Abstract
The Architect EBV antibody panel is a new chemiluminescence immunoassay system used to determine the stage of Epstein-Barr virus (EBV) infection based on the detection of IgM and IgG antibodies to viral capsid antigen (VCA) and IgG antibodies against Epstein-Barr nuclear antigen 1 (EBNA-1). We evaluated its diagnostic accuracy in immunocompetent adolescents and young adults with clinical suspicion of infectious mononucleosis (IM) using the RecomLine EBV IgM and IgG immunoblots as the reference standard. In addition, the use of the antibody panel in a sequential testing algorithm based on initial EBNA-1 IgG analysis was assessed for cost-effectiveness. Finally, we investigated the degree of cross-reactivity of the VCA IgM marker during other primary viral infections that may present with an EBV IM-like picture. High sensitivity (98.3% [95% confidence interval {CI}, 90.7 to 99.7%]) and specificity (94.2% [95% CI, 87.9 to 97.8%]) were found after testing 162 precharacterized archived serum samples. There was perfect agreement between the use of the antibody panel in sequential and parallel testing algorithms, but substantial cost savings (23%) were obtained with the sequential strategy. A high rate of reactive VCA IgM results was found in primary cytomegalovirus (CMV) infections (60.7%). In summary, the Architect EBV antibody panel performs satisfactorily in the investigation of EBV IM in immunocompetent adolescents and young adults, and the application of an EBNA-1 IgG-based sequential testing algorithm is cost-effective in this diagnostic setting. Concomitant testing for CMV is strongly recommended to aid in the interpretation of EBV serological patterns.
INTRODUCTION
Infectious mononucleosis (IM) is an acute syndrome typically characterized by fever, pharyngitis, lymphadenopathy, fatigue, and mononuclear leukocytosis (1). Primary infection with Epstein-Barr virus (EBV) is responsible for a majority of IM cases (2). EBV IM is mostly confined to adolescents and young adults living in higher socioeconomic strata of more economically developed countries, with a peak incidence in the age group of 15 to 24 years old (3). Although EBV IM is usually a self-limiting disease in immunocompetent individuals, accurate and prompt diagnosis is of utmost importance, as other conditions that require enhanced diagnostic procedures and/or expeditious clinical management, such as primary HIV infection, systemic lupus erythematosus, or lymphoma, can mimic its clinical presentation (4). EBV-specific serology is the method of choice for determining the stage of infection in immunocompetent individuals (5, 6). Its primary objective is to diagnose primary EBV infection in subjects presenting with suspected IM, but a reliable distinction between seronegativity and past infection is also desirable in order to identify those still at risk of infection or presenting at a very early stage of infection (7). The qualitative measurement of IgM and IgG antibodies to viral capsid antigen (VCA) and IgG antibodies against Epstein-Barr nuclear antigen-1 (EBNA-1) (8) is usually sufficient to establish patient EBV status using a single acute-phase sample. The combined interpretation of VCA IgM, VCA IgG, and EBNA-1 IgG results in eight possible serological patterns, only three of which are deemed to be clinically relevant (9): the presence of VCA IgM and IgG in the absence of EBNA-1 IgG strongly suggests a current or recent primary infection, the detection of VCA IgG and EBNA-1 IgG in the absence of VCA IgM is consistent with a past infection, thus excluding EBV IM, and seronegativity for all three antibodies usually indicates susceptibility to EBV infection. The remaining antibody profiles are considered inconclusive, and retesting the sample by other methodologies and/or testing further samples is required to achieve resolution. Currently, most diagnostic virology laboratories rely on chemiluminescence immunoassays (CLIAs) performed on automated platforms in order to ensure excellent analytical performance combined with high throughput and rapid turnaround times. The recently available Architect EBV antibody panel (Abbott, Wiesbaden, Germany) consists of three CLIAs for the detection of VCA IgM, VCA IgG, and EBNA-1 IgG antibodies in serum or plasma.
The primary objective of this pilot study was to evaluate the diagnostic accuracy of the Architect EBV antibody panel under routine laboratory conditions in immunocompetent adolescents and young adults with clinically suspected IM. A cost-benefit analysis comparing the performances of the three EBV antibody markers in sequential and parallel testing algorithms was also undertaken. A secondary goal was to assess the extent to which other acute viral infections known to exhibit similar clinical manifestations to EBV IM would generate reactive results in the Architect EBV VCA IgM assay.
MATERIALS AND METHODS
Study samples.
The evaluation panel totaled 223 acute-phase single serum samples analyzed for diagnostic purposes in our laboratory between January 2012 and October 2013. The samples were divided in two groups according to the type of study.
(i) Group I.
