10 retained in the final multivariate models were all highly correlated with each other (see Tables S4 and S5 in the supplemental material). Nevertheless, the fact that IL-10 was the variable with by far the strongest association with infection at both time points is noteworthy, perhaps reflecting the need to optimally control placental inflammation to ensure survival of the fetus.
In the context of their predictive value for maternal anemia at delivery, the variables measured at inclusion that we identified here included, as in our earlier study (18), increased frequencies of both monocytes and HLA-DR-expressing B cells. In addition, here we identified women with significantly higher plasma levels of IL-10 and with higher frequencies of activated Treg cells at inclusion as having a higher risk of anemia at delivery. Although these are independent associations, they may reflect a common cause in the sense that activated Treg cells could themselves be a source of IL-10. Such anti-inflammatory activity may in itself reflect a response to the proinflammatory activity represented by the elevated numbers of monocytes, but exactly how, mechanistically, the ensemble of these factors may lead to a heightened risk of anemia later in pregnancy must remain in the realm of speculation. Infections that arose between inclusion and delivery likely played a role in this context, but this aspect requires further study. It is also entirely unclear to us how the independent association of a lower risk of anemia at delivery with significantly lower plasma concentrations of the cytokine IL-1β at inclusion might be explained. In any case, it is noteworthy that the levels of IL-1β we detected at either time point were very low, frequently at the limit of detection of the assay employed, and thus any interpretation of these findings must necessarily be done with great caution.
In conclusion, the findings we report here add to our knowledge of the pathophysiological changes associated with P. falciparum infections associated with pregnancy. Overall, they strengthen the evidence base for the utility of the concentrations of circulating plasma-borne cytokines (IL-10) and chemokines (IP-10) as biomarkers of such infections, but a reliable specific means of detection of occult submicroscopic infections remains problematic.
Supplementary Material
ACKNOWLEDGMENTS
This paper describes work undertaken in the context of the STOPPAM project. The STOPPAM study is a Small and Medium Scale Collaborative Project supported by the European 7th Framework Programme (contract no. 200889). This publication was made possible through support provided by the AIRD-DPF and Bourse Doctorale de Mobilité Universitaire 2011 of Université Paris Descartes (Paris, France) (Ph.D. research support for S.A.I.).
We are extremely grateful to all of the women who participated in the study. In Benin, we especially thank all of the field and administration staff members of Akodeha, Comé Central, and Ouedèmé Pedah health centers for their contributions. We particularly thank Jacqueline Affedjou, Jean-Claude Sagbo, Bernadette Gandonou, and Gildas Gbaguidi for their hard work. We are also grateful to Sophie Borgella and Parfait Houngbegnon for database management. In Stockholm, we thank Margareta Hagstedt for laboratory contributions. The help of Franck Noumbissié, Thor Theander, and Nicaise Tuikue Ndam in contributing to the design of the study and in providing fruitful comments on the manuscript is much appreciated.
S.A.I., N.T.N., B.T.H., A.M., P.D., M.T.B., N.F., and A.J.F.L. conceived, designed, and coordinated the study. S.A.I., S.B., B.V., C.A., and M.A.Z. participated in the sample collection and processing. S.A.I., S.B., M.T.B., N.F., and A.J.F.L. designed and supervised the immunoassays. S.A.I. and L.B. performed statistical analyses. S.A.I., S.B., B.V., and C.A. carried out the immunoassays. S.A.I. and S.B. drafted the first version of the manuscript. All authors read and approved the final manuscript.
We have no conflicts of interest. The funding sources had no involvement in the design, collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to publish.
Footnotes
Published ahead of print 9 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00009-14.
REFERENCES
- 1.Chaouat G, Cayol V, Mairovitz V, Dubanchet S. 1999. Localization of the Th2 cytokines IL-3, IL-4, IL-10 at the fetomaternal interface during human and murine pregnancy and lack of requirement for Fas/Fas ligand interaction for a successful allogeneic pregnancy. Am. J. Reprod. Immunol. 42:1–13. 10.1111/j.1600-0897.1999.tb00459.x [DOI] [PubMed] [Google Scholar]
- 2.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. 1996. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-γ response and increased production of T helper 2 cytokines. J. Immunol. 156:644–652 [PubMed] [Google Scholar]
- 3.Wegmann TG, Lin H, Guilbert L, Mosmann TR. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14:353–356. 10.1016/0167-5699(93)90235-D [DOI] [PubMed] [Google Scholar]
- 4.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. 2004. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10:347–353. 10.1093/molehr/gah044 [DOI] [PubMed] [Google Scholar]
- 5.Saito S, Shima T, Inada K, Nakashima A. 2013. Which types of regulatory T cells play important roles in implantation and pregnancy maintenance? Am. J. Reprod. Immunol. 69:340–345. 10.1111/aji.12101 [DOI] [PubMed] [Google Scholar]
