Abstract
SUMMARY
The ability to produce water-soluble proteins with the capacity to oligomerize and form pores within cellular lipid bilayers is a trait conserved among nearly all forms of life, including humans, single-celled eukaryotes, and numerous bacterial species. In bacteria, some of the most notable pore-forming molecules are protein toxins that interact with mammalian cell membranes to promote lysis, deliver effectors, and modulate cellular homeostasis. Of the bacterial species capable of producing pore-forming toxic molecules, the Gram-positive pathogen Staphylococcus aureus is one of the most notorious. S. aureus can produce seven different pore-forming protein toxins, all of which are believed to play a unique role in promoting the ability of the organism to cause disease in humans and other mammals. The most diverse of these pore-forming toxins, in terms of both functional activity and global representation within S. aureus clinical isolates, are the bicomponent leucocidins. From the first description of their activity on host immune cells over 100 years ago to the detailed investigations of their biochemical function today, the leucocidins remain at the forefront of S. aureus pathogenesis research initiatives. Study of their mode of action is of immediate interest in the realm of therapeutic agent design as well as for studies of bacterial pathogenesis. This review provides an updated perspective on our understanding of the S. aureus leucocidins and their function, specificity, and potential as therapeutic targets.
INTRODUCTION
Staphylococcus aureus is a major bacterial pathogen that causes a significant disease burden in both hospital and community settings (1, 2). The organism can colonize or infect nearly all host tissues, from the skin and nares to bone, joints, muscle, heart, and lungs (1–3). Invasive infections that disseminate via the bloodstream can lead to devastating clinical consequences if treatment is not rapidly initiated. The ultimate success of S. aureus in multiple disparate host environments and its high incidence among both hospitalized and otherwise healthy individuals make this organism a major concern for public health.
Currently, the clinical standard of care for individuals with invasive S. aureus infections includes aggressive administration of antibiotics (4). However, the recent increase in the incidence of multidrug-resistant isolates such as hospital-acquired methicillin-resistant S. aureus (HA-MRSA) and community-acquired MRSA (CA-MRSA) and the increased dominance of highly virulent clonal lineages that can cause aggressive disease have diminished the success of such therapeutic strategies (5–10). A number of comprehensive reviews addressing the increased incidence of these infectious lineages have already been reported (5, 6, 11–13). It is clear that S. aureus exhibits tremendous adaptability when confronted with aversive stimuli (i.e., antibiotics) and harsh environmental conditions (host tissues that deprive the organism of essential nutrients), allowing it to execute a highly pathogenic life-style. Noteworthy examples of its adaptability are the organism's rapid exchange and/or acquisition of DNA and the mutability of its genome, both of which facilitate unrelenting resistance to antibiotics and promote novel and/or enhanced virulence traits (1, 11, 14–18). Such genetic plasticity has left us with limited options for combating the diverse and sometimes deadly conditions associated with S. aureus infection.
A major pathogenic attribute of S. aureus that facilitates its survival during infection is the ability to secrete a diverse repertoire of immune system evasion factors. Included among these factors are a number of potent cytotoxins (hemolysins, cytolytic peptides, and leucocidins), immunomodulatory proteins (superantigens, superantigen-like proteins, and complement-inhibitory proteins), proteases, and factors that prevent immune cell recognition and killing (protein A, capsule, and catalase, among others) (19–27). Each of these molecules subverts the host immune system in different ways, leaving the organism largely resistant to both innate and adaptive immune defenses. A number of recent reviews and primary research articles serve to highlight the unique mechanisms by which these factors promote immune evasion (11, 19, 25, 28–30). Not only do they work in concert to effectively inhibit clearance of S. aureus by the host immune surveillance, they also prevent the development of immunological memory against this pathogen (31–34). This combined attack on both innate and adaptive immune defenses is believed to be a major reason why current therapeutic and vaccine strategies, which rely on robust innate and adaptive pathogen recognition, have failed (35–38). Clearly, there are major limitations of our current efforts to combat a pathogen such as S. aureus, which has adapted to avoid nearly all immune recognition strategies. As a result, recent initiatives are now under way to better define mechanisms by which S. aureus subverts the host immune system within a therapeutic framework (39). The underlying themes of these studies are to use our knowledge of S. aureus immune evasion tactics to bolster the host immune response, promote natural clearance of bacteria, and enhance the development of immunologic memory against this pathogen.
Of interest in the development of therapeutic modalities aimed at promoting the natural clearance of S. aureus by the host immune system are the bicomponent pore-forming leucocidins (19–21). These toxins consist of two separate water-soluble monomeric subunits that target and kill immune cells by binding to host leukocyte membranes and forming β-barrel pores that span the phospholipid bilayer (Fig. 1). All strains of S. aureus are capable of producing at least three (HlgAB, HlgCB, and LukAB/HG) of the six known leucocidins, while most highly virulent clinical strains currently infecting humans produce five (HlgAB, HlgCB, LukAB/HG, Panton-Valentine leucocidin [PVL], and LukED). One additional toxin, LukMF′, is present in S. aureus lineages that are isolated from ruminants and other mammals but not humans. It is worth noting that other classes of proteinaceous molecules, including the phenol-soluble modulins (PSMs), a family of small amphipathic α-helical peptides with broad-range lytic activities, can also lyse host immune cells, leading to their classification as “leucocidins.” Included within the PSM family are the α- and β-type PSMs as well as the historically characterized delta-toxin, a member of the α-type PSMs. These leukocytolytic molecules are distinct from the classically defined bicomponent β-barrel pore-forming leucocidins discussed in this review (for detailed information on PSMs and their roles in S. aureus immune evasion, see references 29, 40, and 41). Reemerging interest in the bicomponent leucocidins has occurred due to their potential as targets in therapeutic design, which could ultimately promote natural clearance of S. aureus infection and reduce reliance on antibiotic usage in already resistant strains (20). In recent years, our understanding of the bicomponent leucocidins has changed dramatically. This review will serve to consolidate our current understanding of bicomponent leucocidin biology and provide an outlook for future studies and therapeutic development.
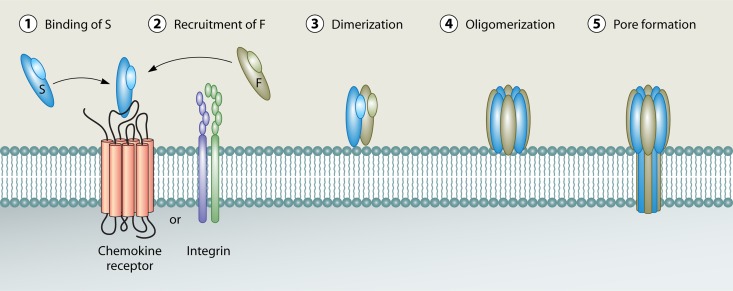
FIG 1.
Current model of leucocidin pore formation. Leucocidin pore formation is believed to occur in a stepwise fashion that begins with toxin recognition of cellular receptors on the surface of target host cells. On most host cells, the “S” subunit recognizes a proteinaceous receptor (either a chemokine receptor [LukED and PVL] or an integrin [LukAB/HG]) to facilitate high-affinity binding to the cell surface (1). The S subunit then recognizes and recruits the “F” subunit (2), leading to dimerization on the host cell surface (3). Dimerization is followed by oligomer formation (4). Toxin oligomers assemble into an octameric prepore structure containing alternating S and F subunits. Following oligomerization, a major structural change occurs in the stem domains of the S and F subunits, leading to membrane insertion and the formation of a β-barrel pore that spans the host cell lipid bilayer (5).
A BRIEF HISTORICAL PERSPECTIVE: IDENTIFICATION AND EARLY CHARACTERIZATION OF THE LEUCOCIDINS
Late 19th Century to Mid-20th Century: Discovery of S. aureus Leukolytic Activity and Identification of Panton-Valentine Leucocidin
The study of S. aureus leucocidins has a history that spans more than 110 years (42). In 1894, Van de Velde published the first studies to demonstrate the leukocidal activity of S. aureus (then called Staphylococcus pyogenes) on primary rabbit leukocytes (42, 43). This leukotoxic substance was given the name “leucocidin.” At that time, the composition of leucocidin was unknown. Furthermore, it was unclear whether leucocidin was a novel secreted substance or whether the activity was a secondary function of the already described hemolysin (later known as alpha-hemolysin). Additionally, it is not absolutely certain whether Van de Velde's description of leukocidal activity was actually caused by the S. aureus leucocidins or whether the observed effects were induced by other toxic molecules present within the complex milieu of bacterial culture supernatant. Observations made in later years, describing a leukocidal activity similar to what was originally described by Van de Velde, support the notion that the “leucocidin” was indeed that of an S. aureus bicomponent leucocidin(s) (44–46). His early observations closely resembled what was later described by Gladstone and van Heyningen and are identical to what we see today upon the intoxication of leukocytes with any of the six known bicomponent leucocidins (Fig. 2) (44, 47). It is therefore likely that the leucocidin described by Van de Velde was that of either one or multiple bicomponent leucocidins. The following year, Denys and Van de Velde also demonstrated that rabbits are able to generate neutralizing antibody against the leucocidin (43). This study was the first to demonstrate the immunogenicity of leucocidin and the utility of neutralizing antibodies in protecting immune cells from the leukocidal activity of S. aureus (43).
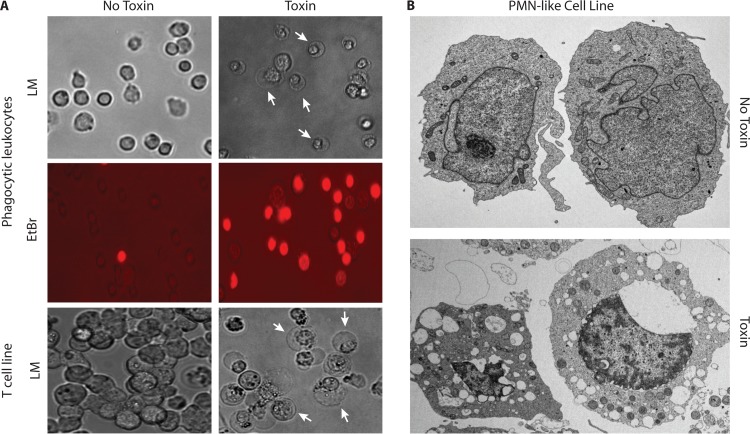
FIG 2.
Morphological changes associated with leucocidin-mediated killing of immune cells. (A) Light and fluorescence microscopy images of murine phagocytic leukocytes (macrophages and neutrophils) and light microscopy (LM) images of the human T cell line HUT-R5 (cell line overexpressing CCR5) after exposure to a 90% lethal dose of LukED (5 μg/ml). Characteristic membrane halos and expansion of cellular nuclei are seen, along with increased permeability to ethidium bromide (EtBr) (red), an indicator of pore formation and membrane damage. Arrows point to characteristic cellular morphology changes upon leucocidin intoxication. (B) Electron microscopy images of the human PMN-like cell line PMN-HL60 after exposure to S. aureus supernatant containing a 100% lethal dose of LukAB/HG (>2.5 μg/ml). All intoxications and microscopic image acquisition were conducted as previously described (47, 97, 227).
In the early 1900s, Neisser and Wechsberg followed up on the work of Van de Velde by performing studies to determine whether the leukocidal and hemolytic activities of S. aureus were caused by independent toxic substances (48). Through adsorption of hemolysin with red blood cells (RBCs) or leucocidin with leukocytes, those authors determined that each substance could be selectively removed from culture supernatants without influencing the activity of the other (48). Similarly, Julianelle performed studies aimed at correlating leukocidal activity with hemolytic activity within a series of strains and found that in a number of instances, the activities of hemolysin and leucocidin did not coincide with one another (46). As the 20th century progressed, however, more detailed investigations, including those by Weld and Gunther, were unable to conclusively validate whether the S. aureus hemolysin (now referred to as alpha-hemolysin) and leucocidin exert functions independently of one another (49, 50). Many researchers were unable to reproduce the adsorption studies of Neisser and Wechsberg, and the conjecture that alpha-hemolysin and leucocidin were independent substances remained dubious at best (48, 50, 51). Much of the reason for the difficulty in deciphering functional differences between alpha-hemolysin and leucocidin would not be appreciated until much later, when the regulatory complexities and breadth of strains expressing more than one leucocidin would be fully evaluated.
In 1932, the conclusion that leucocidin and alpha-hemolysin were unique substances was finally validated by the work of Panton and Valentine. Through detailed phenotypic studies of a number of S. aureus strains, some of which had potent hemolytic activity but no leukocidal activity and vice versa, it was conclusively established that the hemolytic and leukocidal activities of S. aureus were caused by two unique substances (45, 52). Later work validated these landmark studies and prompted a renaming of leucocidin to Panton-Valentine leucocidin or PV leucocidin (PVL), thereby distinguishing it from the suspect Neisser-Wechsberg (NW) leucocidin (which was later found to actually be alpha-hemolysin) (44, 49, 53). Thus, Van de Velde's original definition of leucocidin as a unique entity, insofar as it resembles Panton-Valentine leucocidin, was largely correct. Again, it should be noted that these studies were conducted before it was realized that S. aureus strains often encode more than one leucocidin. It is therefore difficult to ascertain whether the leukocidal activity was strictly caused by PVL or a combination of leucocidins present within culture filtrates. We presume that it was likely the latter circumstance for most studies using S. aureus culture supernatants, prior to the purification of PVL by Woodin in the 1960s (54, 55). Thus, “PVL” remained the primary toxin of study for much of the mid-20th century (1960s to 1970s). As such, it was the first leucocidin to be purified and served to demonstrate a number of the hallmark features of these toxins (54–71). Of his numerous contributions to the leucocidin field, Woodin provided the first evidence that active leucocidins consist of two separate protein components, designated “S,” or slow (LukS-PV), and “F,” or fast (LukF-PV), based on their chromatography elution profiles (Table 1). The bicomponent leucocidins are cytotoxic only when both S and F subunits are combined (54, 55).
TABLE 1.
Characteristics of the leukotoxins of Staphylococcus aureus
| Toxin | Alternate name(s) | Genome location | Toxins with closest similarity (% similarity) | Immune cell type(s) targeted | Strain distribution | Receptor(s) recognized | Role(s) in virulence | Species specificity(ies) | Sublytic activity(ies) | Hemolytic activity |
|---|---|---|---|---|---|---|---|---|---|---|
| LukSF | PVL, LukSF-PV | ϕSa2 | S subunit, HlgC (81.7); F subunit, LukD (82.1) | Monocytes, neutrophils, macrophages | 2–3% of all isolates | C5aR and C5L2 | Debated; (i) pathogenesis of severe necrotizing pneumonia in rabbits, (ii) conflicting role in skin and soft tissue infection in rabbits and mice, and (iii) may influence the pathogenesis of osteomyelitis | Yes; rabbit and human immune cells only | Yes; (i) neutrophil priming, (ii) induction of apoptosis, and (iii) inflammasome activation | No |
| LukED | LukEDv | Pathogenicity island, vSaβ | S subunit, LukM (77.5); F subunit, LukF′ (83.4%) | Monocytes, neutrophils, macrophages, T cells, dendritic cells, NK cells | 70% of all isolates | CCR5, CXCR1, CXCR2 | (i) Contributes to lethality observed during acute sepsis in mice and (ii) influences bacterial burden in murine renal abscess models | No; toxic to immune cells of rabbits, humans, mice, dogs, and carp | Unclear; (i) potential proinflammatory effects and (ii) lymphocyte proliferation | Yes |
| HlgAB | For HlgA, Hlg2 and HγII; for HlgB, Hlg1, HγI, and LukF; for HlgAB, gamma-hemolysin and gamma-toxin | Core genome | S subunit, HlgC (69.8); F subunit, LukD (75.6) | Monocytes, macrophages, T cells, neutrophils | 99% of all isolates | Unknown | (i) Role in pathogenesis of septic arthritis in mice, (ii) influences inflammation and pathogenesis in ocular infection models of rabbits, and (iii) contributes modestly to lethality observed during acute sepsis in mice | Unclear; toxic to immune cells of rabbits, humans, and some cells of murine origin | Not known | Yes |
| HlgCB | For HlgC, LukS; for HlgB, Hlg1, HγI, and LukF; for HlgCB, leucocidin R and leucocidin | Core genome | S subunit, LukS-PV (81.7); F subunit, LukD (75.6) | Monocytes, macrophages, neutrophils | 99% of all isolates | Unknown (evidence suggests shared receptors with LukSF-PV) | (i) Role in pathogenesis of septic arthritis in mice, (ii) influences inflammation and pathogenesis in ocular infection models of rabbits, and (iii) contributes modestly to lethality observed during acute sepsis in mice | Unclear; toxic to immune cells of rabbits, humans, and some cells of murine origin | Induction of Ca2+ release from neuronal intracellular stores | Yes |
| LukAB | LukHG | Core genome | S subunit, LukE (36.1); F subunit, HlgB (40.1) | Neutrophils, macrophages, monocytes, dendritic cells | Unknown; likely a significant proportion of isolates | CD11b | (i) Influences bacterial burden in murine renal abscess models and (ii) promotes inflammation in skin of rabbits | Yes; human and rabbit immune cells only | Unclear; (i) potential proinflammatory effects and (ii) NET formation | No |
| LukMF′ | LukS′-PV, LukF′-PV | ϕSa1 | S subunit, LukE (77.5); F subunit, LukD (83.4) | Bovine neutrophils and macrophages | Limited to lineages infecting ruminants and other small mammals | Unknown | Debated; believed to be involved in pathogenesis of mastitis | Yes; ruminant immune cells | Not known | No |
Mid- to Late 20th Century: Identification of Gamma-Hemolysin
During the same time when PVL was initially being characterized, Smith and Price identified what they described as an alternate hemolysin with distinct biochemical characteristics. This novel hemolysin was derived from an S. aureus strain which did not produce appreciable amounts of alpha-hemolysin (72). As such, it was given the name gamma-hemolysin and would later be identified as one of two bicomponent leucocidins with the ability to lyse both leukocytes as well as red blood cells (the other leucocidin being LukED). After its initial identification in 1938, further studies of gamma-hemolysin were hampered due to the difficulty in separating its activity from those of other hemolysins in S. aureus (by this time, at least three unique hemolysins had been described, alpha-, beta-, and delta-hemolysins) (73–76). It was not until the 1960s and 1970s that purified preparations of gamma-hemolysin were isolated and its lytic activity was demonstrated on red blood cells of diverse species (76–81). Like PVL, gamma-hemolysin was purified as two separate protein subunits that were active only when combined (77, 78, 82, 83). Fackrell and Wiseman determined that, in addition to red blood cells, gamma-hemolysin exhibited lytic activity on human leukocytes and lymphoblasts, and Szmigielski et al. further measured lytic activity on rabbit polymorphonuclear leukocytes (81, 84). Altogether, these studies served to classify gamma-hemolysin as both a bicomponent leucocidin and a hemolysin (Table 1). This dual lytic activity on leukocytes and red blood cells may explain why early work to determine whether or not the hemolytic and leukocidal activities of S. aureus were unique was such a challenging endeavor. It was not until the late 1980s that the gamma-hemolysin genetic locus was cloned and found to actually consist of three genes that comprise two separate toxins, HlgAB and HlgCB. HlgA and HlgC are S components, while HlgB is a common F component (54, 85–90). Given its early identification, gamma-hemolysin, like PVL, has been studied extensively at the biochemical and biophysical levels. Details of the genetic architecture of gamma-hemolysin, its location within the S. aureus genome, and its biological activity are discussed below.