Group I samples (n = 163) were used to estimate the diagnostic accuracy of the Architect EBV antibody panel and to compare the cost-effectiveness of sequential and parallel testing algorithms. These samples derived from immunocompetent adolescents and young adults (mean age, 24 years; median age, 22 years; range, 8 to 49 years; female, 55%; male, 45%) who presented either to the general practitioner (65%) or the hospital (35%) with at least two clinical and/or laboratory findings suggestive of IM (pharyngitis, lymphadenopathy, fever, hepatomegaly, splenomegaly, absolute lymphocytosis [>4,500 cells/μl], relative lymphocytosis [>50%], presence of atypical lymphocytes, or increased alanine aminotransferase [>40 U/liter]). Group I samples were specified by classifying the available pool of eligible samples in three categories (i.e., seronegative, indicative of primary infection, and indicative of past infection) based on the results obtained in the Liaison EBV antibody panel (DiaSorin S.p.A, Saluggia, Italy), followed by random selection from each category until numerically homogeneous subgroups were formed. The etiological causes responsible for the IM clinical picture in patients with stages not compatible with primary infection were unknown for a majority of cases (101/104 [97.1%]); these samples mainly originated from individuals presenting to the general practitioner (85/104 [81.7%]) with a combination of fever, pharyngitis, and/or cervical lymphadenopathy for which a request for EBV serology was ordered prior to knowing the hematological results and in the absence of other virological or bacteriological investigations in serum or other specimens.
(ii) Group II.
Group II samples (n = 60) were used to assess the degree of cross-reactivity of the Architect VCA IgM assay in patients with well-characterized symptomatic primary cytomegalovirus (CMV) (n = 28), HIV-1 (n = 13), and parvovirus B19 (n = 19) infections. A diagnosis of primary viral infection was made by demonstrating IgG seroconversion (CMV, n = 1; parvovirus B19, n = 2), detecting low-avidity IgG antibodies in conjunction with IgM antibodies (CMV, n = 27) or high viral loads (HIV-1, n = 13), or measuring IgM antibodies in the presence of high viral loads (parvovirus B19, n = 17).
In addition to the evaluation panel, the PME202 EBV performance panel (SeraCare Life Sciences, Milford, MA, USA), which included 21 samples (seronegative, n = 1; primary infection, n = 6; past infection, n = 14) and several EBV (seronegative, n = 1; primary infection, n = 2; past infection, n = 4) and non-EBV (CMV IgM, n = 3; parvovirus B19, n = 2) proficiency samples from the United Kingdom National External Quality Assessment Service for Microbiology (UK NEQAS) scheme, were also tested.
Serological methods.
Patient samples were anonymized and recoded prior to starting the study. Before analysis, the samples, which had been stored at −20°C, with the number of freeze-thaw cycles limited to one, were thawed at ambient temperature, homogenized by vortexing (15 s), and centrifuged (12,000 rpm, 5 min) to sediment any particulate matter. The immunoassays were performed and interpreted according to the manufacturers' instructions as detailed in the product inserts. The study samples were tested once over a 3-week period by three different senior health care scientists to reproduce routine working conditions.
Diagnostic accuracy and cost-benefit analysis. (i) Reference standard.
The RecomLine EBV IgM and IgG line immunoassays (Mikrogen Diagnostik, Neuried, Germany) (10–12) were used in combination as the reference standard for establishing the stage of EBV infection. Each test consists of a nitrocellulose strip coated with lines of different recombinant EBV antigens (for IgM, p23, ZEBRA, p138, and p54, and for IgG, p72, p18, p23, BZLF1, p138, and p54) at precise concentrations. A brief description of the method is as follows: the strips were incubated with diluted patient serum/controls (Cosmos Biomedical Ltd., Derbyshire, United Kingdom) for 1 h so that EBV-specific antibodies would bind to their corresponding antigens. After three washes with buffer to remove the unreacted components, the strips were incubated for 45 min with an anti-human IgM or IgG horseradish peroxidase-labeled conjugate to detect the bound antibodies. The unbound conjugate was separated by three further washes with buffer, followed by the addition of the chromogen tetramethylbenzidine. Color development was allowed to proceed for 8 min before stopping the reaction by washing three times with distilled water. The strips were then air dried in darkness for 2 h prior to reading by two operators who were blinded to each other's results. The presence of serum and conjugate addition control bands was required prior to interpreting EBV-specific antibody reactivity. The banding pattern was established by comparing it to a template provided by the manufacturer (Table 1), taking into account that a band was considered positive only when its intensity was at least equal to that of a cutoff control band.
TABLE 1.
Determination of EBV infection stage using RecomLine IgM and IgG immunoblots
| Infection stage | IgM strip resultsa for: |
IgG strip results for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p23 | ZEBRA | p138 | p54 | EBNA-1 | p18 | p23 | BZLF1 | p138 | p54 | |
| Seronegative | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Primary infection | Pos/Neg | Pos | Pos/Neg | Pos/Neg | Neg | Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg |
| Pos/Neg | Pos/Neg | Pos | Pos/Neg | Neg | Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | |
| Pos/Neg | Pos/Neg | Pos/Neg | Pos | Neg | Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | |
| Past infection | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos | Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg |
| Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | Neg | Pos | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | |
| Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | Pos | Pos | Pos/Neg | Pos/Neg | Pos/Neg | Pos/Neg | |
| Indeterminate | Isolated antigen band in 1 strip | Isolated antigen band in 1 strip | Isolated antigen band in 1 strip | Isolated antigen band in 1 strip | Isolated antigen band in 1 strip | Isolated antigen band in 1 strip | Isolated antigen band in 1 strip | |||
Neg, negative; Pos, positive.