- 6.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. 2010. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 7:e1000221. 10.1371/journal.pmed.1000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diouf I, Fievet N, Doucoure S, Ngom M, Andrieu M, Mathieu JF, Gaye A, Thiaw OT, Deloron P. 2007. IL-12 producing monocytes and IFN-γ and TNF-α producing T-lymphocytes are increased in placentas infected by Plasmodium falciparum. J. Reprod. Immunol. 74:152–162. 10.1016/j.jri.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 8.Mens PF, Bojtor EC, Schallig HD. 2010. Molecular interactions in the placenta during malaria infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 152:126–132. 10.1016/j.ejogrb.2010.05.013 [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68:115–119 [PubMed] [Google Scholar]
- 10.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. 1998. Massive chronic intervillositis of the placenta associated with malaria infection. Am. J. Surg. Pathol. 22:1006–1011. 10.1097/00000478-199808000-00011 [DOI] [PubMed] [Google Scholar]
- 11.Abrams ET, Brown H, Chensue SW, Turner GD, Tadesse E, Lema VM, Molyneux ME, Rochford R, Meshnick SR, Rogerson SJ. 2003. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated β chemokine expression. J. Immunol. 170:2759–2764 [DOI] [PubMed] [Google Scholar]
- 12.Fievet N, Moussa M, Tami G, Maubert B, Cot M, Deloron P, Chaouat G. 2001. Plasmodium falciparum induces a Th1/Th2 disequilibrium, favoring the Th1-type pathway, in the human placenta. J. Infect. Dis. 183:1530–1534. 10.1086/320201 [DOI] [PubMed] [Google Scholar]
- 13.Fried M, Muga RO, Misore AO, Duffy PE. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-γ and TNF-α associated with pregnancy outcomes. J. Immunol. 160:2523–2530 [PubMed] [Google Scholar]
- 14.Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, Meshnick SR. 1999. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J. Infect. Dis. 180:1987–1993. 10.1086/315135 [DOI] [PubMed] [Google Scholar]
- 15.Rogerson SJ, Brown HC, Pollina E, Abrams ET, Tadesse E, Lema VM, Molyneux ME. 2003. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect. Immun. 71:267–270. 10.1128/IAI.71.1.267-270.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suguitan AL, Jr, Leke RG, Fouda G, Zhou A, Thuita L, Metenou S, Fogako J, Megnekou R, Taylor DW. 2003. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 188:1074–1082. 10.1086/378500 [DOI] [PubMed] [Google Scholar]
- 17.Bostrom S, Ibitokou S, Oesterholt M, Schmiegelow C, Persson JO, Minja D, Lusingu J, Lemnge M, Fievet N, Deloron P, Luty AJ, Troye-Blomberg M. 2012. Biomarkers of Plasmodium falciparum infection during pregnancy in women living in northeastern Tanzania. PLoS One 7:e48763. 10.1371/journal.pone.0048763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibitokou S, Oesterholt M, Brutus L, Borgella S, Agbowai C, Ezinmegnon S, Lusingu J, Schmiegelow C, Massougbodji A, Deloron P, Troye-Blomberg M, Varani S, Luty AJ, Fievet N. 2012. Peripheral blood cell signatures of Plasmodium falciparum infection during pregnancy. PLoS One 7:e49621. 10.1371/journal.pone.0049621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. 2007. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J. Immunol. 179:557–565 [DOI] [PubMed] [Google Scholar]
- 20.Morva A, Lemoine S, Achour A, Pers JO, Youinou P, Jamin C. 2012. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood 119:106–114. 10.1182/blood-2011-06-360768 [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R. 2007. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J. Exp. Med. 204:1107–1118. 10.1084/jem.20062013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L. 2011. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc. Natl. Acad. Sci. U. S. A. 108:12485–12490. 10.1073/pnas.1103708108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donati D, Zhang LP, Chene A, Chen Q, Flick K, Nystrom M, Wahlgren M, Bejarano MT. 2004. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect. Immun. 72:5412–5418. 10.1128/IAI.72.9.5412-5418.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. 2008. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar. J. 7:26. 10.1186/1475-2875-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh BT, Fievet N, Gbaguidi G, Dechavanne S, Borgella S, Guezo-Mevo B, Massougbodji A, Ndam NT, Deloron P, Cot M. 2011. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am. J. Trop. Med. Hyg. 85:214–220. 10.4269/ajtmh.2011.11-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diallo A, Ndam NT, Moussiliou A, Dos Santos S, Ndonky A, Borderon M, Oliveau S, Lalou R, Le Hesran JY. 2012. Asymptomatic carriage of plasmodium in urban Dakar: the risk of malaria should not be underestimated. PLoS One 7:e31100. 10.1371/journal.pone.0031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adegnika AA, Verweij JJ, Agnandji ST, Chai SK, Breitling LP, Ramharter M, Frolich M, Issifou S, Kremsner PG, Yazdanbakhsh M. 2006. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am. J. Trop. Med. Hyg. 75:798–803 [PubMed] [Google Scholar]
- 28.Malhotra I, Dent A, Mungai P, Muchiri E, King CL. 2005. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J. Clin. Microbiol. 43:3630–3635. 10.1128/JCM.43.8.3630-3635.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong S, Kurtis JD, Pond-Tor S, Kabyemela E, Duffy PE, Fried M. 2012. CXC ligand 9 response to malaria during pregnancy is associated with low-birth-weight deliveries. Infect. Immun. 80:3034–3038. 10.1128/IAI.00220-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suguitan AL, Jr, Cadigan TJ, Nguyen TA, Zhou A, Leke RJ, Metenou S, Thuita L, Megnekou R, Fogako J, Leke RG, Taylor DW. 2003. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 69:574–581 [PubMed] [Google Scholar]
- 31.Wilson NO, Bythwood T, Solomon W, Jolly P, Yatich N, Jiang Y, Shuaib F, Adjei AA, Anderson W, Stiles JK. 2010. Elevated levels of IL-10 and G-CSF associated with asymptomatic malaria in pregnant women. Infect. Dis. Obstet. Gynecol. 2010:pii=317430. 10.1155/2010/317430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.