Late 20th Century to Present: Identification of Leucocidins MF′, ED, and AB/HG
Leucocidin MF′ was first identified in 1995 by Choorit and colleagues (91). The S subunit of the toxin (LukM) was serendipitously isolated during routine purification of the gamma-hemolysin subunits HlgC (LukS) and HlgB (LukF) for functional studies. LukM was initially characterized as an F-type subunit based on functional similarity to HlgB of gamma-hemolysin; however, its high degree of sequence similarity to the gamma-hemolysin S subunits HlgA and HlgC (∼70%) and its cross-reactivity with S subunit antibodies of gamma-hemolysin suggested greater similarity to S-type subunits (91). Follow-up studies, which purified and sequenced a novel F component located immediately downstream of the LukM stop codon, served to identify the corresponding F-type subunit of LukMF′, designated LukF′-PV (LukF-PV P83) (92). The activity and limited host range of LukMF′ are discussed below (Table 1).
The identification of an additional leucocidin, LukED, followed in 1998. Gravet and colleagues performed a series of radial gel immunoprecipitation assays to identify molecules in S. aureus culture filtrates that were immunologically similar to those of other known leucocidins (93). The use of specified medium conditions led to the increased expression of a novel immunologically reactive molecule while diminishing the expression of other known leucocidins, allowing the purification and subsequent molecular characterization of LukED from S. aureus strain Newman (NTCC 8178) (93). Later, Morinaga and colleagues identified what they termed a variant of LukED, LukEDv, that was conserved in a remarkable number (>87%) of S. aureus strains and was lytic when added to both leukocytes and rabbit red blood cells (Table 1) (94). Ten years after these initial discoveries, it was determined that the original genetic sequence of lukED was significantly dissimilar from that of lukED found in all other sequenced S. aureus isolates, including other sources of strain Newman from which the toxin was first identified. This finding suggests that the originally described lukED sequence was likely a sequence anomaly (47). In all other instances, lukEDv is the dominant sequence type and is remarkably conserved in all sequenced strains (47, 95). Thus, it is suggested for ease of reference that LukED be uniformly used as the primary nomenclature for this toxin here (Table 1).
In 2010, an additional leukocytolytic bicomponent toxin with ∼30% amino acid sequence identity to the other known leucocidins was identified (96). Ventura et al. discovered a putative leucocidin during a study that used bacterial cell surface proteomics to identify S. aureus proteins that promote the increased virulence of current epidemic strains of CA-MRSA. Some of the most abundant peptides within their cell surface preparations corresponded to a protein with sequence homology to the S. aureus leucocidins. They named this novel leucocidin LukHG, where LukH corresponds to the S subunit and LukG corresponds to the F subunit (96). LukHG was found to be both surface associated as well as secreted and contributed directly to the lysis of primary human polymorphonuclear leukocytes (PMNs) in vitro, establishing its function as a leucocidin (96). Around the same time, an additional study by DuMont et al. used isogenic leucocidin deletion mutants in S. aureus strain Newman as well as proteomic studies of S. aureus culture filtrates to identify a novel leucocidin responsible for lytic activity of S. aureus culture supernatants derived from bacteria grown in RPMI medium supplemented with Casamino Acids (RPMI-CAS) (97). This toxin, named LukAB (LukA is the S subunit, and LukB is the F subunit) was lytic toward PMNs, macrophages, and dendritic cells; contributed to the cytotoxicity of both methicillin-sensitive S. aureus (MSSA) and MRSA strains; protected S. aureus from PMN killing; and was required for full virulence in murine models of systemic infection. Sequence comparison of the LukAB toxin to LukHG revealed that both toxins were the same (97). LukAB/HG is the last known bicomponent leucocidin to be identified in S. aureus.
Over the past 120 years, our appreciation of the breadth and functionality of the leucocidins produced by S. aureus has progressed from that of a single toxic substance, the “leucocidin,” to the identification of six unique proteins (LukSF-PV, HlgAB, HlgCB, LukED, LukMF′, and LukAB/HG), all capable of exerting potent lytic activity on a variety of host immune cells (Table 1). The majority of these toxins, except for LukAB/HG, exhibit a remarkable degree of sequence conservation and similar mechanisms of action on host cells (Fig. 1 and 3 and Table 1) (21). However, each toxin exhibits a number of unique characteristics that will likely prove key to enhancing our understanding of leucocidin biological functions on host cells and defining mechanisms of action in vivo. Advances in our understanding of both the redundancies and the functional differences among the bicomponent leucocidins, as they relate to toxin lytic activity and cellular signaling, are emphasized in greater detail later in this review.
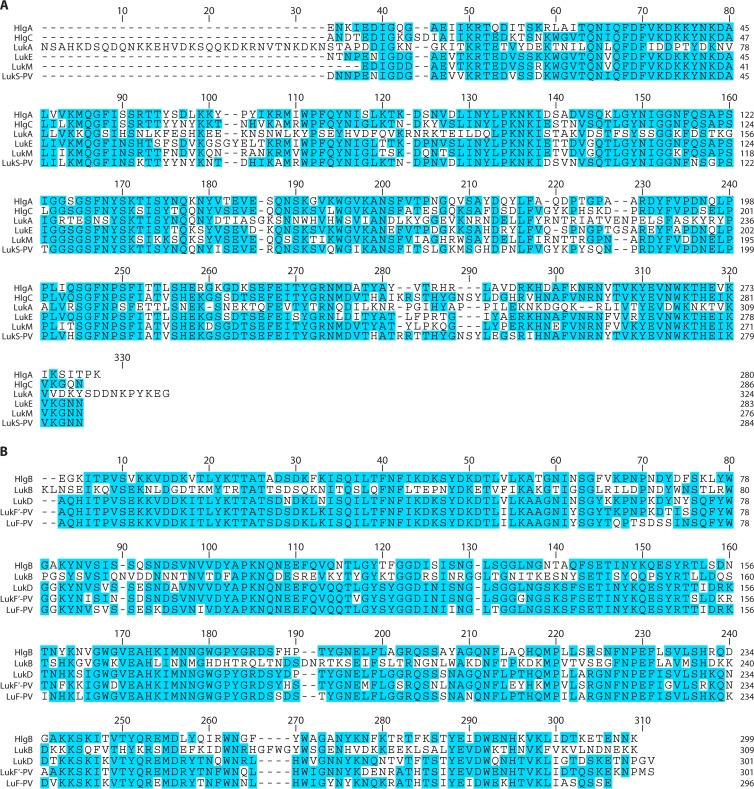
FIG 3.
Amino acid sequence alignment of mature leucocidins without their signal peptide. Amino acid sequence comparisons were generated by ClustalW alignment using Lasergene MegAlign Pro software (DNASTAR). Identical amino acids are shown in blue, and divergent residues are shown in white. (A) Alignment of the leucocidin S subunits HlgA, HlgC, LukA, LukE, LukM, and LukS-PV. Notable distinctions are the unique N- and C-terminal extensions that are present in LukA/H but absent from all other toxins. (B) Alignment of the leucocidin F subunits HlgB, LukB/G, LukD, LukF′-PV, and LukF-PV.
LEUCOCIDIN GENETIC ORGANIZATION AND GENOME DISTRIBUTION
The genetic architecture of the S. aureus leucocidins is largely conserved. Typically, S and F subunits of a given toxin pair are cotranscribed from a single promoter. The open reading frame of the S subunit immediately precedes that of the F subunit, with as little as a single nucleotide separating each open reading frame (Fig. 4). The only known exception to this rule is the gamma-hemolysin locus. Gamma-hemolysin comprises a genetic locus consisting of three open reading frames that reside within the core genome of S. aureus (85, 89, 98). The hlgC and hlgB genes consist of a single operon encoding the HlgCB toxin and are thus similar in genetic organization to other leucocidins (Fig. 4) (85). The independently transcribed hlgA gene sits upstream of the hlgCB operon (Fig. 4). The combination of the gene product of hlgA with that of hlgB, encoding the F subunit of HlgCB, constitutes the HlgAB toxin (85, 98). Thus, HlgAB and HlgCB share an F subunit. It is worth noting that the gamma-hemolysin subunits are not uniformly named in the literature, which is presumably a consequence of their identification in the pregenomic era but also may have been influenced by the presence of sequence variants isolated from the different S. aureus strains used in a variety of studies (90, 99–103). This has led to considerable confusion when interpreting studies of gamma-hemolysin from the late 1980s through the early 1990s. Table 1 provides a list of alternate names used to describe gamma-hemolysin for reference. In this review, the primary HlgACB nomenclature is adopted for ease of reference. The gamma-hemolysin locus is highly conserved in nearly all S. aureus lineages, as the genes are present in 99% of sequenced strains (95, 104). However, there is a degree of genetic diversity within their coding sequences (95). Based on this assessment, it appears that gamma-hemolysin represents a generally conserved set of leucocidins uniformly present in the core genome of S. aureus.
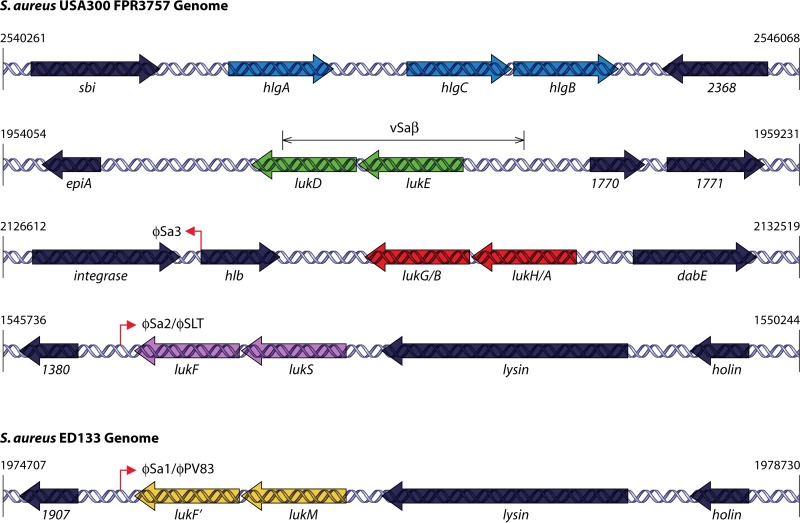
FIG 4.
Genome organization of the S. aureus leucocidins. Shown is a schematic representation of the S. aureus leucocidin genetic loci within the genome of the sequenced USA300 strain FPR3757 (GenBank accession number NC_007793.1) (blue arrows, hlgACB; green arrows, lukED; red arrows, lukAB [lukHG]; purple arrows, lukSF-PV) or the sequenced genome of bovine isolate ED133 (GenBank accession number NC_017337.1) (yellow arrows, lukMF′). Numbers to the right and left indicate the nucleotide base positions of the indicated region designated in the genome repository of the National Center for Biotechnology Information (NCBI). Vertical lines with arrowheads demarcate the location of prophage insertions (ϕSa3, ϕSa2/ϕSLT, and ϕSa1/ϕPV83) within the S. aureus genome. Flanking genes upstream and downstream of the respective leucocidins are supplied with either their designated nomenclature or their gene number. The S. aureus pathogenicity island vSaβ (the site where lukED is located) is indicated with branching arrows above the lukED-containing region.
Like gamma-hemolysin, LukAB/HG is also core genome encoded. The lukAB (lukHG) operon sits adjacent to the hlb gene (Fig. 4). hlb encodes beta-hemolysin, a sphingomyelinase that causes hemolytic activity on blood agar plates in a limited number of S. aureus strains (105–107). The gene is inactivated by lysogenic conversion in most S. aureus lineages that cause clinical disease (108, 109). As a result, the potential role of beta-hemolysin in pathogenesis is largely unknown. It is typically disrupted by the prophage ϕSa3, which carries genes encoding enterotoxins and the immune evasion molecules staphylococcal complement inhibitor protein (SCIN) and chemotaxis-inhibitory protein (CHIP) (95, 108–110). Importantly, it does not appear that prophage insertion into hlb in any way influences the adjacent lukAB (lukHG) genes. Additional studies are needed to determine whether the presence of lukAB (lukHG) is uniformly conserved among all S. aureus isolates, although the genes are found in all publicly available sequenced genomes, suggesting that it is likely stable. Similarly, additional work is needed to fully evaluate the extent and frequency of lukAB (lukHG) sequence variants among S. aureus lineages. It is well established that a number of genes on the core genome, such as spa (encoding protein A), exhibit significant variability within their coding sequences, while others exhibit less frequent variations (lukED) (95, 111). Our preliminary assessment of lukAB (lukHG) suggests that there is genetic diversity within the coding regions of a small subset of isolates, although the extent of this diversity and the functional consequences in terms of cytolytic activity and virulence characteristics remain to be determined (V. J. Torres, unpublished data).
Unlike LukAB/HG, leucocidin ED exhibits little to no sequence diversity among sequenced S. aureus strains (95). While its sequence is highly conserved, the presence of the toxin locus is lineage specific. It is completely absent from some S. aureus lineages, including clonal complexes (CCs) 22, 30, 42, 45, 75, 398, and 431 (95). Two independent studies predicted the overall frequency of the lukED locus to be approximately 70%, with strict lineage dependence (95, 104). Importantly, when present in a given lineage, the gene is fully conserved within all strains of that lineage (95). Higher lukED frequencies were predicted in a 2003 study by Morinaga et al. (87%), although this study may not have surveyed as diverse a pool of S. aureus lineages, leading to an overestimation of the actual frequency (94, 104). These more recent estimates of lukED frequency were derived from sequencing data and are contrary to early studies conducted when LukED was first identified (93). Gravet et al. suggested a lukED gene frequency of approximately 30% (93). We suspect that the methods used to identify lukED (antibody recognition and DNA hybridization) in this work, coupled with the anomalous sequence of the lukED gene, may have led to the underestimated gene frequency (93).
Unlike gamma-hemolysin and LukAB/HG, the lukED genes are located outside the S. aureus core genome (95, 112, 113). The genes are present in what is known as S. aureus pathogenicity island (SaPI) vSaβ (Fig. 4 and Table 1) (112, 114). This pathogenicity island is not a mobile genetic element, and unlike the mobile SaPIs or temperate phages, vSaβ is stable (110, 114–116). However, the gene content of vSaβ is not uniformly conserved (95, 112, 113). It can include a number of genes encoding enterotoxins, LukED, a lantibiotic system, and serine proteases, among other uncharacterized factors (112). The presence or absence of certain genes within any given strain is not universally conserved and is likely a consequence of unique horizontal gene transfer events that occurred within specific S. aureus lineages (95, 112, 113). For example, a high percentage of strains that lack the lukED genes appear to instead contain a locus comprised of enterotoxin-encoding genes (104). Thus, the lukED operon is unique from the other leucocidins described thus far in that it exists on a stable yet variable pathogenicity island that was acquired through prior horizontal gene transfer events.
In contrast to the core genome- and vSaβ-encoded leucocidins, Panton-Valentine leucocidin and LukMF′ are both located within the genomes of temperate phages (117–119). PVL is located on temperate phage ϕSa2 (ϕSLT), while LukMF′ is on phage ϕSa1 (Fig. 4 and Table 1) (110, 120–122). Both PVL and LukMF′ are present in an extremely limited range of S. aureus lineages (95, 123, 124). The prophage carrying PVL is present in only approximately 2 to 3% of all S. aureus isolates (125), although it is found at disproportionately high levels in strains that cause severe necrotizing pneumonia and community-acquired infections (>90%) (126–129). In fact, the predominant strain causing community-acquired disease in the United States, USA300, contains the prophage-carried PVL genes in the majority of isolates (128, 130). Such findings have led to the speculation that acquisition of the ϕSa2 prophage was at least partly responsible for the enhanced virulence of current epidemic and highly pathogenic strains of S. aureus (2, 126, 128, 131). Like LukED, LukSF-PV exhibits limited genetic diversity in its coding sequence among isolates (95, 132). Because of early correlative evidence suggesting a role for PVL in the success of epidemic strains of CA-MRSA, it has become the predominant leucocidin in clinical and epidemiological studies (126–128, 131, 133). However, it is now becoming less clear whether PVL should even be considered a major factor linked to the enhanced virulence of CA-MRSA (6, 113, 129, 134–137). Compelling evidence for the dominant role of other factors, such as the phenol-soluble modulins (PSMs) and alpha-hemolysin (both of which are highly expressed in CA-MRSA strains), in the progression of skin and soft tissue infection (SSTI) and necrotizing pneumonia highlights the alternate possibility that complex influences from multiple virulence factors have led to the increased incidence of highly virulent CA-MRSA strains (6, 17, 29, 138–141). Nonetheless, the potent activity of PVL on human immune cells together with its epidemiological association with severe disease support the notion that PVL could contribute to pathogenesis in a yet-to-be-identified capacity. Like PVL, the prophage encoding LukMF′ is found almost exclusively in strains that are isolated from ruminants, where the toxin is believed to play a significant role in the pathogenesis of mastitis (Table 1) (104, 123, 142–145).
LEUCOCIDIN MECHANISM OF ACTION: PORE FORMATION
Early investigations into the molecular mechanism of action of leucocidins on host cells began with biochemical studies by Woodin in the early 1960s. Although pore formation was not yet known to be the mechanism by which toxin-mediated cell death occurred, Woodin and Wieneke did observe alterations in ion fluxes, primarily potassium and calcium, and extrusion of cellular contents upon treatment with purified PVL (64, 66, 68). Woodin and Wieneke also observed subunit-dependent binding to cellular membranes and presumed that “polymerization” of the toxin was occurring upon interactions with the cell surface (61, 62). While pore formation was not conclusively demonstrated in any of these studies, a review by Rogolsky in 1979 suggested the possibility that PVL was forming a pore in host cell membranes (146). Follow-up studies by Noda et al. in the 1980s confirmed the increased influx of calcium upon treatment with leucocidin (HlgCB) and binding of the toxin to host cell membranes (147). Additional efforts by both Noda et al. and Morinaga et al. began to assess the ability of HlgCB and PVL to recognize host cell membranes with targeted specificity (148–150). However, none of these studies definitively recognized pore formation as a mechanism of toxin action.
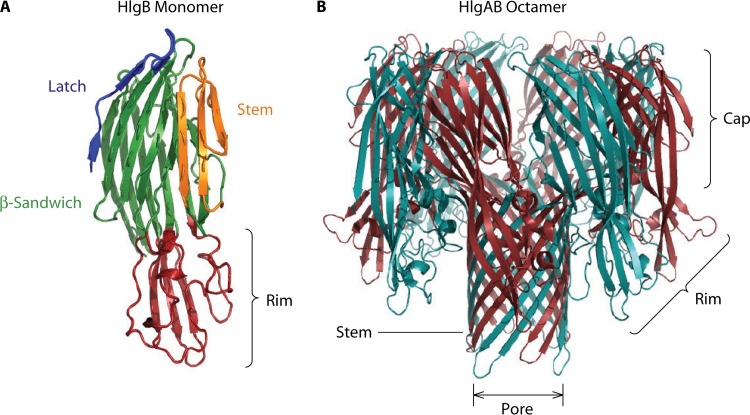
The classification of PVL and subsequent leucocidins as pore-forming toxins was not officially made until 1993, when Finck-Barbancon and colleagues performed a series of kinetic studies to measure calcium and ethidium bromide entry into PMNs (or neutrophils) upon treatment with PVL (151). In this work, it was proposed that ethidium entry into host cells, but not calcium entry, is a definitive marker of pore formation by leucocidins (152, 153). In contrast, calcium entry upon leucocidin treatment is believed to occur prior to pore formation and is mediated by the opening of membrane divalent cation channels (154, 155). In the 10 years following this early assessment of the leucocidin pore, a number of studies using gamma-hemolysin and PVL served to dramatically increase our understanding of the molecular characteristics of pore formation. This work has already been summarized extensively in a series of technical reviews (for details, see references 99–102). Notably, substantial biophysical measures of leucocidin pore formation and structural assembly on host cells were performed (156–159). Key studies included the determination that leucocidins form an octameric pore arranged as alternating S and F subunits (160–163) as well as detailed biochemical characterizations of residues required for toxin activity, including sites of phosphorylation (164, 165), subunit interactions (166–170), membrane recognition (171–174), and prepore-to-pore transition states (175–177). However, it was the crystallographic determination of toxin subunit structures that was transformative in our understanding of the leucocidins and the mechanism of pore formation on host cells (Fig. 5). In 1999, the F components of both gamma-hemolysin (HlgB) and PVL (LukF-PV) were crystallized, followed by the S component of PVL in 2004 and an engineered dimer of HlgAB in 2008 (178–181). Through these crystallographic studies, the major structural features of the leucocidins were described (Fig. 5). The individual S and F subunits were found to closely resemble the core structure of the previously identified alpha-hemolysin (182). Functional domains include the rim domain, composed of a high degree of aromatic residues that recognize and bind to phospholipids and other molecules; a glycine-rich and hydrophobic stem domain, which undergoes dramatic structural shifts and inserts into the membrane of host cells, forming a presumed β-barrel pore-like structure; the β-sandwich domain, containing key residues for intersubunit interactions; and an amino latch, which is believed to be involved in stem domain positioning during the monomeric-to-oligomeric pore transition for alpha-hemolysin, although the function may not be conserved in the leucocidins (Fig. 5) (169, 183–185).