(ii) Index test.
The Architect EBV antibody panel for the Architect i2000SR analyzer was the index method. It is composed of VCA IgM, VCA IgG, and EBNA-1 IgG CLIAs of the indirect format, in which capture antigens are a p18 synthetic peptide for the VCA assays and a p72 synthetic peptide for the EBNA-1 IgG test. A brief description of the method is as follows: patient serum/controls, sample diluent, and antigen-coated paramagnetic particles were combined in a reaction vessel in which the binding of EBV-specific antibodies to capture antigens occurred; the VCA IgM assay included a pretreatment phase to neutralize the effects of rheumatoid factor (RF) and IgG antibodies. After a wash step, bound EBV-specific antibodies were detected by adding anti-human IgM or IgG acridinium-labeled conjugate. Following another wash step, the sequential addition of pretrigger (i.e., hydrogen peroxide) and trigger (i.e., sodium hydroxide) reagents generated a chemiluminescent signal measured in relative light units (RLUs) by a photomultiplier tube. The Architect i system calculates the ratio of the sample RLU to a cutoff RLU (S/CO) and produces a result according to predefined values for individual parameters (VCA IgM: negative, <0.50; equivocal, 0.50 to <1; positive, ≥1; VCA IgG: negative, <0.75; equivocal, 0.75 to <1; positive, ≥1; and EBNA-1 IgG: negative, <0.50; equivocal, 0.50 to <1; positive, ≥1). The EBV serological marker combinations were interpreted according to the manufacturer's recommendations (Table 2).
TABLE 2.
Determination of EBV infection stage using the Architect EBV antibody panel
| Infection stage | Results by antibody typea |
||
|---|---|---|---|
| VCA IgM | VCA IgG | EBNA-1 IgG | |
| Seronegative | Neg | Neg | Neg/Equ |
| Primary infection | Pos/Equ | Pos/Equ | Neg/Equ |
| Past infection | Neg/Equ | Pos/Equ | Pos |
| Suspected primary infectionb | Pos/Equ | Neg | Neg/Equ |
| Transient infectionb | Pos | Pos/Equ | Pos |
| Isolated VCA IgGb | Neg | Pos/Equ | Neg/Equ |
| Isolated EBNA-1 IgGb | Neg | Neg | Pos |
| Unresolvedb | Pos | Neg | Pos |
Neg, negative; Equ, equivocal; Pos, positive.
Inconclusive serological patterns.
Cross-reactivity.
No reference standard was available to verify the analytical specificity of the VCA IgM marker, as capture antigens in the RecomLine EBV IgM immunoblot differed from the one used in the Architect EBV VCA IgM assay. However, to mitigate this deficiency, testing for EBNA-1 IgG antibodies with two CLIAs, the Liaison EBNA IgG and the Architect EBV EBNA-1 IgG, was carried out in patients who tested VCA IgM positive in order to exclude a primary EBV infection. If both EBNA-1 IgG results were positive, the presence of VCA IgM was considered to be unrelated to primary EBV infection, whereas for negative or discordant EBNA-1 IgG results, the RecomLine EBV IgM and IgG assays were performed to investigate the possibility of a primary EBV infection.
Statistical analysis.
Diagnostic accuracy was calculated by comparing the index method (Architect EBV antibody panel) against the reference standard (RecomLine IgM and IgG assays). The MedCalc software (version 9.4; Mariakerke, Belgium) was used to estimate sensitivity and specificity with 95% confidence intervals. Sensitivity was determined as the proportion of primary infections that were correctly identified by the index test, whereas specificity referred to the percentage of nonprimary infections (i.e., seronegative and past infection) that were identified as such by the index test. Three sets of calculations were carried out to improve the understanding of the discriminatory performance of the index method, (i) inconclusive results were excluded (i.e., absence from numerators and denominators) to determine misclassifications occurring among samples displaying a noninconclusive serological profile (i.e., seronegative, primary infection, past infection), (ii) inconclusive results were included and considered the most adverse (i.e., absent from numerators, but present in denominators) to define the minimum expected values for sensitivity and specificity, and (iii) inconclusive results were included and considered the most favorable (i.e., present in numerators and denominators) to establish the maximum expected values for sensitivity and specificity. As the actual values for sensitivity and specificity most likely lie between the minimum and maximum expected values, we tried to refine the diagnostic accuracy estimation by assigning a likelihood ratio to the different types of inconclusive results. Although most of them have a likelihood ratio of around 1, an isolated VCA IgM result confers an increased probability toward a diagnosis of primary infection, as this serological pattern is compatible with a suspected primary infection. Taking this premise into account, a fourth sensitivity estimation, in which this profile was considered the most favorable, was performed. The sensitivity and specificity panels comprised 58 and 104 samples, respectively; assuming an anticipated sensitivity and specificity of 95%, this enables a precision of the sensitivity and the specificity as measured by a 95% confidence interval of within 10% and 5%, respectively.
The cross-reactivity study was treated as a descriptive analysis due to the insufficient sample size for each type of viral infection, and therefore no statistical analyses were conducted.
Ethical considerations.
Ethics approval was not required for this study, as deidentified remnant sera derived from samples collected for routine clinical care were used for kit evaluation purposes.
RESULTS
Diagnostic accuracy and cost-benefit analysis.
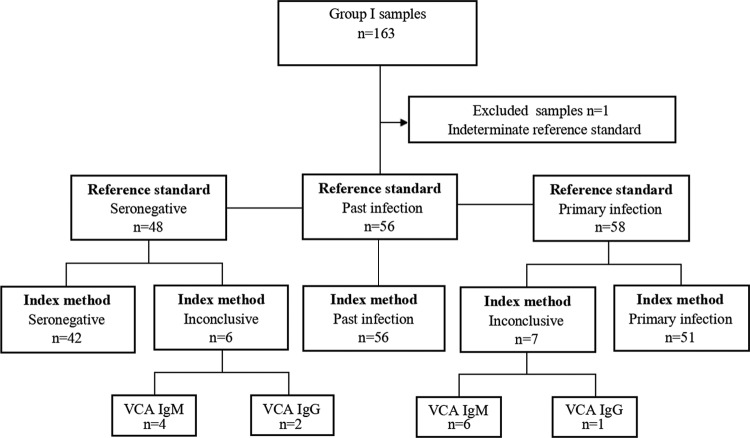
Group I samples were classified as seronegative (n = 48), primary infection (n = 58), past infection (n = 56), and indeterminate (n = 1) based on the results of the reference standard. The indeterminate sample was excluded from the study. The remaining samples (n = 162) were tested by the index method and categorized in accordance with the manufacturer's interpretative criteria (Table 3). A total of 13 (8%) samples produced an inconclusive serological profile, which was either isolated VCA IgM (sensitivity panel, n = 6; specificity panel, n = 4) or isolated VCA IgG (sensitivity panel, n = 1; specificity panel, n = 2). Inconclusive results in the specificity panel occurred exclusively in the seronegative category. A flow diagram of the diagnostic accuracy study is depicted in Fig. 1. Regarding individual markers, the rate and distribution of the equivocal results were 5/162 (3.1%) samples for VCA IgM (seronegative, n = 3; past infection, n = 2), 7/162 (4.3%) samples for VCA IgG (primary infection, n = 7), and 2/162 (1.2%) for EBNA-1 IgG (primary infection, n = 2). Sensitivity was 100% (95% confidence interval [CI], 92.9 to 100%) if the inconclusive results were excluded, 87.9% (95% CI, 76.7 to 95%) if the inconclusive results were included and considered the most adverse, 100% (95% CI, 93.8 to 100%) if the inconclusive results were included and considered the most favorable, and 98.3% (95% CI, 90.7 to 99.7%) if the inconclusive results were included and considered the most adverse, with the exception of isolated VCA IgM. Specificity was 100% (95% CI, 96.3 to 100%) if the inconclusive results were excluded, 94.2% (95% CI, 87.9 to 97.8%) if the inconclusive results were included and considered the most adverse, and 100% (95% CI, 96.5 to 100%) if the inconclusive results were included and considered the most favorable. The results from the PME202 EBV performance panel and EBV UK NEQAS proficiency samples were 100% concordant with results of the providers (data not shown).
TABLE 3.
Architect EBV antibody panel results compared to the reference standard
| Architect infection stage | No. in infection stage by reference standard (total no. in group) |
|||
|---|---|---|---|---|
| Seronegative (48) | Primary infection (58) | Past infection (56) | Total (162) | |
| Seronegative | 42 | 0 | 0 | 42 |
| Primary infection | 0 | 51 | 0 | 51 |
| Past infection | 0 | 0 | 56 | 56 |
| Suspected primary infection | 4 | 6 | 0 | 10 |
| Transient infection | 0 | 0 | 0 | 0 |
| Isolated VCA IgG | 2 | 1 | 0 | 3 |
| Isolated EBNA-1 IgG | 0 | 0 | 0 | 0 |
| Unresolved | 0 | 0 | 0 | 0 |
FIG 1.
Flow diagram of diagnostic accuracy.
Samples classified by the reference standard as having a definite stage of infection (n = 162) were assigned an EBNA-1 IgG status of negative, equivocal, or positive on the basis of the manufacturer's S/CO values for this parameter in the Architect EBV EBNA-1 IgG assay (Table 4). The overall agreement between both the sequential and parallel testing algorithms was 100%, as samples classified as past infection were EBNA-1 IgG positive, whereas samples categorized as seronegative and primary infection were negative or equivocal for this marker. Considering a price per test of £3, testing of the study samples with the three EBV markers in parallel would cost £1458 compared to £1122 for the sequential testing approach. Therefore, a reagent cost savings of £336 (23%) would have been achieved had these samples been analyzed following the sequential testing algorithm.