FIG 5.
Leucocidin structural features. (A) Crystal structure of the monomeric F subunit of gamma-hemolysin (HlgB) (178). Structural information was acquired from the Protein Data Bank (PDB) (accession number 1LKF), and the major structural domains were colored by using PyMOL software. Blue, amino latch; green, β-sandwich; orange, stem domain; red, rim domain. (B) Crystal structure of the HlgAB octamer (190). The S subunit (HlgA) is in cyan, while the F subunit (HlgB) is in red. The stem, rim, and cap as well as the β-barrel pore are shown. Structural information for the HlgAB octamer was acquired from the PDB (accession number 3B07), and the major structural domains were colored by using PyMOL software.
By as early as 2007, a proposed model of leucocidin function on host cells emerged, which remains largely unchallenged today: (i) monomeric water-soluble toxin molecules are secreted by S. aureus; (ii) the S or F components recognize each other as well as proteins and/or lipids on the host surface in a species- and cell type-specific manner; (iii) host cell interactions typically lead to S component recognition and binding to the target cell, followed by F subunit recruitment, although in the case of gamma-hemolysin, the F subunit appears to bind first on red blood cells (186, 187); (iv) the toxin subunits oligomerize into an octameric structure composed of alternating S and F subunits, forming a prepore (188); and (v) dramatic structural shifts occur, leading to stem domain insertion into the host cell membrane and subsequent pore formation (Fig. 1). Additional studies have substantiated the biochemical characteristics and stoichiometry of the leucocidin pore through the use of photobleaching experiments and fluorescently labeled S or F subunits (189). In either case (LukS labeled or LukF labeled), photobleaching occurred in four intervals, confirming that the pore is comprised of four S subunits and four F subunits (189). Thus far, the majority of the biochemical evidence supports the notion that the leucocidins form octameric pores. Additional structural evidence in support of octameric pores was provided with the description of the crystal structure of a gamma-hemolysin (HlgAB) pore (190). The addition of HlgA and HlgB subunits to 2-methyl-2,4-pentanediol led to spontaneous toxin oligomerization in solution (similar to alpha-hemolysin), allowing crystallographic structural determination (182, 190). The HlgAB pore structure confirmed that the structurally favored orientation of gamma-hemolysin is an octamer and laid to rest a series of prior biochemical inconsistencies that had suggested that the leucocidin pore consisted of mixed hexamers and heptamers (161, 166). It is believed that these hexameric and heptameric structures were actually intermediates captured during the formation of a stable octamer rather than bona fide functional pores (190). Furthermore, atomic force microscopy studies confirmed that octameric pores are the most stable conformation of HlgAB within lipid membranes (191). Based on the sequence similarity of gamma-hemolysin, PVL, LukED, and LukMF′, it stands to reason that the octameric structure described for the HlgAB pore will closely resemble the pore structure of these other leucocidins (Fig. 5). A recent crystallographic assessment of the LukE and LukD monomers further supports this hypothesis, although additional structural studies of the LukED pore itself would provide the most conclusive evidence (192). LukAB/HG, the most divergent of the leucocidins, contains unique features that set it apart from the other leucocidins (Fig. 3), including N- and C-terminal extensions not present in any of the other toxins (97). Interestingly, this unique C-terminal extension was recently shown to contain a single amino acid that is absolutely critical for the recognition of the host cell surface by LukAB/HG and therefore its lytic activity (193). Such findings further support the notion that ascribing functional activities to LukAB/HG from prior research on and/or structures of the other leucocidins will be challenging. As a result, predictions of the structure and biochemical function of LukAB/HG will be reserved until further crystallographic studies are conducted. The divergence of this toxin from others in its class will certainly prove informative in understanding the range of functions associated with these molecules.
ROLES OF LEUCOCIDIN IN PATHOGENESIS
LukSF-PV (PVL)
Since its description in 1932, PVL has long been presumed to contribute to the pathogenic potential of S. aureus in humans. Unfortunately, demonstrating this through the use of experimental models has proven exceedingly difficult. Valentine and Butler provided early evidence that rabbits and humans develop antibody titers against leucocidins that are sufficient to block toxin activity in vitro (194, 195). Similarly, toxoid preparations of PVL administered to both humans and rabbits demonstrated specific antigenic recognition of PVL by the immune system, suggesting potential efficacy in promoting the clearance of S. aureus infection, although immunization as a preventative strategy was not tested (56, 58, 59, 196, 197). From these studies, it is difficult to ascertain whether PVL is responsible for the significant disease burden caused by S. aureus despite the fact that neutralizing antibodies are readily generated and some degree of infection resolution is observed upon the induction of an antibody response. A detailed discussion of the prospects of immunization with leucocidin toxoids as treatment modalities to promote S. aureus infection resolution is provided later in this review. Nonetheless, these immunization studies served as an early indicator that the functions of the leucocidins are likely to have relevance to pathogenesis in humans.
Studies of the role of PVL in virulence using animal models and isogenic pvl deletion mutants have likewise proven difficult to interpret (6, 19, 198). Murine models, which were originally used in a number of virulence studies, have since been proven unreliable in establishing virulence-associated functions of PVL. This is a direct consequence of the species specificity associated with PVL targeting of host cells (PVL cannot efficiently recognize its cellular receptor, C5aR, on murine cells) (199, 200). Even before the identification of the species-dependent recognition of the PVL cellular receptor, the relative ineffectiveness of the toxin in terms of its lytic activity on murine cells had been noted (201). Such studies presumably should have cautioned against the use of murine models to assess the virulence-associated characteristics of PVL (201). Regardless, murine models were still heavily implemented to evaluate the role of PVL in pathogenesis in vivo (139, 202–208). These murine studies are not discussed in great detail, given their exceedingly contradictory results. Instead, readers are encouraged to interpret murine studies of PVL pathogenesis with caution in light of the fact the PVL is unable to exert lytic activity on murine cells, and as such, the biological effects of active toxin are not being considered. Despite its lack of lytic activity on murine cells, there is compelling evidence indicating that PVL is capable of eliciting a proinflammatory response from murine immune cells. Thus, murine studies may be useful toward evaluating the proinflammatory effects of PVL independent of its lytic activity. In fact, the proinflammatory activity of PVL has been linked directly to the pathogenic characteristics of necrotizing pneumonia seen in mouse models (203, 206, 209, 210). Similar effects are seen in mouse models of severe skin and soft tissue infection (SSTI), where muscle tissue injury appears to be a direct consequence of the proinflammatory activity of PVL (205).
In rabbit models of necrotizing pneumonia, where PVL is fully lytic on host immune cells, this toxin dramatically increases proinflammatory responses in the lung (Table 1) (211). Injection of purified recombinant PVL leads to increased immune cell recruitment and increased architectural destruction of the lung due to toxin-mediated recruitment and subsequent lysis of immune cells (211). Compared to wild-type (wt) S. aureus, an isogenic deletion mutant of pvl exhibits reduced bacterial burden and inflammation in the lungs and leads to an overall increase in rabbit survival (211). Other models of infection using rabbits, such as SSTI, have led to more ambiguous results (Table 1). While some groups have reported a modest but significant role for PVL in the pathogenesis of early-stage SSTIs, others have seen no discernible contribution of PVL to disease (212, 213). In rabbit bacteremia models, PVL plays a negligible role in virulence (214). In contrast, PVL may contribute to S. aureus persistence in a model of osteomyelitis (Table 1) (215). Additional studies in primates were unable to uncover a role for PVL in a pneumonia model (216). The discrepancy in virulence characteristics associated with pneumonia in rabbits and primates likely stems from the fact that primate PMNs are also relatively insensitive to the lytic functions of the toxin (200). In summary, based on a wide variety of animal models, it remains difficult to attribute a specific pathogenic role to PVL. Thus far, it can only be firmly stated that PVL is proinflammatory in vivo and directly influences the severity of a limited number of conditions, including necrotizing pneumonia and osteomyelitis in rabbit models. Most other infection models remain largely inconclusive.
From an epidemiological perspective, strains containing the pvl genes are associated with more severe invasive disease and poor prognosis and are more likely to be isolated from community rather than hospital settings (2, 126–129, 131, 133). It is important to make the distinction that a higher incidence of PVL-positive (PVL+) strains causing community-associated infections and invasive disease does not necessarily imply that PVL alone is the factor responsible for the maintenance of certain infectious isolates within the population or their hypervirulence. In fact, studies now suggest that additional global alterations, including increased expression levels of core genome-encoded factors such as alpha-hemolysin and the phenol-soluble modulins, as well as acquisition of additional mobile genetic elements that confer a metabolic advantage and antibiotic resistance also contribute to the success of current epidemic strains (6, 11, 17, 29, 138–141, 217–219). In line with this supposition, recent studies have identified PVL-negative S. aureus strains, including pandemic isolates from South Korea, that are capable of persisting within communities despite their lack of the pvl-carrying prophage (113, 198, 218, 220). Thus, in these pandemic strains, other factors are most certainly responsible for the increased virulence and strain maintenance. Clearly, our understanding of PVL as a virulence factor is far from complete. Furthermore, it stands to reason that studies of PVL may directly benefit from investigations of other leucocidins, which have received relatively less attention but whose influence on pathogenesis is more easily evaluated using small-animal models and in vitro systems.
Gamma-Hemolysin (HlgAB and HlgCB)
In contrast to PVL, the contribution of gamma-hemolysin to S. aureus pathogenesis is less studied (Table 1). In ex vivo whole-blood infection models, a gamma-hemolysin mutant (hlgACB) is more susceptible to bacterial killing than wt S. aureus strains, although this manifests only as modest reductions in mortality rates during systemic infection of mice (221). Additionally, hlgACB mutants have been shown to contribute to the pathogenesis of septic arthritis, particularly when alpha-hemolysin (hla) is also deleted (222). Relative to wt strains, strains lacking both hlgACB and hla cause reduced frequency of arthritis and decreased weight loss and elicit lower proinflammatory cytokine (interleukin-6 [IL-6]) levels (222).
The remaining studies evaluating the contribution of gamma-hemolysin to pathogenesis concern ocular models of infection and inflammation (Table 1) (223–226). Intraocular injection of purified gamma-hemolysin into rabbit eyes leads to increased inflammation, hemorrhage, swelling, and retinal necrosis (225). The proinflammatory and pathological characteristics associated with the administration of purified toxin imply a functional role for the toxin in mediating intraocular pathogenesis. Experiments conducted with live bacteria confirm this supposition, as S. aureus gamma-hemolysin mutants exhibit reduced pathology and inflammation in a keratitis model of ocular infection as well as reduced eyelid inflammation and CFU recovered from infected vitreous fluid (223, 224, 226). However, hla is believed to contribute to an even greater degree to the pathogenesis of ocular infection and causes significant epithelial erosion during corneal infection (224). Together, these studies demonstrate that gamma-hemolysin is responsible for some of the pathological outcomes of ocular infection and associated inflammatory conditions and highlight the major proinflammatory properties of the toxin. Whether the proinflammatory activity of gamma-hemolysin occurs as a result of sublytic cellular engagement and subsequent activation of proinflammatory signaling cascades or is a consequence of overt cellular damage caused by toxin-mediated lysis is not clear from these studies. Additionally, the contributions of gamma-hemolysin to a wide range of infection conditions, including skin and soft tissue infection, osteomyelitis, and pneumonia, have yet to be investigated. More in-depth studies are warranted in this regard.
In addition to defining a role for gamma-hemolysin in the pathogenesis of ocular infection and septic arthritis, these infection models highlight an important point regarding bacterial toxin synergism and redundant functions as they relate to pathogenesis. Both alpha-hemolysin and gamma-hemolysin were shown to influence the pathogenic outcomes associated with ocular infection in similar ways (223, 224). Thus, by virtue of their redundant mechanisms of action in vivo, these two unique S. aureus cytotoxins have similar influences on overall pathogenic outcomes during infection. Such redundancies have the potential to complicate the assessment of the role of any one virulence factor in a specific disease model and as a result may significantly misrepresent in vivo contributions to infection. Later in this review, we describe recent efforts to decipher potential nonredundant functions of leucocidins and evaluate how these unique functional characteristics may drive pathogenic outcomes.
LukED
The implementation of murine infection models has proven particularly useful for studies of LukED function in vivo. This is primarily because LukED is one of the only leucocidins to exhibit broad activity on a wide variety of cell types from various species, including mice (HlgCB and HlgAB exhibit specificity for some but not all target murine cells) (Table 1) (47, 94, 227–229). The lytic activity of LukED on murine cells is similar to that on both rabbit and human leukocytes (47, 94). As a result, both murine and rabbit models have been informative in deciphering the mechanism of action of LukED in vivo. Purified recombinant LukED elicits inflammation and dermonecrosis upon injection into the skin of rabbits (94). Thus, like PVL and gamma-hemolysin (and possibly all leucocidins), LukED is capable of inducing a proinflammatory response in vivo. In murine models of systemic infection, LukED contributes significantly to animal mortality, as mice infected with a lukED mutant survive normally lethal acute infection (47, 227, 230). Compared to animals infected systemically with wt S. aureus, lukED mutant strains exhibit a reduced ability to replicate in target organs (liver and kidneys) and have decreased levels of circulating proinflammatory cytokines in their sera (47, 230). LukED is directly lytic to infiltrating phagocytic leukocytes in vivo, as evidenced by decreased cellular viability measured by flow cytometry (47, 227, 230). Much of the ability of LukED to influence pathogenesis in vivo is a direct consequence of its targeted killing of specific immune cells, including neutrophils, T cells, macrophages, NK cells, and dendritic cells (Table 1) (47, 227, 230). We discuss mechanisms of leucocidin targeting later in this review.
There is some correlative evidence to suggest that LukED-producing strains are isolated at a greater frequency from patients with impetigo, antibiotic-associated diarrhea, furuncles, and invasive blood-borne infections (104, 133, 231, 232). However, these studies were conducted when the lukED genes were believed to be present in ∼30% of all S. aureus strains. Since then, additional studies have indicated that the gene itself is lineage specific, but unlike PVL (present in only 2 to 3% of all S. aureus strains), it is present in a greater proportion (∼70%) of strains (47, 95). Thus, the early clinical associations of lukED with impetigo, antibiotic-associated diarrhea, and furuncles are intriguing but should be interpreted with caution, as they were based on a perceived lower global strain distribution of the toxin. It is clear that epidemiological studies of lukED (and most other leucocidins other than PVL) are in the early stages, and more detailed assessments of the breadth of strains harboring these genes will certainly provide novel insights into their association, or lack thereof, with various pathological outcomes.
LukAB/HG
Like PVL, the use of murine infection models to evaluate LukAB/HG pathogenesis is complicated by its limited lytic activity on murine immune cells (Table 1) (233). However, in low-dose murine systemic infection models, deletion of LukAB/HG leads to a reduction in bacterial burden (97). Similarly, deletion of lukAB (lukHG) in a strain of S. aureus containing a deletion in the gene encoding the transcription factor repressor of toxins (Rot), which leads to increased toxin production and hypervirulence, modestly reduces the hypervirulence associated with this strain (47). These data suggest that while LukAB/HG exhibits limited lytic activity on murine cells, other yet-to-be-determined functions may still facilitate pathogenic outcomes in murine infection models. In contrast, when using a murine systemic infection survival model, no differences were seen between the wt and a lukAB (lukHG) mutant (233). Thus, there is conflicting evidence from these models concerning the contribution of lukAB (lukHG) to pathogenesis using murine infection models. As with PVL, it appears that caution should be taken when interpreting the potential role of LukAB/HG in models where the lytic activity of the toxin is suboptimal. Like the other leucocidins, injection of LukAB/HG into rabbit skin induces inflammation at the site of inoculation albeit to a lesser degree than PVL (233). In contrast, measurements of abscess size and bacterial burden in rabbits infected with wt S. aureus or an isogenic lukAB (lukHG) or lukAB (lukHG)-pvl mutant showed no measureable differences (233). However, it should be noted that LukAB/HG is ∼10-fold less potent on rabbit immune cells than on human immune cells; thus, rabbit models may also prove suboptimal for measuring the potential contributions of LukAB/HG to pathogenesis (233).
Ex vivo infection models using primary human cells offer an alternate perspective into the perceived virulence potential of LukAB/HG (97, 234). wt S. aureus exhibits greater survival upon infection of PMNs than a lukAB (lukHG) deletion mutant (97, 234, 235). This is due largely to the greater PMN damage inflicted by LukAB/HG-producing strains (96, 97, 234, 235). One mechanism by which LukAB/HG promotes bacterial survival amid PMNs is by facilitating bacterial escape after phagocytosis. S. aureus lukAB (lukHG) deletion mutants escape less efficiently from phagocytosed PMNs, leading to reduced bacterial rebound after phagocytosis (234, 235). Similar escape defects are observed with the cytolytic phenol-soluble modulins (236). Such findings again highlight that the redundant effects imparted by cytotoxic molecules produced by S. aureus (in this case, PSMs and LukAB) are likely to synergize to promote optimal bacterial escape after PMN phagocytosis. Altogether, ex vivo studies demonstrate that LukAB/HG likely has major roles in promoting bacterial survival in the presence of infiltrating PMNs. Unfortunately, the described issues of species specificity make further in vivo analyses a challenge. LukAB/HG targets rabbit and mouse immune cells with significantly less potency than human and primate cells; thus, both models appear to grossly underestimate the influences of LukAB/HG on pathogenesis and have thus far led to conflicting conclusions (233). The development of more appropriate animal infection models will help further investigations of LukAB/HG pathogenic functions. Such models are discussed in greater detail later in this review.
LukMF′
LukMF′ is not found in any S. aureus isolates of human origin and is seen predominantly in strains isolated from cases of bovine mastitis (Table 1) (123, 124, 142). However, whether LukMF′ contributes to the pathogenesis of bovine disease is not well appreciated at this time. Purified recombinant LukMF′ is toxic to bovine neutrophils and macrophages and is capable of binding to murine neutrophils, macrophages, and T cells (123, 237, 238). It is the most potent leucocidin on cells of bovine origin and can be isolated from the mammary glands of cows with severe mastitis (239). Infected cows can elicit an immunological response to LukMF′, as antibody can be isolated from serum during the course of infection (239). Whether these associations indicate that LukMF′ plays a significant role in the pathogenesis of mastitis remains to be determined. Unlike the other leucocidins, which elicit strong proinflammatory responses upon administration, injection of LukMF′ into the mammary glands of cows does not lead to a pronounced proinflammatory response, as measured by leukocyte infiltration into the toxin-infused mammary glands (240). The administration of sublethal amounts of toxin to bovine mammary macrophages is unable to elicit the production of the proinflammatory cytokine IL-8 or IL-1β, in contrast to what is often seen for other leucocidins (240). Despite its clear association with strains of bovine origin and its absence from human S. aureus isolates, it remains difficult to conclude whether LukMF′ is a major virulence factor involved in the progression of mastitis or other bovine diseases. In fact, the presence of LukMF′ is not exclusively a defining characteristic of bovine isolates, as (i) not all bovine strains contain LukMF′ and (ii) ewes, goats, squirrels, and other mammals can be colonized by LukMF′-producing strains (123, 144, 241).
UNDERSTANDING SPECIES AND CELLULAR SPECIFICITY OF THE LEUCOCIDINS
As is clear from the previous section, studies aimed at evaluating the contribution of S. aureus leucocidins to pathogenesis have been hampered due to the limited host range associated with many of the toxins. In nearly all instances, this has negatively influenced the ability to interpret data, establish optimal infection models, and formulate firm conclusions. Additionally, most early studies focused largely on the similarities among the leucocidins, their ability to target PMNs, and the complications of interpreting outcomes caused by functionally redundant molecules. However, it is now becoming apparent that each toxin has evolved to recognize specific cellular subpopulations that may dictate unique pathogenic outcomes (Table 1). A number of recent studies have now more clearly defined the species and cellular specificity of the leucocidins and have had a major impact on our understanding of leucocidin biological functions and their potential diverse roles in infection biology. These studies are summarized below.
Leucocidin Cellular Receptors Dictate Cell and Species Specificity
LukED and CCR5, CXCR1, and CXCR2.
As mentioned above, LukED exhibits a broad host range in regard to its tropism for and lytic activity on immune cells. The toxin is capable of killing rabbit, murine, and human leukocytes with similar potency and is also able to target the lymphocytes of canines and fish (Table 1) (47, 94, 227–229). The mechanisms by which LukED, or any other leucocidin, exerts its cellular and/or species specificity have gone largely unrecognized over the past century. Most leucocidins are known to preferentially recognize certain cellular membrane lipids, and this lipid recognition is believed to facilitate the prepore-to-pore transition (61, 99, 178, 180, 242, 243). However, it is difficult to ascertain how membrane lipid recognition could also lead to the precise targeting of well-defined cellular subsets. Thus, it has been proposed that, rather than lipids, the leucocidins recognize distinct proteinaceous receptors on the cell surface to target and kill immune cells. Evidence for leucocidin cellular receptors other than membrane lipids is supported by a number of studies which demonstrate specific and saturable binding of the toxins on target cells (173, 174, 244). In line with this evidence, LukED was recently found to target the chemokine receptor and HIV coreceptor CCR5 to kill inflammatory macrophages, T cells, and dendritic cells (227). Thus, while phospholipid composition may play a role in some aspects of leucocidin cell surface recognition, the demonstration of LukED recognition of CCR5 indicates that the leucocidins are likely to target specific cellular subsets through binding of proteinaceous receptors. In agreement with previous studies that used fluorescently labeled toxins to demonstrate saturable binding kinetics on the surface of host cells, the LukE subunit binds specifically and saturably to CCR5-expressing cells. Binding of the S subunit (LukE) fits all previous models, which had predicted initial binding to the host cell surface via the S subunit of the toxin followed by F subunit recruitment (173, 227). Targeted killing of CCR5+ cells is fully recapitulated using primary human peripheral blood mononuclear cells (PBMCs) as well as upon the isolation of CCR5+ macrophages and T cells from S. aureus-infected mice (227). Additional in vivo studies indicate that LukED is capable of directly targeting CCR5+ macrophages during acute infection to promote S. aureus immune escape and facilitate disease progression (227). Remarkably, clinically approved CCR5 receptor antagonists block the interaction of LukED with its receptor, indicating that toxin-receptor interactions can be directly targeted in order to prevent leukocyte killing (227). The therapeutic potential of blocking of LukED targeting with pharmacological antagonists was not assessed in in vivo infection models due to the limited activity of the antagonists on murine receptors. However, the prospects of leucocidin inhibition using small molecules are promising given the potent inhibitory activity of the antagonists in in vitro models (245).
Interestingly, it has been known for some time that LukED targets and kills PMNs, yet these cells do not express CCR5 on their cell surface (47, 93, 94, 230). Murine models of systemic infection suggest that LukED is capable of influencing pathogenesis in a manner that is partially dependent but also independent of CCR5 surface expression (230). Thus, it stands to reason that LukED influences pathogenesis via additional mechanisms that are independent of its activity against CCR5+ cells. It was found that, in addition to CCR5, LukED targets CXCR1/2 on primary human neutrophils, monocytes, NK cells, and a subset of CD8+ T cells (230). Directed targeting of CXCR1 and CXCR2 on host cells is the mechanism by which LukED kills PMNs during systemic infection of mice. Similar to CCR5-dependent targeting of macrophages, CXCR1 and CXCR2 targeting of PMNs leads to more efficient immune escape by the bacterium and enhanced pathogenesis (230). Through the combined targeting of these three cellular receptors, LukED is able to facilitate the disarming of both innate and adaptive arms of the immune system. Interestingly, like gamma-hemolysin, LukED is the only other leucocidin known to lyse red blood cells (Table 1). It remains to be determined whether RBC lysis by LukED is a result of receptor-specific recognition or whether the targeting of RBCs represents a promiscuous nonspecific activity shared by LukED, HlgAB, and HlgCB.
PVL and C5aR and C5L2.
PVL and LukED share a high degree of sequence identity (>75%) (Table 1), yet various lines of evidence from early biochemical and receptor-specific studies support the notion that LukED and PVL do not share similar receptors (21). This evidence is as follows: (i) PVL has a more limited host range than LukED (it is not lytic on murine immune cells); (ii) PVL exhibits a more restricted immune cell-targeting profile, as it kills primarily neutrophils, monocytes, and macrophages; (iii) of the leucocidin subunits, only HlgC is capable of competing with fluorescently labeled LukS-PV for binding of the surface of primary leukocytes; and (iv) the most divergent regions of PVL and LukED occur in the rim domain of the protein believed to be most critical for receptor recognition and cellular specificity (Fig. 3 and 5) (153, 174, 200, 230). Interestingly, the ability of HlgC to successfully compete with labeled LukS-PV for cell surface binding suggests that PVL and HlgCB of gamma-hemolysin may target similar receptors on the cell surface, although this has not yet been validated experimentally (174).
A recent study by Spaan et al. used a leukocyte receptor antibody screening strategy to identify cellular factors for which PVL has a high affinity (199). They assessed the ability of LukS-PV to displace fluorescently labeled antibodies against leukocyte receptors present on the surface of host immune cells. These studies led to the identification of the seven-transmembrane G-protein-coupled receptors (GPCRs) C5aR and C5L2 as the receptors required for cellular targeting by PVL. C5aR/C5L2 recognition by PVL is mediated by both the core membrane-spanning portions of the receptors as well as their extracellular N termini (199). The toxin interaction occurs through the S subunit, is highly specific for human C5aR, and is less so for those of other species, including macaque and murine receptors. Thus, C5aR targeting by PVL provides a rationale for the species specificity associated with its lytic activity (199, 200). C5aR also serves as a receptor for the chemotaxis-inhibitory protein of S. aureus (CHIPS), which binds to the receptor and prevents neutrophil activation and recruitment (246–251). Indeed, CHIPS is capable of blocking the PVL interaction with C5aR on host cells (199). In this study, CXCR2 was used as the negative control to which PVL binding of C5aR was compared. In all cases, CXCR2 expression did not promote binding of PVL to the cell surface (199). Such findings are in line with the presumption that LukED (which targets CXCR2) and PVL, despite sharing a remarkable degree of sequence conservation, do not target the same receptors on host cells (199, 230). It is interesting that both LukED and PVL target seven-transmembrane GPCRs. As a result, it was speculated that GPCRs may be a conserved receptor class targeted by these highly similar toxins (199). By virtue of binding C5aR, LukS-PV not only exerts its lytic activity on target host cells but also can facilitate the priming of primary human PMNs for activation by proinflammatory stimuli such as N-formyl-Met-Leu-Phe (fMLP). This finding fully supports previous studies that suggested that PVL could promote the proinflammatory responsiveness of PMNs when applied at sublytic concentrations (199, 252, 253).
LukAB/HG and CD11b.
LukAB/HG shares only 30 to 40% sequence homology with the other S. aureus leucocidins (Fig. 3 and Table 1) (97). Thus, it stands to reason that its marked sequence diversity could result in unique functional characteristics that set LukAB/HG apart from other bicomponent leucocidins. Thus far, early evidence for unique biochemical and/or biological features is limited to three observations. First, unlike the other leucocidins, LukAB/HG appears to associate with the bacterial cell surface in certain bacteriological growth media (tryptic soy broth [TSB] and growth medium containing digested casein or Casamino Acids, yeast extract, and glycerophosphate [CCY medium]) (96, 234). LukAB/HG is the only bicomponent leucocidin known to establish what appears to be a functional reservoir of toxin on the bacterial cell surface. The precise role for surface-associated LukAB/HG is not yet completely defined; therefore, we will reserve speculation on the possible biological significance of active toxin reservoirs in the pathogenesis of S. aureus at this time. However, this novel characteristic of LukAB/HG suggests that the toxin is unlikely to behave in the exact manner as the other leucocidins. Second, a critical amino acid required for LukAB/HG receptor recognition was recently identified within a 10-amino-acid C-terminal extension that is not found in any of the other leucocidins. When the glutamic acid at position 323 of LukAB/HG is mutated to an alanine, the toxin is no longer able to recognize its cellular receptor (CD11b) (discussed below), rendering it inactive on host cells (193). Finally, evidence was recently provided to suggest that LukAB/HG exists as a stable heterodimer in solution. The formation of stable heterodimers contrasts with that of the other leucocidins, which are believed to exist primarily as water-soluble monomers in solution. It has been proposed that such heterodimers may enhance the potency of LukAB/HG by eliminating the need for a cell surface dimerization step during pore formation (193). Together, these early findings strongly suggest that there are a number of major structural and functional differences between LukAB/HG and the other bicomponent leucocidins. Additional studies will provide important insights into the ways in which this diversity influences the toxin's activity and role in pathogenesis.
LukED and PVL both recognize GPCRs that are important for immune cell activation and chemotaxis (199, 227, 230). It is thus tempting to speculate that highly similar leucocidins (PVL, LukED, HlgCB, HlgAB, and LukMF′) will also use GPCRs to recognize and kill host cells. In contrast, recent studies indicate that LukAB/HG does not target a GPCR, in line with the hypothesis that the sequence divergence of this toxin is likely to dictate unique functional attributes. A biochemical approach was recently used to identify the cellular receptor targeted by LukAB to promote its lytic activity on immune cells (235). A pulldown of solubilized membrane proteins incubated with LukAB showed that CD11b, a component of the αM/β2 integrin Mac-1, is required for LukAB targeting of primary human neutrophils (235). The toxin interacts specifically with the I-domain of CD11b, the primary site of endogenous ligand binding. Binding to the I-domain is entirely species dependent, as the affinity of LukAB for the I-domain from murine CD11b is negligible (235). This finding serves to explain why murine leukocytes, many of which express significant levels of CD11b, are largely resistant to even high doses of the toxin (233, 235). Thus, like PVL, the ability of LukAB to recognize its cellular receptor on the host cell membrane dictates cell type and species specificity. The identification of CD11b as the receptor for LukAB has provided mechanistic insight into the toxin's ability to facilitate S. aureus intracellular escape upon phagocytosis by PMNs. It was found that after phagocytosis, CD11b is accessible for LukAB-mediated targeting of S. aureus-containing phagosomes. This intracellular targeting facilitates rapid bacterial escape mediated by neutrophil lysis (235). The precise biochemical mechanism by which LukAB engages its receptor remains to be determined.
Gamma-hemolysin.
No proteinaceous receptors have been reported for either HlgAB or HlgCB of gamma-hemolysin. Previous studies suggested that HlgCB and PVL likely share a similar receptor based upon the ability of HlgC to compete with LukS-PV for cell surface binding (174). Such direct competition implies that HlgC and LukS-PV share C5aR and/or C5L2 as a cellular receptor, although this has not been experimentally validated. Beyond this assessment, no additional studies have investigated the possibility that gamma-hemolysin targets a proteinaceous receptor to mediate host cell killing. In contrast, a number of studies have suggested that gamma-hemolysin recognizes and binds to the membranes of red blood cells through direct interactions with host lipids and glycolipid derivatives (150, 187, 242, 254). As early as 1980, Noda and colleagues identified the ganglioside GM1 as an efficient inhibitor of HlgCB-dependent (referred to as LukSF or leucocidin here) killing of rabbit leukocytes in vitro (150). GM1 is a sialic acid-containing oligosaccharide linked to a ceramide lipid and is found at significant levels on cells within the nervous system but can be found in the membranes of many cell types (255). Preincubation with GM1 for as little as 5 min was sufficient to inhibit toxin activity at a molar ratio of 1:1 (150). This interaction occurred specifically through the S subunit of the toxin, HlgC (150). The isolation of a truncated mutant of HlgC lacking 17 amino acids at its C terminus prevented the recognition of GM1 with the toxin, indicating a potential role for these residues in ganglioside recognition (256). Similar work with HlgAB indicated that GM1 is also capable of inhibiting activity through binding of the S subunit, HlgA, at a similar 1:1 molar ratio (257). The inhibition studies with HlgAB were conducted by using rabbit erythrocytes rather than leukocytes, due to the perceived principal role of HlgAB as a hemolysin at that time. Thus, the influences of GM1 on leukocyte targeting by HlgAB are not known. The fact that GM1 is abundant on the outer leaflet of most cell types further supports the notion that gamma-hemolysin could be using this ganglioside to associate with cell membranes; however, it does not explain the cell type specificity of the toxin (erythrocytes and immune cells) (255). Based on the recent identification of the proteinaceous receptors for LukED, LukAB/HG, and PVL, it seems unlikely that GM1 is a primary receptor required for gamma-hemolysin targeting. Rather, it may serve as an accessory surface association motif that promotes stabilization of the toxin on the cell surface to facilitate membrane insertion. Further studies of gamma-hemolysin are needed to more clearly incorporate the roles of GM1 and potential proteinaceous receptors into a framework of cellular targeting and pore formation. Regardless, GM1 was shown to display remarkable inhibitory activity against gamma-hemolysin and has proven a useful tool for evaluating regions of the toxin required for cell surface recognition.
Additional studies evaluated the possibility that membrane lipids might serve as major cell surface recognition sites for gamma-hemolysin. Given data that demonstrate saturable and specific binding kinetics on target host cells, it seems unlikely that gamma-hemolysin recognizes lipid motifs exclusively to cause cell lysis. However, enrichment of certain lipids in raft-like microdomains or under artificial conditions may allow for an enhanced pore-forming capacity in a manner that is independent of receptor recognition (191, 258). In fact, synthetic vesicles composed of defined lipids can lead to HlgAB-mediated pore formation in the absence of a protein receptor or GM1 (242). The efficiency of pore formation in this synthetic model is increased in the presence of phosphocholine-containing lipids as well as phosphatidylethanolamine but not sphingomyelin (242). Phosphatidylcholine has been shown to bind specifically to the F component (HlgB) with a molar ratio of 1:1, like that of GM1 with HlgA and HlgC, and the addition of exogenous phosphatidylcholine prevents the lytic activity of HlgCB on rabbit leukocytes (150, 243). The solved crystal structures of both HlgB and LukF-PV further support the notion that lipids are likely recognized by the bicomponent leucocidins, as both structures were cocrystallized in the presence of phosphatidylcholine (178, 179). While the identification of optimal lipid compositions required for pore formation are useful in terms of deducing how lipid chain length and membrane flexibility modulate pore-forming capacity, such investigation bypasses important influences that may occur due to proteinaceous receptor-dependent recognition by gamma-hemolysin on host cells. Based on the evidence provided, it seems likely that a combination of both optimal lipid microenvironments and membrane receptor recognition motifs on host cells dictates the activity of gamma-hemolysin on host cells, although additional studies are needed to determine whether or not this is actually the case.
INFLUENCES ON CELL SIGNALING AND INFLAMMATION
Inflammation Induced by Lysis
Given that leucocidins exhibit potent lytic activity on host immune cells, it is reasonable to predict that a robust inflammatory response will be induced in response to the cellular damage and release of cytosolic contents associated with cell killing. This toxin-mediated proinflammatory induction of the immune system is believed to be responsible for the pathological features of severe necrotizing pneumonia caused by PVL-producing S. aureus (127, 203, 204, 206, 211). Treatment of leukocytes with lytic concentrations of PVL leads to the release of potent proinflammatory mediators such as IL-8, histamine, and leukotrienes (259, 260). IL-8 is a major chemotactic cytokine that influences neutrophil recruitment, and histamine is most commonly associated with proinflammatory allergic reactions and vasodilatation, while leukotrienes, along with prostaglandins (metabolites of arachidonic acid), contribute to acute inflammation (261–263). Beyond proinflammatory mediators, the lytic activity of the leucocidins also leads to the release of major cytoplasmic enzymes that can act locally to cause tissue damage and further elicit proinflammatory mediators (68, 259). Thus, by virtue of their lytic activity on host immune cells, the leucocidins engage in two activities: (i) they prevent host immune cells from phagocytosing and killing S. aureus, and (ii) they induce substantial inflammation and cellular damage through the release of proinflammatory mediators and tissue-damaging enzymes, both of which presumably contribute to the severity of disease.
Proinflammatory Receptor Engagement
The lytic capacity of leucocidins is certainly critical to their primary roles in immune cell killing and pathogenesis. However, a substantial body of evidence now suggests that most, if not all, leucocidins have bona fide immune cell-activating properties and/or additional sublytic functions that occur in the absence of cell lysis (Fig. 6) (210, 233, 252, 253, 264–266). Most studies evaluating the proinflammatory signaling properties of the leucocidins stem from work done with PVL and gamma-hemolysin (210, 252, 253, 264–266). To evaluate proinflammatory signaling, the toxins are typically applied at sublytic concentrations or as single subunits so that overt cell lysis does not appreciably obscure other mechanisms by which the proinflammatory response is activated. Noda et al. demonstrated that HlgC of gamma-hemolysin was capable of inducing neutrophil chemotaxis as well as phospholipase A2 activity, which leads to the subsequent release of arachidonic acid and prostaglandins (147). Arachidonic acid is the major metabolite of proinflammatory prostaglandins and leukotrienes; thus, their release by HlgC-treated leukocytes is likely to have significant influences on host inflammation (267, 268). Colin and Monteil first examined the capacity of leucocidins to engage in what was referred to as PMN priming (265). They showed that various combinations of gamma-hemolysin and PVL, when applied at sublytic concentrations (HlgAB, HlgA–LukF-PV, LukSF-PV, and HlgC–LukF-PV), are capable of priming neutrophils for increased production of H2O2 upon treatment with fMLP, although at higher concentrations, the leucocidin combinations were perceived to be inhibitory (Table 1) (265). Later studies confirmed that sublytic addition of PVL to primary human PMNs leads to priming for inflammatory reactivity (252). Such enhanced responsiveness includes increased reactive oxygen species production upon the addition of fMLP that is independent of Toll-like receptor 2 (TLR2) and cluster of differentiation 14 (CD14) signaling, increased phagocytosis and killing of S. aureus, and increased production of major proinflammatory mediators (Fig. 6) (252). Much of this proinflammatory priming is likely due to engagement of the C5a receptor, the cellular target of LukS-PV (199). Spaan et al. demonstrated that pretreatment with LukS-PV enhances reactive oxygen species production in response to fMLP in a C5aR-dependent manner (199). Thus, toxin-receptor interactions appear to be key to the induction of the nonlytic proinflammatory activities of PVL. In contrast to PVL, LukAB/HG does not appear to induce neutrophil priming, as treatment with the toxin does not lead to an increased production of reactive oxygen intermediates and does not influence phagocytosis or bactericidal activity, although it may indirectly influence inflammation through the induction of neutrophil extracellular trap (NET) formation and increased CD11b expression (269).