TABLE 4.
Architect EBV EBNA-1 IgG results compared to the reference standard
| Architect EBNA-1 IgG result | No. in infection stage by reference standard (total no. in group) |
|||
|---|---|---|---|---|
| Seronegative (48) | Primary infection (58) | Past infection (56) | Total (162) | |
| Negative | 48 | 56 | 0 | 104 |
| Equivocal | 0 | 2 | 0 | 2 |
| Positive | 0 | 0 | 56 | 56 |
Cross-reactivity.
Sixty samples from patients with acute viral infections other than EBV (CMV, n = 28; HIV-1, n = 13; parvovirus B19, n = 19) were tested with the Architect EBV VCA IgM assay (Table 5). A total of 17/28 (60.7%) samples from patients with CMV IM showed some degree of reactivity in the VCA IgM test, with 7/17 (41.2%) reactive samples displaying S/CO values in the high-positive range (>2). Although a negative result in the VCA IgM assay was the most common finding in patients with acute retroviral syndrome, 3/13 (23.1%) samples produced S/CO values in the equivocal range. Finally, 6/19 (31.6%) samples tested reactive for VCA IgM with acute parvovirus B19 infection; a weak signal in the equivocal or low-positive range was seen in five samples, whereas one produced a high S/CO value (5.36). All cross-reacting samples tested positive for EBNA-1 IgG in two assays, thus virtually ruling out a primary EBV infection. The UK NEQAS proficiency samples (CMV, n = 3; parvovirus B19, n = 3) tested negative in the VCA IgM assay (data not shown).
TABLE 5.
Architect EBV VCA IgM results in selected primary viral infections
| Architect VCA IgM result | Results (no. [%]) by virus type (total no. in group) |
||
|---|---|---|---|
| CMV (28) | HIV-1 (13) | Parvovirus B19 (19) | |
| Negative | 11 (39.3) | 10 (76.9) | 13 (68.4) |
| Equivocal | 1 (3.6) | 3 (23.1) | 4 (21.1) |
| Positive | 16 (57.1) | 0 (0) | 2 (10.5) |
DISCUSSION
In the diagnostic accuracy study, we challenged the reference and index methods with a selection of precharacterized samples derived from immunocompetent adolescents and young adults with clinical suspicion of IM. Although exclusion of the inconclusive results from sensitivity and specificity calculations artificially improves the accuracy of the test, this approach allowed us to investigate the occurrence of misclassifications in samples that had produced a conclusive serological profile in the index test. A perfect correlation was found, as all samples that were classified as seronegative, indicative of primary infection, and indicative of past infection by the Architect EBV antibody panel matched the corresponding reference standard result. When the inconclusive results were included in the analysis, sensitivity ranged from 87.9% (95% CI, 76.7 to 95%) to 100% (95% CI, 93.8 to 100%), depending on whether these results were considered the most adverse or the most favorable. However, we believed that treating samples with an isolated VCA IgM profile (6/58 [10.3%]) as being indicative of a primary infection would produce a better estimate of sensitivity of 98.3 (95% CI, 90.7 to 99.7%), as this serological pattern is typically found in early primary infection; also, its occurrence in clinical practice usually promotes further testing, either the detection of EBV DNA in the same sample or the documentation of VCA IgG seroconversion in a later sample. The likely bona fide nature of the VCA IgM results as shown by S/CO values in the high-positive range (4.5 to 38.3) and confirmed initial reactivity after retesting in duplicate supported this approach. An acceptable specificity was estimated even if the inconclusive results were considered the most adverse (94.2% [95% CI, 87.9 to 97.8%]), thus indicating the reliability of the test to rule out primary infection. However, the occurrence of isolated VCA IgM (4/104 [3.8%]) and isolated VCA IgG (2/104 [1.9%]) results in seronegative individuals deserves further investigation. It was important to clarify the nature of an isolated VCA IgM result, as this finding may signify a very early infection not detected by the reference standard but revealed by the index method due to the increased analytical sensitivity of chemiluminescent over chromogenic detection methods (13) or a false-positive result. Samples with this profile had significantly lower S/CO values than those measured in the sensitivity panel (0.6 to 1.6 versus 4.5 to 38.3, respectively). In addition, initial reactivity was confirmed after retesting in duplicate in only two samples, which would imply a false positivity rate of ≥50%. No later samples were available from patients who repeatedly tested reactive, thus precluding further analysis. Samples with an isolated VCA IgG profile produced S/CO values in the high-positive range (2.9 to 8.3), which persisted after retesting in duplicate. Although not an aim of this study, it is worth mentioning that the misclassification of EBV-naive individuals as seropositive would compromise the use of the Architect VCA IgG assay for pretransplant serology screens because the exclusion of seronegative recipients from surveillance schemes could have detrimental consequences to the prevention of posttransplant lymphoproliferative disease (14).