FIG 6.
Sublytic effects of S. aureus leucocidins. Sublytic activities of leucocidins have been investigated primarily for PVL and gamma-hemolysin. Some sublytic functions are shown. (1) Priming of PMNs through the engagement of cellular receptors and other mechanisms yet to be defined that lead to increased reactive oxygen species formation, enhanced granule exocytosis, robust phagocytosis, and increased bactericidal activity of host neutrophils. (2) Induction of the NLRP3 inflammasome and subsequent IL-1β release mediated by potassium efflux from the cytosol due to pore formation. (3) Stimulation of immune cell chemotaxis and NF-κB activation as a result of calcium influx. The subsequent activation of cellular kinases leads to IκB phosphorylation and targeted degradation, followed by translocation of NF-κB to the nucleus and induction of proinflammatory gene expression. (4) Engagement of Toll-like receptors (TLR2 and TLR4) to stimulate the same canonical NF-κB activation pathway described above (3). (5) Activation of apoptosis via mitochondrial disruption potentially caused by pore formation.
Despite the perceived TLR2/CD14-independent role of PVL in PMN priming, Zivkovic and colleagues reported an in vivo immunomodulatory response that appears to rely upon toxin engagement of TLR2, leading to the activation of NF-κB through the canonical pathway (IκB-α phosphorylation that leads to proteasomal degradation and translocation of NF-κB to the nucleus, followed by subsequent NF-κB-dependent gene expression) (Fig. 6) (270). Furthermore, a direct interaction of LukS-PV with TLR2 was demonstrated by enzyme-linked immunosorbent assays (ELISAs), and knockout of both TLR2 and CD14 rendered cells largely unresponsive to the toxin (270, 271). A similar study proposed that HlgCB interacts with Toll-like receptor 4 (TLR4) (a pattern recognition receptor known for recognizing lipopolysaccharide [LPS] of Gram-negative bacteria to induce inflammation) to induce the production of IL-12-p40 and tumor necrosis factor alpha (TNF-α) from murine bone marrow-derived dendritic cells (Fig. 6) (272, 273). Thus, it appears from these studies that the leucocidins are likely to engage classical pattern recognition receptors, in nonclassical ways, to induce inflammatory responses. More in-depth investigation will serve to validate these findings and determine how such altered proinflammatory signaling through TLR2 and TLR4 impacts global immune responses to S. aureus.
Inflammasome Activation
In addition to its described proinflammatory priming of PMNs, PVL is also known to bind to both monocytes and macrophages (likely due to its recognition of C5aR on the cell surface) and elicit cellular responses (174, 253). When PVL binds to the surface of monocytes and macrophages, significant increases in IL-1β release are observed (Table 1). IL-1β is a major proinflammatory cytokine that is produced during a cellular process known as inflammasome activation (Fig. 6) (253). This cytokine can activate neutrophils and induce the expression of additional proinflammatory cytokines such as TNF and IL-6 (for detailed information on the inflammasome, the production of IL-1β, and its influence on the host response to infection, see references 274–278). One major inflammasome complex, known to respond to pore-forming toxins to induce the release of IL-1β, is the NLRP3 inflammasome (278–280). PVL-dependent induction of IL-1β release from monocytes and macrophages appears to be directly dependent on NLRP3 inflammasome activation, similar to what has been observed for alpha-hemolysin (Fig. 6) (253, 281). In support of this study, others have determined that PVL is the primary leucocidin responsible for the release of IL-1β by primary human macrophages, although it was found that HlgCB also induces IL-1β release albeit to a lesser extent (266). Leucocidin synergism with other toxic molecules produced by S. aureus was also found to effectively enhance the release of IL-1β induced by PVL, highlighting the complex nature of the inflammatory response that likely occurs during S. aureus infection (266). The increased IL-1β release by macrophages in response to a sublytic administration of PVL was shown to stimulate cocultured cells (in this case, alveolar epithelial cells) to release the proinflammatory cytokines IL-8 and macrophage chemotactic protein 1 (MCP-1) via cytokine-dependent activation of the IL-1 receptor (266). This study is particularly informative, as it directly links the functional consequences of IL-1β release by immune cells in response to PVL to the induction of proinflammatory signaling by epithelial cells, which ultimately leads to increased immune cell recruitment during infection. An important difference between the above-described two studies is their use of moderately lytic concentrations (253) versus sublytic concentrations (266) of PVL to induce IL-1β production. The fact that sublytic toxin concentrations are capable of inducing IL-1β release supports the hypothesis that toxin-mediated signaling events occur through direct cellular recognition strategies and are not simply an effect of overt toxin-induced lysis. Signaling by leucocidins to induce the release of IL-1β by immune cells may also extend into the neuronal compartment, as microglia are known to produce IL-1β in a manner that is partially dependent on the presence of secreted gamma-hemolysin (282). Thus, in addition to the lytic activity of the S. aureus leucocidins, the capacity to induce proinflammatory signaling may also have dramatic influences on ultimate infection outcomes.
Other Accessory Toxin Effects
Thus far, PVL and gamma-hemolysin have been most intensely studied in terms of their nonlytic effects on host cells. However, LukED has been demonstrated to inhibit lymphocyte proliferation at high concentrations but to stimulate lymphocyte proliferation at low concentrations (229). The mechanism by which this occurs is unknown, as this study was conducted on carp lymphocytes, which, to our knowledge, have not been tested for susceptibility to LukED or for receptor recognition (229). Given the known receptor-dependent targeting of lymphocytes of both murine and human origins, it is possible that LukED may also target carp lymphocytes in a receptor-dependent manner to elicit a lymphoproliferative response at sublytic concentrations. Similar studies conducted on canine lymphocytes demonstrate that high concentrations of LukED limit lymphocyte proliferation, although this is likely due to the lytic capacity of LukED on these cells (228). With the recent identification of the receptors required for LukED immune cell targeting, more detailed studies of the potential influence on cell signaling can be conducted. Thus far, there is no indication that the LukE subunit alone can elicit signaling events through either CCR5 or CXCR1/2 insofar as toxin treatment is unable to elicit calcium signaling through receptor recognition (227, 230). However, recent proteomic studies indicate that the addition of lytic concentrations of LukED to PMNs induces the production of major proinflammatory proteins and support the notion that most, if not all, leucocidins are capable of inducing inflammation to some degree (Table 1) (283).
A unique activity of PVL is its ability to induce apoptosis at sublytic concentrations (Fig. 6) (284). The administration of PVL at low doses leads to characteristic morphological changes associated with apoptosis, including chromatin condensation and cell rounding (284). Intoxicated cells stain positive for annexin V but are not permeable to propidium iodide, a phenotypic hallmark of apoptotic cells. These apoptotic characteristics are linked to mitochondrial disruption and activation of the proapoptotic caspases caspase-3 and caspase-9 (284). Localization of recombinant PVL to the mitochondria after subcellular fractionation suggests that the toxin may exert deleterious effects on the mitochondrial membrane, leading to the induction of apoptosis. While the implications of PVL-dependent initiation of apoptosis are intriguing, it is important to note that studies describing the toxin's proapoptotic effects were limited to the use of recombinant PVL and bacterial culture supernatants. Additional work is needed to evaluate whether PVL-dependent apoptosis is a biologically relevant sublytic function and whether mitochondrial membrane disruption is a direct consequence of pore formation at the level of the mitochondrion or a downstream consequence of pore formation at the cellular membrane.
A novel sublytic activity of the gamma-hemolysin pair HlgCB is its ability to induce the release of calcium from internal stores within neuronal cells (285). Sublytic concentrations of HlgCB, and, to a lesser extent, HlgAB and PVL, are capable of stimulating glutamate release from cerebellar granular neurons. The release of glutamate was subsequently determined to be a direct consequence of alterations in intracellular calcium release that were induced by leucocidin cellular engagement (285). As a result of this study, it has been suggested that the leucocidins could foreseeably play a role in neuronal tissue damage, thereby influencing the perception of pain during invasive infection with S. aureus. A recent study suggested that S. aureus is indeed able to directly induce sensations of pain through the action of a pore-forming toxin (in this case, alpha-hemolysin) on nociceptive neurons; however, the bicomponent leucocidins were not implicated (286). Thus, the biological functions of glutamate release caused by HlgCB on granular neurons remain to be determined.
MIXED PORES AND TOXIN SYNERGISM
The genes encoding the S and F subunits of a given leucocidin (LukED, LukAB, LukSF-PV, LukMF′, and HlgCB) sit directly adjacent to one another in the S. aureus chromosome and are cotranscribed (Fig. 4). The pairing of the S subunit with its genetically linked F subunit always results in the formation of an active toxin on target host immune cells. As such, native pairings of leucocidins are assumed to be the most common biologically active toxins seen in vivo. However, the high degree of sequence similarity among the leucocidins and the unconventional pairing of HlgA with HlgB, despite being translated from independent transcripts, imply that other mixed pairings of leucocidins may be able to form biologically active toxins capable of causing lytic activity or influencing the responses of host cells. Indeed, Prevost et al. performed studies with mixed-subunit pairings of gamma-hemolysin and PVL and found that while hemolytic activity was restricted to pairings of gamma-hemolysin subunits only, PMN lytic activity was not (98). Combinations of LukF-PV and either HlgA or HlgC and combinations of HlgB and LukS-PV caused lysis of primary PMNs (98). In addition, Colin and Monteil provided compelling evidence that mixed pairings of gamma-hemolysin and PVL subunits can induce immune cell activation by priming neutrophils with similar capacities (265). Upon the identification of LukED, additional combinations of leucocidin subunits were found to have lytic activity on both RBCs and PMNs. Hemolytic activity was seen for a number of nonconventional subunit combinations, including LukE plus HlgB, HlgA plus LukD, and HlgA plus LukF-PV (93). An even greater repertoire of toxin combinations exhibited activity on PMNs, as determined by induction of calcium mobilization. However, measurement of ethidium bromide uptake as a readout for pore formation demonstrated that mixed subunits of gamma-hemolysin and PVL were the only toxin combinations capable of inducing robust cell lysis, consistent with what had previously been described (93). In contrast, Morinaga et al. demonstrated that their identified “variant” of LukED (later determined to be highly conserved in nearly all sequenced strains of S. aureus) could form active toxins through nonconventional subunit pairings of both PVL and gamma-hemolysin subunits, indicating that the overall diversity of active toxins can become quite large (94). It was further demonstrated by Meyer et al. that S subunits efficiently recruit nonconventional F subunits to the surface of host cells (244). For example, HlgB and LukD can be recruited to the surface of cells that have been pretreated with LukS-PV, while LukS-PV or HlgC can recruit LukF-PV to the cell surface (244). This study did not measure whether these mixed pairings form functional toxins; however, it provides further evidence that within mixed populations, leucocidin subunits are capable of assembling the constituents of active toxins albeit comprised of nonconventional subunit pairs. Upon the identification of LukAB/HG, it was also found that the pairing of either the S or F subunit with the respective F or S subunit of PVL leads to the formation of an active toxin on the human macrophage-like cell line THP1 (266). Still, there are major outstanding questions concerning whether nonconventional leucocidin pairings occur in vivo and the exact functional consequences of these pairings as they relate to leucocidin synergism and expanding the diversity of immune cell targets.
LEUCOCIDIN REGULATION
Influences of Medium Composition and Growth Conditions
Environmental factors/conditions play a significant role in dictating differential leucocidin expression profiles and can have major influences on the ultimate cytotoxic activity of S. aureus (Fig. 7). Early investigations into the complex environmental stimuli that influence leucocidin expression were provided by Panton and Valentine, Woodin, and Gladstone and coworkers, who demonstrated that specific growth conditions and medium compositions can promote abundant secretion of PVL by S. aureus (44, 45, 64, 287). These studies defined optimal conditions that lead to an increased abundance of PVL in culture supernatants for purification purposes. The use of CCY medium strongly induced the production of PVL, in particular by S. aureus strain V8 (44, 55, 59). This medium continues to be used by a number of research groups to purify the toxin from culture filtrates (98, 288, 289). Brain heart infusion (BHI) medium also leads to increased expression levels of PVL in broth culture, with comparatively lower expression levels of the other leucocidins, although gamma-hemolysin gene expression and toxin activity can also be measured in this medium (221). In medium containing yeast extract, Casamino Acids, and sodium pyruvate (YCP medium), the expression level of LukED is increased (93). Growth of strain Newman in YCP medium sufficiently increased the production of LukED over that of gamma-hemolysin to allow its initial purification and identification from S. aureus culture supernatants (93). In contrast, LukED is expressed at low levels in most other media, including BHI medium, RPMI medium supplemented with Casamino Acids (RPMI-CAS), tryptic soy broth (TSB), as well as lysogeny broth (LB) (47, 97, 221). The level of LukAB/HG expression is highest during growth in RPMI-CAS, whereas other leucocidins are expressed at comparatively low levels in this medium (97, 234). In LB medium, PVL is more abundantly expressed than LukAB/HG, while in TSB, both PVL and LukAB/HG are produced in relatively similar abundances compared to other leucocidins, which are minimally expressed (234). LukAB/HG is also produced at high levels in CCY medium, as evidenced by its contribution, along with PVL, to the cytolytic activity of CCY culture filtrates in vitro (96). Additionally, early efforts to purify PVL demonstrated a dependence on CO2 for optimal leucocidin expression (290). A CO2 percentage as high as 40% was used for the generation of preparative cultures of S. aureus for PVL purification (290). While CO2 may enhance the production of toxins, it does not appear to be required for abundant secretion of PVL, as most other studies cultured S. aureus under normal atmospheric conditions. Interestingly, DuMont et al. used luciferase reporter strains of S. aureus to demonstrate that the lukAB (lukHG) promoter is significantly upregulated over other leucocidins when cultured in the presence of primary human neutrophils. Such findings indicate that additional host-derived signals may directly influence leucocidin gene expression (234). In all cases, the media and growth conditions described above are relatively complex; thus, it is unknown what specific factors may be promoting the production of one leucocidin over another. These studies do highlight a number of critical considerations: (i) in vitro growth conditions must be carefully considered when evaluating in vitro leucocidin activity, (ii) the perceived dominance of any leucocidin under one growth condition is not necessarily the same under another, (iii) extrapolation of the relevance of one leucocidin to pathogenesis based solely on in vitro studies should be made with caution, and (iv) it is likely that complex environmental cues within an infected host may have effects similar to those seen in broth culture experiments, as the diversity of leucocidins in one infectious site may vary considerably from that in another. The described variations in toxin gene expression in complex media are likely a direct consequence of the diverse regulatory inputs known to exist in S. aureus, as many of these systems are believed to be highly responsive to environmental stimuli. Some major regulatory systems known to influence leucocidin gene expression are discussed below.
FIG 7.
Leucocidin gene regulation. The molecular details of leucocidin gene regulation have not been extensively defined, but a number of master regulators and external signals provide major inputs into their altered gene expression in various environments. Some major regulatory inputs are shown. (1) The Agr quorum-sensing system is activated upon reaching a high bacterial density, leading to AgrA-dependent activation of the P3 promoter, which encodes the regulatory RNA, RNAIII. RNAIII negatively regulates the translation of the leucocidin repressor Rot, leading to increased leucocidin production. (2) The global regulator SarA indirectly facilitates leucocidin expression by positively regulating the expression of the P3 promoter, leading to a similar repression of Rot translation and increased leucocidin production. (3) Rot is believed to bind directly to leucocidin promoters to inhibit toxin gene expression. (4) The SaeRS two-component system recognizes external stimuli from the environment, leading to direct binding of SaeR to leucocidin promoters and subsequent enhancement of gene expression. (5) Other environmental stimuli positively and negatively regulate leucocidin gene expression, although the precise stimuli and their mechanism(s) of activation/repression are yet to be defined.
Regulation at the Transcriptional Level
Bacterial gene regulation of the bicomponent leucocidins is complex (Fig. 7). Multiple inputs from a variety of global regulatory systems feed into one another to exert precise control over toxin gene expression. It is becoming clear that while there are dominant inputs, the leucocidins display differential regulatory patterns that still require substantial investigation. Semiquantitative reverse transcriptase PCR (semi-qRT-PCR) studies confirmed previous studies indicating that YCP medium increases leucocidin expression compared to that in BHI medium (291). These transcript studies also demonstrated growth phase-dependent expression of the leucocidins, with greater transcript abundances of hlgA, hlgCB, lukED, and lukSF-PV as the bacteria entered late exponential and early stationary phases (291). Such growth phase-dependent gene expression is suggestive of regulatory input imparted by the accessory gene regulatory (Agr) system in S. aureus (Fig. 7) (292). The Agr system (composed of 4 genes, agrBCDA) is a quorum-sensing system that becomes activated upon reaching a high bacterial density (292). agrC and agrA encode a histidine kinase and a response regulator, respectively, like other canonical two-component regulatory systems. AgrC is activated upon reaching a high bacterial density due to the increased effective concentration of a small peptide ligand processed from AgrD by AgrB (293–295). This small peptide is known as an autoinducing peptide (AIP) that interacts with AgrC to activate the AgrCA two-component system (294, 296). Agr activation leads to the increased transcription of two RNAs, RNAII (encoding AgrBCDA) and RNAIII, a regulatory RNA that inhibits and/or promotes the translation of multiple gene products, including a transcription factor known as repressor of toxins (Rot) (292, 297–300). Rot is so named due to its ability to act as a repressor at S. aureus toxin promoters; however, it is known to also activate promoters of other virulence genes (Fig. 7) (301–304). Rot is negatively regulated at the translational level by RNAIII, such that when the Agr system is activated, RNAIII inhibits Rot translation due to antisense binding to the rot transcript, thereby increasing the expression levels of toxins (305–307). Thus, on a global level, the activation of the Agr system leads to increased expression levels of major S. aureus virulence factors, including proteases, cytotoxins, and cytolytic peptides. Indeed, it was found that when agr is deleted, S. aureus expresses lower overall levels of leucocidin transcripts (291, 300). This equates to largely reduced leucocidin abundance at the protein level, although some toxins are more dramatically influenced than others. For example, in strain Newman, levels of LukED and Hla are dramatically reduced in an agr mutant background, while the abundances of LukAB/HG and HlgCB are reduced but to a lesser degree (47). As described above, the primary input responsible for the reduced production of toxins in an agr mutant is believed to be increased repression of gene expression imparted by Rot, which is produced in a greater abundance in an agr mutant (in an agr mutant, RNAIII levels are low, and thus, Rot translation is derepressed). Indeed, the generation of an agr rot double mutant restores most leucocidins to levels similar to or greater than those of wild-type S. aureus (47). This suggests that the Agr-Rot axis is critical for optimal leucocidin regulation, although it is not the only regulatory input dictating leucocidin gene expression.
In addition to Agr and Rot, the staphylococcal accessory regulatory protein (SarA) and the S. aureus exoprotein (Sae) two-component system has also been shown to positively regulate the expression of a number of S. aureus cytotoxins at the transcriptional level (Fig. 7) (300, 308–310). SarA has been shown to positively regulate the gamma-hemolysin genes hlgCB and the gene encoding alpha-hemolysin, hla, as determined by microarray analysis (300). Like Agr, SarA is a global regulator with influences on the expression of many S. aureus virulence genes, including the Agr system itself (311–314). Thus, SarA may impose significant indirect influences on leucocidin gene expression via its positive modulation of the Agr-Rot axis.