At a time when pathology laboratories are experiencing significant financial constraints, the development of cost-effective testing algorithms is an important task for laboratory diagnosticians. The stage of EBV infection in immunocompetent individuals is usually established by parallel testing of VCA IgM, VCA IgG, and EBNA-1 IgG antibodies. Alternatively, a sequential testing algorithm (Fig. 2) is advocated by some European microbiological organizations (15), in which a positive result for EBNA-1 IgG precludes further testing, as the presence of this marker is consistent with past infection, whereas negative or equivocal results for this parameter are followed by VCA IgM and IgG tests. The scientific rationale behind this approach is based on several facts: first, EBNA-1 IgG appears, at the earliest, 4 weeks after the onset of EBV IM (16), its presence indicating either a resolving or resolved primary infection; second, EBNA-1 IgG develops in ≥95% of individuals after a primary infection and persists indefinitely (17); third, viral reactivation is deemed clinically silent (18); and finally, the EBNA-1 IgG seropositivity rate is high in the target population, in which about 70% of the samples originate from patients with past EBV infection (5). An essential prerequisite for the successful application of this approach is the use of an EBNA-1 IgG assay with exquisite analytical specificity in order to maximize the detection of the stages of infection that require further analysis with VCA markers, namely, seronegativity and primary infection. In addition, high assay sensitivity is also recommended in order to minimize unnecessary testing for VCA antibodies in patients with past infection. Our results showed perfect agreement between the use of the Architect VCA IgM, VCA IgG, and EBNA-1 IgG assays in sequential and parallel testing algorithms, thus highlighting its commutability. However, the implementation of the former diagnostic strategy was associated with significant reagent savings, suggesting it may be a cost-effective alternative to the simultaneous testing of the three markers. Importantly, as our study was performed in a group intentionally enriched with patients with EBV stages other than past infection, we can anticipate that the use of this algorithm in a typical population would produce more significant cost savings.
FIG 2.
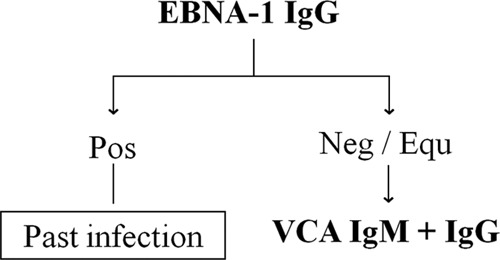
EBV sequential testing algorithm.
Antibodies to VCA IgM are usually detectable at the time of presentation due to the long incubation period of EBV IM (19). However, its presence in serological profiles compatible with primary infection should be interpreted with caution because false-positive results for this marker may occur during the course of other acute infections with overlapping clinical features (20), such as primary infections with CMV, HIV-1, parvovirus B19, rubella virus, or Toxoplasma gondii (21–23). The main pathogen interfering with the serological diagnosis of primary EBV infection is CMV. Two reasons account for this: first, primary CMV infection is responsible for a significant minority (5 to 7%) of IM cases in adolescents and young adults living in Western societies (24), and second, reactive VCA IgM results are frequently detected across different serological platforms during primary CMV infection (25), a finding most likely explained by the generation of VCA IgM antibodies during CMV-induced EBV immunoreactivation (26). In our study population, a majority of patients with CMV IM tested reactive in the Architect VCA IgM assay, with a high proportion of S/CO values in the high-positive range. The substantial amount of reactive results for this marker during primary CMV infection indicates that concomitant testing for CMV is important for the proper interpretation of EBV serological profiles. Fortunately, most primary CMV infections occur in individuals with past EBV infection (27), who are characterized by the presence of EBNA-1 IgG; thus, the detection of this marker, which was documented in 98% of our subjects, facilitates the interpretation of VCA IgM-positive serological patterns. Primary HIV-1 is an unusual cause of IM (<1%) (28, 29). Some authors have described falsely reactive VCA IgM results during primary HIV-1 infection (30). In our study population, 3/13 (23.1%) tested reactive in the Architect VCA IgM assay, with all S/CO values in the equivocal range. Despite the fact that parvovirus B19 is a rare cause of IM (31), 3/19 (15.8%) of our patients presented with this syndrome. Acute infection with this virus has been associated with false-positive IgM results against a variety of heterologous viruses (32), including with VCA IgM (33). Our results showed reactive VCA IgM results in 6/19 (31.6%) patients with acute parvovirus B19 infection, which were mostly confined to the equivocal or low-positive range.