In contrast to the presumed indirect influences of SarA on leucocidin gene expression, the S. aureus exoprotein regulatory system (SaePQRS) is known to play a direct role in the regulation of a number of S. aureus leucocidin genes (Fig. 7) (309, 315). SaeRS is a two-component system that responds to host environmental cues, including PMNs, to upregulate the expression of a diverse repertoire of S. aureus secreted proteins, many of which are major virulence factors (304, 309, 310, 316–321). SaeR binds to a consensus sequence within virulence factor promoters to induce gene expression (309, 321). SaeR binding sites have been identified within the promoter regions of nearly all leucocidin genes, including lukSF, hlgA, hlgCB, lukED, and lukAB (lukHG) (309, 321). Experimentally, an sae deletion mutant of S. aureus has significantly reduced transcript levels of all known leucocidins and is dramatically attenuated in murine infection models (308–310, 315).
In addition to the Agr, SarA, and Sae regulatory inputs, the master regulator of iron acquisition, Fur, provides additional regulatory control over leucocidin gene expression (322). S. aureus fur mutants significantly upregulate the production of LukED and HlgCB in broth culture (322). Whether the regulation of leucocidins by Fur occurs via direct or indirect mechanisms is yet to be determined. Nevertheless, the induction of LukED and HlgCB in a fur mutant links leucocidin production with nutrient acquisition and suggests that leucocidins may be responsive to other forms of metabolic and nutritional signals. In summary, while we have gained a better appreciation for the fundamental regulatory inputs that influence leucocidin gene expression, the diverse signals and mechanistic details underlying the optimal activity of these complex regulatory pathways remain to be uncovered.
Regulation at the Posttranslational Level
While understanding leucocidin gene regulation at the transcriptional level has been a primary research focus, a small number of studies have assessed the possibility that posttranslational modifications may serve to modulate leucocidin activity. Two studies by Kamio and colleagues suggest that HlgC is phosphorylated in a protein kinase A-dependent manner and that this phosphorylation event is required for the activity of HlgCB on host cells (164, 165). There is a predicted consensus phosphorylation sequence (KRST) within the C terminus of HlgC that is believed to be recognized by protein kinase A (164). In initial studies, it was determined that the threonine at position 246 could be phosphorylated in the presence of protein kinase A but only when HlgC was first denatured (164). When this threonine at position 246 was mutated to an alanine, phosphorylation of denatured HlgC did not occur, and the toxin had no cytolytic activity. However, in this initial study, native HlgC was unable to be phosphorylated in a manner similar to that of the denatured protein, which largely calls into question whether phosphorylation of HlgC at T246 is a biologically relevant phenomenon. However, it is clear from these studies that regardless of the phosphorylation status, T246 is a critical residue for HlgCB function, as mutation to alanine eliminates toxin activity. Follow-up studies using radiolabeled phosphate in the presence of human PMNs demonstrated that HlgC is indeed phosphorylated in the presence of PMNs and that this phosphorylation occurs at T246 (165). Inhibition of phosphorylation by using protein kinase A inhibitors revealed that HlgCB lytic activity is reduced and that this reduction in activity correlates with reductions in levels of phosphorylation. Thus, it appears that phosphorylation at T246 is a relevant posttranslational modification that confers optimal activity to HlgCB in the presence of target cells. Interestingly, LukS-PV contains the same consensus phosphorylation sequence, while LukE and HlgA do not (102). Thus, phosphorylation is not a conserved mechanism by which leucocidin lytic activity is modulated. It is also worth noting that no additional studies have followed up on phosphorylation as a requirement for the activity of HlgCB or any other leucocidin, so readers are cautioned that the relevance of such activity would seemingly benefit from more rigorous investigation.
Recently, studies aimed at identifying the role of S. aureus proteases in secreted protein functions demonstrated that leucocidin abundance is likely to be directly influenced by the proteolytic activity of certain S. aureus strains. Upon the deletion of all secreted protease-encoding genes, Kolar et al. found that the abundances of LukAB/HG, LukSF-PV, LukED (LukE), and gamma-hemolysin (HlgA) were increased in bacterial supernatants compared to wild-type S. aureus (323). Thus, it is clear that proteases are likely to influence leucocidin stability in some capacity. As these studies were conducted with a protease-null mutant of S. aureus, it is not possible to attribute leucocidin degradation to the activity of one specific protease. Further investigation is needed to provide additional mechanistic detail and support the biological significance of these authors' findings as they relate to leucocidin activity (323).
TARGETING OF LEUCOCIDINS AS A THERAPEUTIC MODALITY
The leucocidins potently target both innate and adaptive immune cells to promote immune evasion by S. aureus. Thus, it stands to reason that blocking of leukocytolytic activity may prove beneficial in promoting natural clearance of S. aureus by host immune cells that would have normally been disabled or killed by the toxin. This perspective is not necessarily novel, as in the late 1800s and early 1900s, the potential of Panton-Valentine leucocidin as a therapeutic target was already being realized. Van de Velde et al., Valentine et al., and Gladstone et al. all devised methods by which they demonstrated the immunogenicity of “leucocidin” and the neutralization of leukocytolytic activity by specific antibodies (42, 43, 45, 52, 57, 194, 197, 324). Gladstone and colleagues even conducted a series of studies on both rabbits and humans by using a PVL toxoid (56–59). While there appeared to be some efficacy, as measured by a reduced time to infection resolution, the PVL toxoid left much to be desired in terms of its use as a promising therapeutic for S. aureus (56, 58). Insomuch as these PVL immunization studies by Gladstone et al. did not culminate in the development of an effective anti-S. aureus therapeutic agent, they did provide suitable evidence that an immune response can be mounted against PVL in humans. Interestingly, much of the immunization efforts by Gladstone and colleagues were conducted on patients with already established skin and soft tissue infections (SSTIs) (56). Thus, immunization during such active infections may have had limited value. Likewise, it is difficult to ascertain whether infection resolution was due to the administration of the toxoid or simply to natural clearance over the course of infection. It was also unknown whether or not the subjects of that study were infected with strains of S. aureus that contained the pvl genes. More recent research that calls into question the role of PVL in the pathogenesis of SSTIs further limits the interpretation of toxoid efficacy in the studies by Gladstone et al., as the majority of these patients had SSTIs. Regardless, the work of Gladstone and colleagues was at least suggestive of the potential therapeutic value associated with leucocidin immunization and supported further investigation. Importantly, these studies were conducted at a time when there was little appreciation for the diversity of leucocidins present within a given strain of S. aureus (19–21). Given the redundant and nonredundant activities of individual leucocidins, it is perhaps not surprising that the administration of a toxoid composed strictly of PVL displayed limited efficacy (19–21). Our current appreciation for the redundancies in cytotoxic molecules expressed by S. aureus makes it clear that an effective strategy to promote enhanced resolution of infection by the immune system is going to require the targeting of more than one toxic molecule (19). In particular, the diverse repertoire of immune evasion molecules produced by S. aureus will certainly necessitate the consideration of multicomponent therapeutics and vaccines (22, 23). In the past 10 years, our increased knowledge of leucocidin diversity, directed targeting of host immune cells, and their unique cellular specificities has left researchers better poised to test whether the development of novel therapeutic agents targeting multiple S. aureus leucocidins will prove to be an efficacious treatment strategy.
In the last 50 years, few studies have sought to directly evaluate the therapeutic potential of antileucocidin-based treatment modalities. One study, which immunized rabbits with PVL in order to prevent the pathological outcomes of mastitis, showed no efficacy (325). In contrast, another study suggested that the administration of intravenous immunoglobulin (IVIG) might have potential therapeutic value, as it is likely to contain a number of antitoxin antibodies. Gauduchon et al. demonstrated that IVIG is capable of neutralizing the toxic activity of PVL in vitro and suggested that its use in conjunction with antibiotics may improve outcomes in patients with invasive S. aureus infection (326). However, IVIG is currently not recommended as a routine course of therapy, even for invasive S. aureus infection, despite its ability to neutralize PVL quite efficiently (4). This is due in part to conflicting evidence surrounding whether or not inhibition of PVL by IVIG is beneficial during infection. In 2010, Yoong and Pier showed that the administration of antisera, isolated from rabbits immunized with PVL, to S. aureus-infected mice led to worse disease outcomes (209). This study suggested that the proinflammatory activity of PVL on host immune cells, rather than being detrimental, as described for rabbit pneumonia models, is actually beneficial and assists in establishing more productive immune responses and better infection resolution. When PVL is neutralized (or deleted from the S. aureus genome), the overall virulence of S. aureus was actually enhanced rather than diminished (208–210, 233). These studies are in direct opposition to work that was reported the year prior indicating that immunization of mice with PVL led to reduced pathological outcomes in models of necrotizing pneumonia and skin and soft tissue infection (204). Similarly, the administration of anti-PVL antibodies to the eyes of S. aureus-infected mice using a keratitis model demonstrated increased efficacy for the resolution of CA-MRSA (327). An additional study showed that immunization of mice with rationally designed mutants of PVL leads to significantly improved outcomes in a lethal systemic infection model, although the protection observed in this model is likely due to the broadly neutralizing capacity of the sera (lytic factors other than PVL were blocked by sera obtained from PVL-immunized animals) (328). Currently, it is difficult to reconcile the conflicting evidence both in favor of and against leucocidin immunization strategies as viable therapeutic options. As noted above, the majority of PVL-based virulence studies were conducted by using suboptimal murine infection models. PVL is not lytic toward murine leukocytes, although it is capable of eliciting immune-activating properties on murine cells (200, 210). Thus, a major biological function of the toxin is not considered when using murine models to assess virulence features attributed to PVL and the efficacy of antibody-mediated neutralization of the toxin.
Studies with human subjects have demonstrated that children can produce remarkably high levels of anti-PVL antibodies at a very young age, yet they still remain susceptible to SSTI caused by S. aureus (329). Such findings suggest that neutralizing antibody alone does not necessarily protect against S. aureus infection. However, it is possible that the presence of PVL antibodies may still have the ability to reduce adverse infection outcomes without necessarily preventing disease. A recent study by Rasigade et al. found that the time to death of patients with necrotizing pneumonia who had previously had PVL+ infections was significantly longer than that of patients who had not previously had a PVL+ S. aureus infection (131). However, while the acute onset and pathological outcomes associated with disease were far more rapid in patients who had not experienced PVL+ S. aureus infections in the past, both patient groups still showed the same number of deaths overall (131). Thus, PVL antibodies may have slowed the progression of disease but did not prevent the mortality associated with severe S. aureus necrotizing pneumonia. Importantly, both murine and human studies exclusively assessed the therapeutic efficacy of neutralizing the activity of only one leucocidin, PVL. Given the likelihood that any given clinically relevant S. aureus strain will produce at least three bicomponent leucocidins, which possess both overlapping and distinct immune evasion functions, it is perhaps not surprising that such low efficacy was witnessed. In an additional study of children with S. aureus infection, it was found that those with invasive disease generated a high-titer antibody response to LukAB/HG. The antibodies generated have significant neutralizing capabilities in vitro (330). However, like PVL, whether this antibody response to LukAB/HG alone is capable of conferring protection against infection with S. aureus remains to be determined. In this study, the titers of LukAB/HG antibody were higher than those of any other leucocidin tested, implying that it may be a dominant antigen seen during infection (330).
When injected into the vitreous of the eyes of rabbits, PVL and gamma-hemolysin are both capable of inducing endophthalmitis (225, 226, 331, 332). Recently, Laventie et al. demonstrated that the administration of LukS-PV and LukF-PV monovalent and divalent heavy-chain-only diabodies are capable of reducing the inflammatory outcomes associated with PVL administration to the rabbit eye (332). Additionally, they demonstrated that one of these neutralizing diabodies, which was originally designed to target only PVL, could also bind to and neutralize HlgCB of gamma-hemolysin (332). Thus, not only are anti-PVL antibodies capable of reducing PVL-induced inflammation in in vivo rabbit models, it is also possible to generate antibody molecules that neutralize more than one leucocidin pair. Work by Karauzum and colleagues also demonstrated that the generation of broadly neutralizing antibodies after immunization with PVL can have dramatic effects on pathogenic outcomes using a lethal murine systemic infection model (328). It is likely that antibodies with cross-neutralizing capabilities such as these will prove far more efficacious, highlighting promise toward the development of antitoxin molecules that may be able to target multiple toxins at the same time. By using this same ocular intoxication model, a series of small molecules with broad therapeutic applications known as calixarenes, or SCns (p-sulfonato-calix[n]arenes), were also tested for their ability to neutralize the activities of both PVL and HlgAB (331, 333). In the presence of the small molecules, the inflammatory pathology associated with toxin administration to rabbit eyes was significantly reduced (331). It has been proposed that this neutralizing capacity of the calixarenes in rabbit endophthalmitis models stems from the ability of the inhibitors to bind LukS subunits with high affinity, thereby preventing cell surface recognition and toxin-mediated killing. The implications of leucocidin-specific calixarenes for use in the treatment of other S. aureus infectious conditions have yet to be examined.
The identification of the cellular receptors required for cell surface recognition by LukAB/HG, PVL, and LukED has the potential to further the development of high-affinity leucocidin inhibitors. There is evidence for likely success in this endeavor, in that clinically approved CCR5 receptor antagonists, such as the HIV drug maraviroc, block the cytolytic activity of LukED on CCR5-expressing cells (227, 245). Additionally, the use of antibodies and/or natural ligands as competitors for toxin binding for each of the identified toxin receptors, including CCR5 (LukE), CXCR1/CXCR2 (LukE), C5aR/C5L2 (LukS-PV), and CD11b (LukAB/HG), indicates that blocking of the initial interaction with the cell membrane is a specific and potent means of inhibiting leucocidin activity (199, 227, 230, 235). Further studies will certainly benefit from a more refined biochemical definition of toxin-receptor interactions. This includes more in-depth investigations into structural features of each toxin that dictate receptor specificity. Importantly, we suggest that receptor recognition motifs within individual toxins are likely to be better therapeutic targets than the receptors themselves. This is due to the fact that normal signaling through the cellular receptors of the leucocidins is, in most cases, critical for normal immune cell function, including phenomena such as chemotaxis to infected tissue and the induction of optimal inflammatory responses (334). Thus, directed targeting of the leucocidins rather than their receptors is likely to prevent negative outcomes associated with diminishing optimal immune responses that could be brought upon by receptor inhibition. Unfortunately, a major complication in the evaluation of the potential efficacy of any leucocidin-based inhibitor in vivo continues to be the lack of an appropriate animal model. However, the identification of leucocidin receptors suggests considerable potential toward the development of more appropriate small-animal models to mitigate the complications of species specificity and facilitate therapeutic testing in vivo.
CONCLUDING REMARKS
Our understanding of leucocidin function has progressed from the identification of a single toxic substance, the “leucocidin,” to the identification of six unique toxic molecules whose biological functions are only now being fully appreciated. It is clear that the study of the leucocidins did not follow a simple path. An initial lack of appreciation for the diversity of leukocidal molecules present within S. aureus confounded many early studies, complicated nomenclature, and often led to phenotypic discrepancies among research groups. Similarly, species specificity associated with cellular targeting significantly slowed the pace of novel discovery as it relates to pathogenesis and infection outcomes. Such complications, along with complex epidemiological associations, have left many puzzling over the true roles of the leucocidins in human disease. In contrast, biochemical and biophysical studies have been met with greater success. Over the course of the past 20 years, a comprehensive model of leucocidin pore formation has been developed, which remains unchallenged today. Although PVL is often considered a mainstay in leucocidin research, it is now becoming clear that other leucocidins are equally capable of exerting potent lytic activity in vitro and in vivo and are certainly deserving of our future research efforts.
In the past 5 years, the leucocidins have received a considerable resurgence in attention. Studies have (i) identified and characterized a novel leucocidin (LukAB/HG), (ii) determined that the leucocidins dictate cellular specificity through the recognition of proteinaceous receptors, (iii) applied murine models to investigate leucocidin lytic activity in vivo, (iv) uncovered previously unappreciated proinflammatory functions that occur irrespective of cell lysis, and (v) proposed a number of potential therapeutic methodologies for targeted inhibition of toxin activity. These recent discoveries have opened considerable avenues for future investigation. Some areas of immediate interest include the development of small-animal models that are sensitive to the lytic function of all S. aureus leucocidins, investigation into the precise mode of action of all leucocidins in diverse infection settings, further determination of sublytic and accessory leucocidin functions that are influenced by receptor-dependent and -independent targeting, and investigation into the therapeutic potential of leucocidin inhibition toward promoting natural clearance of S. aureus infection.
Thus, despite having been identified over 120 years ago, current studies of the bicomponent leucocidins continue to provide the S. aureus research community with novel insights into the complex underpinnings of toxin-based immune evasion. We are now better poised than ever to develop novel strategies to explore their mode of action in vivo, provide a more concrete picture of their contribution to pathogenesis, and determine the therapeutic efficacy of antileucocidin-based treatment strategies.
ACKNOWLEDGMENTS
We thank the members of the Torres laboratory for critically reading the manuscript.
This work was supported by funds from the AHA (09SDG2060036) and the NIH NIAID (R56 AI091856, R01 AI099394, and R01 AI105129) and by NYUMLC development funds to V.J.T. F.A. was initially supported by an NIH NIAID training grant (5T32-AI0007180) and later by an NIH NIAID NRSA postdoctoral fellowship (F32-AI098395).
F.A. and V.J.T. are listed as inventors on patent applications filed by New York University School of Medicine, which are currently under commercial license.
Biographies

Francis Alonzo III is a postdoctoral fellow in the Department of Microbiology at New York University School of Medicine. He received his B.S. degree from the University of Illinois at Urbana-Champaign in 2005 and subsequently received his Ph.D. at the University of Illinois at Chicago College of Medicine in the laboratory of Dr. Nancy Freitag in 2010. While in Chicago, he studied factors that influence proper secretion, localization, and folding of major virulence factors produced by the intracellular pathogen Listeria monocytogenes. In January 2011, he joined the laboratory of Victor J. Torres, where he currently studies mechanisms underlying the subversion of host immune defenses by Staphylococcus aureus.

Victor J. Torres is an Associate Professor of Microbiology at New York University School of Medicine. He received his B.S. degree in Industrial Microbiology in 2000 from the University of Puerto Rico Mayagüez campus and a Ph.D. in Microbiology and Immunology from Vanderbilt University School of Medicine under the mentorship of Timothy L. Cover in 2004. From 2005 to 2008, he completed postdoctoral training on the pathogenesis of Staphylococcus aureus under the tutelage of Eric P. Skaar, also at Vanderbilt University School of Medicine. In 2008, he joined the faculty of the Department of Microbiology at NYU School of Medicine as an assistant professor. His current research focuses on how (i) S. aureus coordinates the production of toxins in response to signals encountered during infection; (ii) bicomponent, pore-forming leukotoxins target and kill host cells; and (iii) leukotoxins subvert host responses during infection of the mammalian host.
REFERENCES
- 1.David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23:616–687. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core Surveillance MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R, National Nosocomial Infections Surveillance System 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin. Infect. Dis. 42:389–391. 10.1086/499367 [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Ryback MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 5.Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, Mackenzie FM. 2012. Methicillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 39:273–282. 10.1016/j.ijantimicag.2011.09.030 [DOI] [PubMed] [Google Scholar]
- 6.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. 10.1016/S0140-6736(09)61999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marty FM, Yeh WW, Wennersten CB, Venkataraman L, Albano E, Alyea EP, Gold HS, Baden LR, Pillai SK. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 44:595–597. 10.1128/JCM.44.2.595-597.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meka VG, Pillai SK, Sakoulas G, Wennersten C, Venkataraman L, DeGirolami PC, Eliopoulos GM, Moellering RC, Jr, Gold HS. 2004. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23s rRNA gene and loss of a single copy of rRNA. J. Infect. Dis. 190:311–317. 10.1086/421471 [DOI] [PubMed] [Google Scholar]
- 9.Luh KT, Hsueh PR, Teng LJ, Pan HJ, Chen YC, Lu JJ, Wu JJ, Ho SW. 2000. Quinupristin-dalfopristin resistance among Gram-positive bacteria in Taiwan. Antimicrob. Agents Chemother. 44:3374–3380. 10.1128/AAC.44.12.3374-3380.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowzicky M, Talbot GH, Feger C, Prokocimer P, Etienne J, Leclercq R. 2000. Characterization of isolates associated with emerging resistance to quinupristin/dalfopristin (Synercid) during a worldwide clinical program. Diagn. Microbiol. Infect. Dis. 37:57–62. 10.1016/S0732-8893(99)00154-6 [DOI] [PubMed] [Google Scholar]
- 11.Thurlow LR, Joshi GS, Richardson AR. 2012. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol. Med. Microbiol. 65:5–22. 10.1111/j.1574-695X.2012.00937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skov R, Christiansen K, Dancer SJ, Daum RS, Dryden M, Huang YC, Lowy FD. 2012. Update on the prevention and control of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA). Int. J. Antimicrob. Agents 39:193–200. 10.1016/j.ijantimicag.2011.09.029 [DOI] [PubMed] [Google Scholar]
- 13.Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. 2012. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 15:588–595. 10.1016/j.mib.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Amador-Miranda R, Bertran-Pasarell J, Gonzalez M, Conde A. 2008. Methicillin-resistant Staphylococcus aureus in the community. Bol. Asoc. Med. P. R. 100:21–23 [PubMed] [Google Scholar]
- 15.Boyle-Vavra S, Daum RS. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Invest. 87:3–9. 10.1038/labinvest.3700501 [DOI] [PubMed] [Google Scholar]
- 16.Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16:361–369. 10.1016/j.tim.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator Agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 79:1927–1935. 10.1128/IAI.00046-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery CP, Boyle-Vavra S, Daum RS. 2010. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 5:e15177. 10.1371/journal.pone.0015177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenesch F, Lina G, Henry T. 2012. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2:12. 10.3389/fcimb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonzo F, III, Torres VJ. 2013. Bacterial survival amidst an immune onslaught: the contribution of the Staphylococcus aureus leukotoxins. PLoS Pathog. 9:e1003143. 10.1371/journal.ppat.1003143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoong P, Torres VJ. 2013. The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Curr. Opin. Microbiol. 16:63–69. 10.1016/j.mib.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster TJ. 2004. The Staphylococcus aureus “superbug.” J. Clin. Invest. 114:1693–1696. 10.1172/JCI23825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster TJ. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958. 10.1038/nrmicro1289 [DOI] [PubMed] [Google Scholar]
- 24.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. 2013. Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev. 26:422–447. 10.1128/CMR.00104-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falugi F, Kim HK, Missiakas DM, Schneewind O. 2013. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 4(5):e00575–13. 10.1128/mBio.00575-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Pulgarin S, Dominguez-Bernal G, Orden JA, de la Fuente R. 2009. Simultaneous lack of catalase and beta-toxin in Staphylococcus aureus leads to increased intracellular survival in macrophages and epithelial cells and to attenuated virulence in murine and ovine models. Microbiology 155:1505–1515. 10.1099/mic.0.025544-0 [DOI] [PubMed] [Google Scholar]
- 27.Das D, Bishayi B. 2009. Staphylococcal catalase protects intracellularly survived bacteria by destroying H2O2 produced by the murine peritoneal macrophages. Microb. Pathog. 47:57–67. 10.1016/j.micpath.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 28.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. 2013. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu. Rev. Microbiol. 67:629–650. 10.1146/annurev-micro-092412-155746 [DOI] [PubMed] [Google Scholar]
- 29.Peschel A, Otto M. 2013. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 11:667–673. 10.1038/nrmicro3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 34:237–259. 10.1007/s00281-011-0295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. 2012. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 15:92–99. 10.1016/j.mib.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. 2010. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J. Exp. Med. 207:1863–1870. 10.1084/jem.20092514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HK, Kim HY, Schneewind O, Missiakas D. 2011. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 25:3605–3612. 10.1096/fj.11-187963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HK, Emolo C, DeDent AC, Falugi F, Missiakas DM, Schneewind O. 2012. Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice. Infect. Immun. 80:3460–3470. 10.1128/IAI.00230-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagnoli F, Bertholet S, Grandi G. 2012. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front. Cell. Infect. Microbiol. 2:16. 10.3389/fcimb.2012.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proctor RA. 2012. Challenges for a universal Staphylococcus aureus vaccine. Clin. Infect. Dis. 54:1179–1186. 10.1093/cid/cis033 [DOI] [PubMed] [Google Scholar]
- 37.Proctor RA. 2012. Is there a future for a Staphylococcus aureus vaccine? Vaccine 30:2921–2927. 10.1016/j.vaccine.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 38.Otto M. 2010. Novel targeted immunotherapy approaches for staphylococcal infection. Expert Opin. Biol. Ther. 10:1049–1059. 10.1517/14712598.2010.495115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zecconi A, Scali F. 2013. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 150:12–22. 10.1016/j.imlet.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 40.Cheung GY, Joo HS, Chatterjee SS, Otto M. 26 December 2013. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. [Epub ahead of print.] 10.1111/1574-6976.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee SS, Otto M. 2013. How can Staphylococcus aureus phenol-soluble modulins be targeted to inhibit infection? Future Microbiol. 8:693–696. 10.2217/fmb.13.37 [DOI] [PubMed] [Google Scholar]
- 42.Van de Velde H. 1894. Etude sur le mécanisme de la virulence du Staphylocoque pyogene. Cellule 10:401–410 [Google Scholar]
- 43.Denys J, Van de Velde H. 1895. Sur la production d'une antileucocidine chez les lapin vaccinés contre le Staphylocoque pyogène. Cellule 11:359–372 [Google Scholar]
- 44.Gladstone GP, Vanheyningen WE. 1957. Staphylococcal leucocidins. Br. J. Exp. Pathol. 38:123–137 [PMC free article] [PubMed] [Google Scholar]
- 45.Panton PN, Valentine FCO. 1932. Staphylococcal toxin. Lancet i:506–508 [Google Scholar]
- 46.Julianelle LA. 1922. Studies of hemolytic staphylococci: hemolytic activity—biochemical reactions—serologic reactions. J. Infect. Dis. 31:256–284. 10.1093/infdis/31.3.256 [DOI] [Google Scholar]
- 47.Alonzo F, III, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. 2012. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol. Microbiol. 83:423–435. 10.1111/j.1365-2958.2011.07942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neisser M, Wechsberg F. 1901. Ueber das staphylotoxin. Med. Microbiol. Immunol. 36:299–349 [Google Scholar]
- 49.Wright J. 1936. Staphylococcal leucocidin (Neisser-Wechsberg type) and antileucocidin. Lancet i:1002–1004 [Google Scholar]
- 50.Weld JTP, Gunther A. 1931. Differentiation between certain toxic properties of filtrates of hemolytic Staphylococcus aureus. J. Exp. Med. 54:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnet F. 1929. The exotoxins of Staphylococcus pyogenes aureus. J. Pathol. Bacteriol. 32:717–734. 10.1002/path.1700320402 [DOI] [Google Scholar]
- 52.Valentine FCO. 1936. Further observations on the role of the toxin in staphylococcal infection. Lancet i:526–531 [Google Scholar]
- 53.Proom H. 1937. The interrelationships of staphylococcal leucocidins. J. Pathol. Bacteriol. 44:425–429. 10.1002/path.1700440217 [DOI] [Google Scholar]
- 54.Woodin AM. 1960. Purification of the 2 components of leucocidin from Staphylococcus aureus. Biochem. J. 75:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woodin AM. 1959. Fractionation of a leucocidin from Staphylococcus aureus. Biochem. J. 73:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gladstone GP. 1973. Improved leukocidin toxoid. Br. J. Exp. Pathol. 54:255–259 [PMC free article] [PubMed] [Google Scholar]
- 57.Soucková-Stĕpánová J, Gladstone GP, Vanecek R. 1965. Immunisation of rabbits with staphylococcal leucocidin toxoid with a report on pathological histology of their internal organs. Br. J. Exp. Pathol. 46:384–407 [PMC free article] [PubMed] [Google Scholar]
- 58.Mudd S, Gladstone GP, Lenhart NA. 1965. Antigenicity in man of staphylococcal leucocidin toxoid with notes on therapeutic immunization in chronic osteomyelitis. Br. J. Exp. Pathol. 46:455–472 [PMC free article] [PubMed] [Google Scholar]
- 59.Gladstone GP. 1965. Staphylococcal leucocidin toxoid. Br. J. Exp. Pathol. 46:292–307 [PMC free article] [PubMed] [Google Scholar]
- 60.Woodin AM, Wieneke AA. 1968. Role of leucocidin and triphosphoinositide in control of potassium permeability. Nature 220:283–286. 10.1038/220283a0 [DOI] [PubMed] [Google Scholar]
- 61.Woodin AM, Wieneke AA. 1967. Participation of phospholipids in interaction of leucocidin and cell membrane of polymorphonuclear leucocyte. Biochem. J. 105:1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodin AM, Wieneke AA. 1966. Interaction of leucocidin with cell membrane of polymorphonuclear leucocyte. Biochem. J. 99:479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodin AM. 1966. Impedance of leucocyte suspensions treated with leucocidin or streptolysin O. Exp. Cell Res. 43:311–318. 10.1016/0014-4827(66)90058-9 [DOI] [PubMed] [Google Scholar]
- 64.Woodin AM. 1965. Staphylococcal leukocidin. Ann. N. Y. Acad. Sci. 128:152–164 [DOI] [PubMed] [Google Scholar]
- 65.Woodin AM, Wieneke AA. 1963. Incorporation of radioactive phosphorus in leucocytes during extrusion of protein induced by staphylococcal leucocidin. Biochem. J. 87:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodin AM, Wieneke AA. 1963. Accumulation of calcium by polymorphonuclear leucocytes treated with staphylococcal leucocidin and its significance in extrusion of protein. Biochem. J. 87:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodin AM, Marchesi VT, French JE. 1963. Morphological changes associated with extrusion of protein induced in polymorphonuclear leucocytes by staphylococcal leucocidin. Biochem. J. 87:567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodin AM. 1962. Extrusion of protein from rabbit polymorphonuclear leucocytes treated with staphylococcal leucocidin. Biochem. J. 82:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodin AM. 1961. Effect of staphylococcal leucocidin on leucocytes. Biochem. J. 80:562–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodin AM. 1961. Assay of 2 components of staphylococcal leucocidin and their antibodies. J. Pathol. Bacteriol. 81:63–68. 10.1002/path.1700810108 [DOI] [PubMed] [Google Scholar]
- 71.Szmigielski S, Jeljaszewicz J, Wilczynski J, Korbecki M. 1966. Reaction of rabbit leucocytes to staphylococcal (Panton-Valentine) leucocidin in vivo. J. Pathol. Bacteriol. 91:599–604. 10.1002/path.1700910237 [DOI] [PubMed] [Google Scholar]
- 72.Smith ML, Price SA. 1938. Staphylococcus gamma haemolysin. J. Pathol. Bacteriol. 47:379–393. 10.1002/path.1700470303 [DOI] [Google Scholar]
- 73.Marks J. 1951. The standardization of staphylococcal alpha-antitoxin, with special reference to anomalous haemolysins including delta-lysin. J. Hyg. (Lond.) 49:52–66. 10.1017/S0022172400015369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elek SD, Levy E. 1950. Distribution of haemolysins in pathogenic and non-pathogenic staphylococci. J. Pathol. Bacteriol. 62:541–554. 10.1002/path.1700620405 [DOI] [PubMed] [Google Scholar]
- 75.Elek SD. 1959. Staphylococcus pyogenes and its relation to disease. Livingstone, Edinburgh, United Kingdom [Google Scholar]
- 76.Jackson AW. 1963. Staphylococcal gamma lysin and its differentiation from delta lysin. In Gibbons NE. (ed), Recent progress in microbiology: symposia. University of Toronto Press, Toronto, Ontario, Canada [Google Scholar]
- 77.Guyonnet F, Plommet M. 1970. Staphylococcal gamma lysin: purification and properties. Ann. Inst. Pasteur (Paris) 118:19–33 [PubMed] [Google Scholar]
- 78.Guyonnet F, Plommet M, Bouillane C. 1968. Purification de l'hemolysine gamma de Staphylococcus aureus. C. R. Hebd. Seances Acad. Sci. 267:1180–1182 [Google Scholar]
- 79.Mollby R, Wadstrom T. 1971. Separation of gamma hemolysin from Staphylococcus aureus Smith 5R. Infect. Immun. 3:633–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor AG, Bernheimer AW. 1974. Further characterization of staphylococcal gamma-hemolysin. Infect. Immun. 10:54–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fackrell HB, Wiseman GM. 1976. Properties of the gamma haemolysin of Staphylococcus aureus ‘Smith 5R'. J. Gen. Microbiol. 92:11–24. 10.1099/00221287-92-1-11 [DOI] [PubMed] [Google Scholar]
- 82.Bezard G, Plommet M. 1973. Production de la toxine staphylococcique gamma brute. Ann. Rech. Vet. 4:355–358 [Google Scholar]
- 83.Fackrell HB, Wiseman GM. 1976. Production and purification of the gamma haemolysin of Staphylococcus aureus ‘Smith 5R'. J. Gen. Microbiol. 92:1–10. 10.1099/00221287-92-1-1 [DOI] [PubMed] [Google Scholar]
- 84.Szmigielski S, Jeljaszewicz J, Kobus M, Luczak M, Ludwicka A, Mollby R, Wadstrom T. 1976. Cytotoxic effects of staphylococcal alpha-, beta- and gamma-haemolysins, p 202–208 Jeljaszewicz J. (ed), Staphylococci and staphylococcal infections. S. Karger, Basel, Switzerland [Google Scholar]
- 85.Cooney J, Kienle Z, Foster TJ, O'Toole PW. 1993. The gamma-hemolysin locus of Staphylococcus aureus comprises three linked genes, two of which are identical to the genes for the F and S components of leukocidin. Infect. Immun. 61:768–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahman A, Izaki K, Kamio Y. 1993. Gamma-hemolysin genes in the same family with lukF and lukS genes in methicillin resistant Staphylococcus aureus. Biosci. Biotechnol. Biochem. 57:1234–1236. 10.1271/bbb.57.1234 [DOI] [PubMed] [Google Scholar]
- 87.Kamio Y, Rahman A, Nariya H, Ozawa T, Izaki K. 1993. The two staphylococcal bi-component toxins, leukocidin and gamma-hemolysin, share one component in common. FEBS Lett. 321:15–18. 10.1016/0014-5793(93)80611-W [DOI] [PubMed] [Google Scholar]
- 88.Plommet M. 1988. Preparation and purification of gamma-hemolysin of staphylococci. Methods Enzymol. 165:8–16. 10.1016/S0076-6879(88)65005-1 [DOI] [PubMed] [Google Scholar]
- 89.Cooney J, Mulvey M, Arbuthnott JP, Foster TJ. 1988. Molecular cloning and genetic analysis of the determinant for gamma-lysin, a two-component toxin of Staphylococcus aureus. J. Gen. Microbiol. 134:2179–2188 [DOI] [PubMed] [Google Scholar]
- 90.Supersac G, Prevost G, Piemont Y. 1993. Sequencing of leucocidin R from Staphylococcus aureus P83 suggests that staphylococcal leucocidins and gamma-hemolysin are members of a single, two-component family of toxins. Infect. Immun. 61:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choorit W, Kaneko J, Muramoto K, Kamio Y. 1995. Existence of a new protein component with the same function as the LukF component of leukocidin or gamma-hemolysin and its gene in Staphylococcus aureus p83. FEBS Lett. 357:260–264. 10.1016/0014-5793(94)01372-8 [DOI] [PubMed] [Google Scholar]
- 92.Kaneko J, Muramoto K, Kamio Y. 1997. Gene of LukF-PV-like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with lukM. Biosci. Biotechnol. Biochem. 61:541–544. 10.1271/bbb.61.541 [DOI] [PubMed] [Google Scholar]
- 93.Gravet A, Colin DA, Keller D, Girardot R, Monteil H, Prevost G. 1998. Characterization of a novel structural member, LukE-LukD, of the bi-component staphylococcal leucotoxin family. FEBS Lett. 436:202–208. 10.1016/S0014-5793(98)01130-2 [DOI] [PubMed] [Google Scholar]
- 94.Morinaga N, Kaihou Y, Noda M. 2003. Purification, cloning and characterization of variant LukE-LukD with strong leukocidal activity of staphylococcal bi-component leukotoxin family. Microbiol. Immunol. 47:81–90. 10.1111/j.1348-0421.2003.tb02789.x [DOI] [PubMed] [Google Scholar]
- 95.McCarthy AJ, Lindsay JA. 2013. Staphylococcus aureus innate immune evasion is lineage-specific: a bioinfomatics [sic] study. Infect. Genet. Evol. 19:7–14. 10.1016/j.meegid.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 96.Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. 2010. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634. 10.1371/journal.pone.0011634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol. Microbiol. 79:814–825. 10.1111/j.1365-2958.2010.07490.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prevost G, Cribier B, Couppie P, Petiau P, Supersac G, Finck-Barbancon V, Monteil H, Piemont Y. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC-49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63:4121–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menestrina G, Dalla Serra M, Comai M, Coraiola M, Viero G, Werner S, Colin DA, Monteil H, Prevost G. 2003. Ion channels and bacterial infection: the case of beta-barrel pore-forming protein toxins of Staphylococcus aureus. FEBS Lett. 552:54–60. 10.1016/S0014-5793(03)00850-0 [DOI] [PubMed] [Google Scholar]
- 100.Menestrina G, Serra MD, Prevost G. 2001. Mode of action of beta-barrel pore-forming toxins of the staphylococcal alpha-hemolysin family. Toxicon 39:1661–1672. 10.1016/S0041-0101(01)00153-2 [DOI] [PubMed] [Google Scholar]
- 101.Prevost G, Mourey L, Colin DA, Menestrina G. 2001. Staphylococcal pore-forming toxins. Curr. Top. Microbiol. Immunol. 257:53–83. 10.1007/978-3-642-56508-3_4 [DOI] [PubMed] [Google Scholar]
- 102.Kaneko J, Kamio Y. 2004. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68:981–1003. 10.1271/bbb.68.981 [DOI] [PubMed] [Google Scholar]
- 103.Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. 1999. Leukocidal toxins of staphylococci. Zentralbl. Bakteriol. 289:185–201. 10.1016/S0934-8840(99)80105-4 [DOI] [PubMed] [Google Scholar]