Our study had several limitations, which were mainly due to its retrospective design: first, detailed clinical information was not available or was insufficient for many subjects; in particular, the time elapsed from the onset of clinical manifestations to the date of collection of the acute-phase sera, which impacts on the antibody concentration of the three EBV markers at presentation, was lacking for a majority of patients. Second, a decrease in antibody concentrations during thawing, especially those of VCA IgM, could not be ruled out. Third, the sample size was limited due to the nature of the study. Finally, a selection bias was introduced, as only samples displaying conclusive serological patterns in the Liaison platform were used for the diagnostic accuracy study. However, a prospective study, including one with a consecutive series of patients who satisfy the eligibility criteria, is planned to confirm the results of this preliminary verification study (34).
In conclusion, the Architect EBV serology panel is an acceptable system for determining the stage of EBV infection in immunocompetent adolescents and young adults with clinical suspicion of IM.
The use of the Architect EBV serology panel in a sequential testing algorithm based on initial EBNA-1 IgG analysis is cost-effective for the staging of EBV infection in immunocompetent adolescents and young adults with clinical suspicion of IM.
The high rate of reactive Architect VCA IgM results found during primary CMV infection strongly recommends concomitant testing for CMV and EBV in immunocompetent adolescents and young adults presenting with clinically suspected IM.
ACKNOWLEDGMENT
We thank Colette Smith for the help provided with the statistical analysis of data.
Footnotes
Published ahead of print 2 April 2014
REFERENCES
- 1.Sprunt TP, Evans FA. 1920. Mononucleosis leukocytosis in reaction to acute infections (infectious mononucleosis). Bull. Johns Hopkins Hosp. 31:410–417 [Google Scholar]
- 2.Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly H. 1974. Infectious mononucleosis at the United States military academy. A prospective study of a single class over four years. Yale J. Biol. Med. 47:182–195 [PMC free article] [PubMed] [Google Scholar]
- 3.Luzuriaga K, Sullivan JL. 2010. Infectious mononucleosis. N. Engl. J. Med. 362:1993–2000. 10.1056/NEJMcp1001116 [DOI] [PubMed] [Google Scholar]
- 4.Hurt C, Tammaro D. 2007. Diagnostic evaluation of mononucleosis-like illnesses. Am. J. Med. 120:911.e1–911.e8. 10.1016/j.amjmed.2007.08.018 [DOI] [PubMed] [Google Scholar]
- 5.Hess RD. 2004. Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J. Clin. Microbiol. 42:3381–3387. 10.1128/JCM.42.8.3381-3387.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tselis A, Merline JR, Storch GA. 2006. Epstein-Barr virus disease-serologic and virologic diagnosis, p 126–146 In Tselis A, Jenson HB. (ed), Epstein-Barr virus, 1st ed. Taylor & Francis Group, New York, NY [Google Scholar]
- 7.Gärtner BC, Hess RD, Bandt D, Kruse A, Rethwilm A, Roemer K, Mueller-Lantzsch N. 2003. Evaluation of four commercially available Epstein–Barr virus enzyme immunoassays with an immunofluorescence assay as the reference method. Clin. Diagn. Lab. Immunol. 10:78–82. 10.1128/CDLI.10.1.78-82.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middeldorp JM, Herbrink P. 1988. Epstein-Barr virus specific marker molecules for early diagnosis of infectious mononucleosis. J. Virol. Methods 21:133–146. 10.1016/0166-0934(88)90060-2 [DOI] [PubMed] [Google Scholar]
- 9.Kieff E, Rickinson AB. 2007. Epstein-Barr virus, p 2655–2700 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 10.Bauer G. 2001. Simplicity through complexity: immunoblot with recombinant antigens as new gold standard in Epstein-Barr virus serology. Clin. Lab. 47:223–230 [PubMed] [Google Scholar]
- 11.Gärtner BC, Fischinger JM, Roemer K, Mak M, Fleurent B, Mueller-Lanztsch N. 2001. Evaluation of a recombinant line blot for diagnosis of Epstein-Barr virus compared with ELISA, using immunofluorescence as reference method. J. Virol. Methods 93:89–96. 10.1016/S0166-0934(00)00301-3 [DOI] [PubMed] [Google Scholar]
- 12.Crowley A, Connell J, Schaffer K, Hall W, Hassan J. 2012. Is there diagnostic value in detection of immunoglobulin G antibodies to the Epstein–Barr virus early antigen? Biores. Open Access 1:291–295. 10.1089/biores.2012.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weeks I, Kricka LJ, Wild D. 2013. Signal generation and detection systems (excluding homogeneous assays), p 267–285 In Wild D, John R, Sheehan C, Binder S, He J. (ed), The immunoassay handbook, 4th ed. Elsevier Ltd., Oxford, United Kingdom [Google Scholar]
- 14.Green M, Michaels MG. 2013. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am. J. Transplant. 13:41–54. 10.1111/ajt.12004 [DOI] [PubMed] [Google Scholar]
- 15.Health Protection Agency. 2012. UK standards for microbiology investigations: Epstein-Barr virus serology. Health Protection Agency, London, United Kingdom: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317131313375 [Google Scholar]
- 16.Bauer G. 2009. Epstein-Barr virus diagnostics: a challenge to test method and tester. Current diagnostics in infectious and autoimmune diseases, p 1–5. 7th Mikrogen spring symposium. [Google Scholar]
- 17.Henle G, Henle W. 1979. The virus as the etiologic agent of infectious mononucleosis, p 279–320 In Epstein MA, Achong BG. (ed), The Epstein-Barr virus, 1st ed. Springer-Verlag, Berlin, Germany [Google Scholar]
- 18.Obel N, Høier-Madsen M, Kangro H. 1996. Serological and clinical findings in patients with serological evidence of reactivated Epstein-Barr virus infection. APMIS 104:424–428. 10.1111/j.1699-0463.1996.tb00737.x [DOI] [PubMed] [Google Scholar]
- 19.Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA, Marklung G, Rymo L, Wellinder C, Straus SE. 1987. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc. Natl. Acad. Sci. U. S. A. 84:570–574. 10.1073/pnas.84.2.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berth M, Bosmans E. 2010. Comparison of three automated immunoassay methods for the determination of Epstein-Barr virus-specific immunoglobulin M. Clin. Vaccine Immunol. 17:559–563. 10.1128/CVI.00372-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiedbrauk DL, Bassin S. 1993. Evaluation of five enzyme immunoassays for detection of immunoglobulin M antibodies to Epstein-Barr virus viral capsid antigens. J. Clin. Microbiol. 1993. 31:1339–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber B, Brunner M, Preiser W, Doerr HW. 1996. Evaluation of 11 enzyme immunoassays for the detection of immunoglobulin M antibodies to Epstein-Barr virus. J. Virol. Methods 57:87–93. 10.1016/0166-0934(95)01971-5 [DOI] [PubMed] [Google Scholar]
- 23.Robertson P, Beynon S, Whybin R, Brennan C, Vollmer-Conna U, Hickie I, Lloyd A. 2003. Measurement of EBV-IgG anti-VCA avidity aids the early and reliable diagnosis of primary EBV infection. J. Med. Virol. 70:617–623. 10.1002/jmv.10439 [DOI] [PubMed] [Google Scholar]
- 24.Evans AS. 1978. Infectious mononucleosis and related syndromes. Am. J. Med. Sci. 276:325–339. 10.1097/00000441-197811000-00010 [DOI] [PubMed] [Google Scholar]
- 25.de Ory F, Guisasola ME, Sanz JC, García-Bermejo I. 2011. Evaluation of four commercial systems for the diagnosis of Epstein-Barr virus primary infections. Clin. Vaccine Immunol. 18:444–448. 10.1128/CVI.00486-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aalto SM, Linnavuori K, Peltola H, Vuori E, Weissbrich B, Schubert J, Hedman L, Hedman K. 1998. Immunoreactivation of Epstein-Barr virus due to cytomegalovirus primary infection. J. Med. Virol. 56:186–191. [DOI] [PubMed] [Google Scholar]
- 27.Klemola E, Von Essen R, Henle G, Henle W. 1970. Infectious mononucleosis-like disease with negative heterophil agglutination test. Clinical features in relation to Epstein-Barr virus and cytomegalovirus antibodies. J. Infect. Dis. 121:608–614 [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg ES, Caliendo AM, Walker BD. 1999. Acute HIV infection among patients tested for mononucleosis. N. Engl. J. Med. 340:969. 10.1056/NEJM199903253401217 [DOI] [PubMed] [Google Scholar]
- 29.Naito T, Kudo N, Inui A, Matsumoto N, Takeda N, Isonuma H, Dambara T, Hayashida Y. 2006. Causes of infectious mononucleosis-like syndrome in adult patients. Intern. Med. 45:833–834. 10.2169/internalmedicine.45.1725 [DOI] [PubMed] [Google Scholar]
- 30.Post JJ, Chan MK, Whybin LR, Shi Q, Rawlinson WD, Cunningham P, Robertson PW. 2011. Positive Epstein-Barr virus and cytomegalovirus IgM assays in primary HIV infection. J. Med. Virol. 83:1406–1409. 10.1002/jmv.22109 [DOI] [PubMed] [Google Scholar]
- 31.Jones JW, Pether JV, Frost RW. 1994. Human parvovirus B19. Hard to differentiate from infectious mononucleosis. BMJ 308:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa E, Tormo N, Clari MA, Bravo D, Muñoz-Cobo B, Navarro D. 2009. Performance of the Epstein-Barr virus and herpes simplex virus immunoglobulin M assays on the Liaison platform with sera from patients displaying acute parvovirus B19 infection. Clin. Vaccine Immunol. 16:1247–1248. 10.1128/CVI.00142-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berth M, Bosmans E. 2009. Acute parvovirus B19 infection frequently causes false-positive results in Epstein-Barr virus- and herpes simplex virus-specific immunoglobulin M determinations done on the Liaison platform. Clin. Vaccine Immunol. 16:372–375. 10.1128/CVI.00380-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Paschale M, Clerici P. 2012. Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J. Virol. 1:31–43. 10.5501/wjv.v1.i1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]