- 104.von Eiff C, Friedrich AW, Peters G, Becker K. 2004. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 49:157–162. 10.1016/j.diagmicrobio.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 105.Projan SJ, Kornblum J, Kreiswirth B, Moghazeh SL, Eisner W, Novick RP. 1989. Nucleotide sequence: the beta-hemolysin gene of Staphylococcus aureus. Nucleic Acids Res. 17:3305. 10.1093/nar/17.8.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernheimer AW, Avigad LS, Kim KS. 1974. Staphylococcal sphingomyelinase (beta-hemolysin). Ann. N. Y. Acad. Sci. 236:292–306. 10.1111/j.1749-6632.1974.tb41499.x [DOI] [PubMed] [Google Scholar]
- 107.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16–34. 10.1128/CMR.13.1.16-34.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coleman DC, Sullivan DJ, Russell RJ, Arbuthnott JP, Carey BF, Pomeroy HM. 1989. Staphylococcus aureus bacteriophages mediating the simultaneous lysogenic conversion of beta-lysin, staphylokinase and enterotoxin A: molecular mechanism of triple conversion. J. Gen. Microbiol. 135:1679–1697 [DOI] [PubMed] [Google Scholar]
- 109.van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. 2006. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 188:1310–1315. 10.1128/JB.188.4.1310-1315.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, Broker BM, Doskar J, Wolz C. 2009. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J. Bacteriol. 191:3462–3468. 10.1128/JB.01804-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shopsin B, Gomez M, Montgomery SO, Smith DH, Waddington M, Dodge DE, Bost DA, Riehman M, Naidich S, Kreiswirth BN. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. 2008. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J. Bacteriol. 190:300–310. 10.1128/JB.01000-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y, Chatterjee SS, Porcella SF, Yu YS, Otto M. 2013. Complete genome sequence of a Panton-Valentine leukocidin-negative community-associated methicillin-resistant Staphylococcus aureus strain of sequence type 72 from Korea. PLoS One 8:e72803. 10.1371/journal.pone.0072803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gill SR. 2009. Genomics of the staphylococci, p 19–30 In Crossley KB, Jefferson KK, Archer G, Fowler VG., Jr (ed), Staphylococci in human disease. Wiley-Blackwell, Oxford, United Kingdom [Google Scholar]
- 115.Bae T, Baba T, Hiramatsu K, Schneewind O. 2006. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 62:1035–1047. 10.1111/j.1365-2958.2006.05441.x [DOI] [PubMed] [Google Scholar]
- 116.Novick RP, Subedi A. 2007. The SAPIs: mobile pathogenicity islands of Staphylococcus. Chem. Immunol. Allergy 93:42–57. 10.1159/000100857 [DOI] [PubMed] [Google Scholar]
- 117.Zou D, Kaneko J, Narita S, Kamio Y. 2000. Prophage, phiPV83-pro, carrying Panton-Valentine leukocidin genes, on the Staphylococcus aureus P83 chromosome: comparative analysis of the genome structures of phiPV83-pro, phiPVL, phi11, and other phages. Biosci. Biotechnol. Biochem. 64:2631–2643. 10.1271/bbb.64.2631 [DOI] [PubMed] [Google Scholar]
- 118.van der Vijver JC, van Es-Boon M, Michel MF. 1972. Lysogenic conversion in Staphylococcus aureus to leucocidin production. J. Virol. 10:318–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wirtz C, Witte W, Wolz C, Goerke C. 2009. Transcription of the phage-encoded Panton-Valentine leukocidin of Staphylococcus aureus is dependent on the phage life-cycle and on the host background. Microbiology 155:3491–3499. 10.1099/mic.0.032466-0 [DOI] [PubMed] [Google Scholar]
- 120.Narita S, Kaneko J, Chiba J, Piemont Y, Jarraud S, Etienne J, Kamio Y. 2001. Phage conversion of Panton-Valentine leukocidin in Staphylococcus aureus: molecular analysis of a PVL-converting phage, phiSLT. Gene 268:195–206. 10.1016/S0378-1119(01)00390-0 [DOI] [PubMed] [Google Scholar]
- 121.Kaneko J, Kimura T, Kawakami Y, Tomita T, Kamio Y. 1997. Panton-Valentine leukocidin genes in a phage-like particle isolated from mitomycin C-treated Staphylococcus aureus V8 (ATCC 49775). Biosci. Biotechnol. Biochem. 61:1960–1962. 10.1271/bbb.61.1960 [DOI] [PubMed] [Google Scholar]
- 122.Kaneko J, Kimura T, Narita S, Tomita T, Kamio Y. 1998. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene 215:57–67. 10.1016/S0378-1119(98)00278-9 [DOI] [PubMed] [Google Scholar]
- 123.Rainard P, Corrales JC, Barrio MB, Cochard T, Poutrel B. 2003. Leucotoxic activities of Staphylococcus aureus strains isolated from cows, ewes, and goats with mastitis: importance of LukM/LukF′-PV leukotoxin. Clin. Diagn. Lab. Immunol. 10:272–277. 10.1128/CDLI.10.2.272-277.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Delgado S, Garcia P, Fernandez L, Jimenez E, Rodriguez-Banos M, del Campo R, Rodriguez JM. 2011. Characterization of Staphylococcus aureus strains involved in human and bovine mastitis. FEMS Immunol. Med. Microbiol. 62:225–235. 10.1111/j.1574-695X.2011.00806.x [DOI] [PubMed] [Google Scholar]
- 125.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172–179. 10.1086/499632 [DOI] [PubMed] [Google Scholar]
- 126.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132. 10.1086/313461 [DOI] [PubMed] [Google Scholar]
- 127.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piemont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759. 10.1016/S0140-6736(02)07877-7 [DOI] [PubMed] [Google Scholar]
- 128.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O'Boyle C, Danila RN, Lynfield R. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984. 10.1001/jama.290.22.2976 [DOI] [PubMed] [Google Scholar]
- 129.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. 2013. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 13:43–54. 10.1016/S1473-3099(12)70238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978–984. 10.3201/eid0908.030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rasigade JP, Sicot N, Laurent F, Lina G, Vandenesch F, Etienne J. 2011. A history of Panton-Valentine leukocidin (PVL)-associated infection protects against death in PVL-associated pneumonia. Vaccine 29:4185–4186. 10.1016/j.vaccine.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 132.Berglund C, Prevost G, Laventie BJ, Keller D, Soderquist B. 2008. The genes for Panton Valentine leukocidin (PVL) are conserved in diverse lines of methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Microbes Infect. 10:878–884. 10.1016/j.micinf.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 133.Baba-Moussa L, Sina H, Scheftel JM, Moreau B, Sainte-Marie D, Kotchoni SO, Prevost G, Couppie P. 2011. Staphylococcal Panton-Valentine leucocidin as a major virulence factor associated to furuncles. PLoS One 6:e25716. 10.1371/journal.pone.0025716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shallcross LJ, Williams K, Hopkins S, Aldridge RW, Johnson AM, Hayward AC. 2010. Panton-Valentine leukocidin associated staphylococcal disease: a cross-sectional study at a London hospital, England. Clin. Microbiol. Infect. 16:1644–1648. 10.1111/j.1469-0691.2010.03153.x [DOI] [PubMed] [Google Scholar]
- 135.Boakes E, Kearns AM, Badiou C, Lina G, Hill RL, Ellington MJ. 2012. Do differences in Panton-Valentine leukocidin production among international methicillin-resistant Staphylococcus aureus clones affect disease presentation and severity? J. Clin. Microbiol. 50:1773–1776. 10.1128/JCM.06421-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamilton SM, Bryant AE, Carroll KC, Lockary V, Ma Y, McIndoo E, Miller LG, Perdreau-Remington F, Pullman J, Risi GF, Salmi DB, Stevens DL. 2007. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin. Infect. Dis. 45:1550–1558. 10.1086/523581 [DOI] [PubMed] [Google Scholar]
- 137.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, Tonthat GT, Rude TH, Barriere SL, Corey R, Fowler VG., Jr 2008. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J. Clin. Microbiol. 46:678–684. 10.1128/JCM.01822-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. 10.3390/toxins5061140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405–1406. 10.1038/nm1207-1405 [DOI] [PubMed] [Google Scholar]
- 140.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514. 10.1038/nm1656 [DOI] [PubMed] [Google Scholar]
- 141.Bubeck Wardenburg J, Schneewind O. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205:287–294. 10.1084/jem.20072208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamada T, Tochimaru N, Nakasuji S, Hata E, Kobayashi H, Eguchi M, Kaneko J, Kamio Y, Kaidoh T, Takeuchi S. 2005. Leukotoxin family genes in Staphylococcus aureus isolated from domestic animals and prevalence of lukM-lukF-PV genes by bacteriophages in bovine isolates. Vet. Microbiol. 110:97–103. 10.1016/j.vetmic.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 143.Fueyo JM, Mendoza MC, Rodicio MR, Muniz J, Alvarez MA, Martin MC. 2005. Cytotoxin and pyrogenic toxin superantigen gene profiles of Staphylococcus aureus associated with subclinical mastitis in dairy cows and relationships with macrorestriction genomic profiles. J. Clin. Microbiol. 43:1278–1284. 10.1128/JCM.43.3.1278-1284.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le Marechal C, Seyffert N, Jardin J, Hernandez D, Jan G, Rault L, Azevedo V, Francois P, Schrenzel J, van de Guchte M, Even S, Berkova N, Thiery R, Fitzgerald JR, Vautor E, Le Loir Y. 2011. Molecular basis of virulence in Staphylococcus aureus mastitis. PLoS One 6:e27354. 10.1371/journal.pone.0027354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Monecke S, Kuhnert P, Hotzel H, Slickers P, Ehricht R. 2007. Microarray based study on virulence-associated genes and resistance determinants of Staphylococcus aureus isolates from cattle. Vet. Microbiol. 125:128–140. 10.1016/j.vetmic.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 146.Rogolsky M. 1979. Nonenteric toxins of Staphylococcus aureus. Microbiol. Rev. 43:320–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Noda M, Kato I, Hirayama T, Matsuda F. 1982. Mode of action of staphylococcal leukocidin: effects of the S and F components on the activities of membrane-associated enzymes of rabbit polymorphonuclear leukocytes. Infect. Immun. 35:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Noda M, Hirayama T, Kato I, Matsuda F. 1980. Crystallization and properties of staphylococcal leukocidin. Biochim. Biophys. Acta 633:33–44. 10.1016/0304-4165(80)90035-5 [DOI] [PubMed] [Google Scholar]
- 149.Morinaga N, Nagamori M, Kato I. 1988. Changes in binding of staphylococcal leukocidin to HL-60 cells during differentiation induced by dimethyl sulfoxide. Infect. Immun. 56:2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Noda M, Kato I, Hirayama T, Matsuda F. 1980. Fixation and inactivation of staphylococcal leukocidin by phosphatidylcholine and ganglioside GM1 in rabbit polymorphonuclear leukocytes. Infect. Immun. 29:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Finck-Barbancon V, Duportail G, Meunier O, Colin DA. 1993. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta 1182:275–282. 10.1016/0925-4439(93)90069-D [DOI] [PubMed] [Google Scholar]
- 152.Miles G, Cheley S, Braha O, Bayley H. 2001. The staphylococcal leukocidin bicomponent toxin forms large ionic channels. Biochemistry 40:8514–8522. 10.1021/bi010454o [DOI] [PubMed] [Google Scholar]
- 153.Meunier O, Falkenrodt A, Monteil H, Colin DA. 1995. Application of flow cytometry in toxinology: pathophysiology of human polymorphonuclear leukocytes damaged by a pore-forming toxin from Staphylococcus aureus. Cytometry 21:241–247. 10.1002/cyto.990210304 [DOI] [PubMed] [Google Scholar]
- 154.Baba Moussa L, Werner S, Colin DA, Mourey L, Pedelacq JD, Samama JP, Sanni A, Monteil H, Prevost G. 1999. Discoupling the Ca(2+)-activation from the pore-forming function of the bi-component Panton-Valentine leucocidin in human PMNs. FEBS Lett. 461:280–286. 10.1016/S0014-5793(99)01453-2 [DOI] [PubMed] [Google Scholar]
- 155.Staali L, Monteil H, Colin DA. 1998. The staphylococcal pore-forming leukotoxins open Ca2+ channels in the membrane of human polymorphonuclear neutrophils. J. Membr. Biol. 162:209–216. 10.1007/s002329900358 [DOI] [PubMed] [Google Scholar]
- 156.Nguyen VT, Kamio Y, Higuchi H. 2003. Single-molecule imaging of cooperative assembly of gamma-hemolysin on erythrocyte membranes. EMBO J. 22:4968–4979. 10.1093/emboj/cdg498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sugawara N, Tomita T, Kamio Y. 1997. Assembly of Staphylococcus aureus gamma-hemolysin into a pore-forming ring-shaped complex on the surface of human erythrocytes. FEBS Lett. 410:333–337. 10.1016/S0014-5793(97)00618-2 [DOI] [PubMed] [Google Scholar]
- 158.Kaneko J, Ozawa T, Tomita T, Kamio Y. 1997. Sequential binding of staphylococcal gamma-hemolysin to human erythrocytes and complex formation of the hemolysin on the cell surface. Biosci. Biotechnol. Biochem. 61:846–851. 10.1271/bbb.61.846 [DOI] [PubMed] [Google Scholar]
- 159.Gouaux E, Hobaugh M, Song L. 1997. Alpha-hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein Sci. 6:2631–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sugawara N, Tomita T, Sato T, Kamio Y. 1999. Assembly of Staphylococcus aureus leukocidin into a pore-forming ring-shaped oligomer on human polymorphonuclear leukocytes and rabbit erythrocytes. Biosci. Biotechnol. Biochem. 63:884–891. 10.1271/bbb.63.884 [DOI] [PubMed] [Google Scholar]
- 161.Sugawara-Tomita N, Tomita T, Kamio Y. 2002. Stochastic assembly of two-component staphylococcal gamma-hemolysin into heteroheptameric transmembrane pores with alternate subunit arrangements in ratios of 3:4 and 4:3. J. Bacteriol. 184:4747–4756. 10.1128/JB.184.17.4747-4756.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miles G, Movileanu L, Bayley H. 2002. Subunit composition of a bicomponent toxin: staphylococcal leukocidin forms an octameric transmembrane pore. Protein Sci. 11:894–902. 10.1110/ps.4360102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jayasinghe L, Bayley H. 2005. The leukocidin pore: evidence for an octamer with four LukF subunits and four LukS subunits alternating around a central axis. Protein Sci. 14:2550–2561. 10.1110/ps.051648505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nariya H, Nishiyama A, Kamio Y. 1997. Identification of the minimum segment in which the threonine246 residue is a potential phosphorylated site by protein kinase A for the LukS-specific function of staphylococcal leukocidin. FEBS Lett. 415:96–100. 10.1016/S0014-5793(97)01100-9 [DOI] [PubMed] [Google Scholar]
- 165.Nishiyama A, Nariya H, Kamio Y. 1998. Phosphorylation of LukS by protein kinase A is crucial for the LukS-specific function of the staphylococcal leukocidin on human polymorphonuclear leukocytes. Biosci. Biotechnol. Biochem. 62:1834–1838. 10.1271/bbb.62.1834 [DOI] [PubMed] [Google Scholar]
- 166.Joubert O, Viero G, Keller D, Martinez E, Colin DA, Monteil H, Mourey L, Dalla Serra M, Prevost G. 2006. Engineered covalent leucotoxin heterodimers form functional pores: insights into S-F interactions. Biochem. J. 396:381–389. 10.1042/BJ20051878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Viero G, Cunaccia R, Prevost G, Werner S, Monteil H, Keller D, Joubert O, Menestrina G, Dalla Serra M. 2006. Homologous versus heterologous interactions in the bicomponent staphylococcal gamma-haemolysin pore. Biochem. J. 394:217–225. 10.1042/BJ20051210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moussa LB, Werner S, Coraiola M, Colin DA, Keller D, Sanni A, Serra MD, Monteil H, Prevost G. 2006. Site-directed mutagenesis to assess the binding capacity of class S protein of Staphylococcus aureus leucotoxins to the surface of polymorphonuclear cells. J. Biomed. Biotechnol. 2006:80101. 10.1155/JBB/2006/80101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Joubert O, Voegelin J, Guillet V, Tranier S, Werner S, Colin DA, Dalla Serra M, Keller D, Monteil H, Mourey L, Prevost G. 2007. Distinction between pore assembly by staphylococcal alpha-toxin versus leukotoxins. J. Biomed. Biotechnol. 2007:25935. 10.1155/2007/25935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meunier O, Ferreras M, Supersac G, Hoeper F, Baba-Moussa L, Monteil H, Colin DA, Menestrina G, Prevost G. 1997. A predicted beta-sheet from class S components of staphylococcal gamma-hemolysin is essential for the secondary interaction of the class F component. Biochim. Biophys. Acta 1326:275–286. 10.1016/S0005-2736(97)00031-X [DOI] [PubMed] [Google Scholar]
- 171.Ferreras M, Hoper F, Dalla Serra M, Colin DA, Prevost G, Menestrina G. 1998. The interaction of Staphylococcus aureus bi-component gamma-hemolysins and leucocidins with cells and lipid membranes. Biochim. Biophys. Acta 1414:108–126. 10.1016/S0005-2736(98)00160-6 [DOI] [PubMed] [Google Scholar]
- 172.Kaneko J, Mascarenas AL, Huda MN, Tomita T, Kamio Y. 1998. An N-terminal region of LukF of staphylococcal leukocidin/gamma-hemolysin crucial for the biological activity of the toxin. Biosci. Biotechnol. Biochem. 62:1465–1467. 10.1271/bbb.62.1465 [DOI] [PubMed] [Google Scholar]
- 173.Colin DA, Mazurier I, Sire S, Finck-Barbancon V. 1994. Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: sequential binding and subsequent activation. Infect. Immun. 62:3184–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gauduchon V, Werner S, Prevost G, Monteil H, Colin DA. 2001. Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect. Immun. 69:2390–2395. 10.1128/IAI.69.4.2390-2395.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nguyen VT, Higuchi H, Kamio Y. 2002. Controlling pore assembly of staphylococcal gamma-haemolysin by low temperature and by disulphide bond formation in double-cysteine LukF mutants. Mol. Microbiol. 45:1485–1498. 10.1046/j.1365-2958.2002.03125.x [DOI] [PubMed] [Google Scholar]
- 176.Miles G, Jayasinghe L, Bayley H. 2006. Assembly of the bi-component leukocidin pore examined by truncation mutagenesis. J. Biol. Chem. 281:2205–2214. 10.1074/jbc.M510842200 [DOI] [PubMed] [Google Scholar]
- 177.Comai M, Dalla Serra M, Coraiola M, Werner S, Colin DA, Monteil H, Prevost G, Menestrina G. 2002. Protein engineering modulates the transport properties and ion selectivity of the pores formed by staphylococcal gamma-haemolysins in lipid membranes. Mol. Microbiol. 44:1251–1267. 10.1046/j.1365-2958.2002.02943.x [DOI] [PubMed] [Google Scholar]
- 178.Olson R, Nariya H, Yokota K, Kamio Y, Gouaux E. 1999. Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat. Struct. Biol. 6:134–140. 10.1038/5821 [DOI] [PubMed] [Google Scholar]
- 179.Pedelacq JD, Maveyraud L, Prevost G, Baba-Moussa L, Gonzalez A, Courcelle E, Shepard W, Monteil H, Samama JP, Mourey L. 1999. The structure of a Staphylococcus aureus leucocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure 7:277–287. 10.1016/S0969-2126(99)80038-0 [DOI] [PubMed] [Google Scholar]
- 180.Guillet V, Roblin P, Werner S, Coraiola M, Menestrina G, Monteil H, Prevost G, Mourey L. 2004. Crystal structure of leucotoxin S component: new insight into the staphylococcal beta-barrel pore-forming toxins. J. Biol. Chem. 279:41028–41037. 10.1074/jbc.M406904200 [DOI] [PubMed] [Google Scholar]
- 181.Roblin P, Guillet V, Joubert O, Keller D, Erard M, Maveyraud L, Prevost G, Mourey L. 2008. A covalent S-F heterodimer of leucotoxin reveals molecular plasticity of beta-barrel pore-forming toxins. Proteins 71:485–496. 10.1002/prot.21900 [DOI] [PubMed] [Google Scholar]
- 182.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. 1996. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274:1859–1866. 10.1126/science.274.5294.1859 [DOI] [PubMed] [Google Scholar]
- 183.Valeva A, Pongs J, Bhakdi S, Palmer M. 1997. Staphylococcal alpha-toxin: the role of the N-terminus in formation of the heptameric pore—a fluorescence study. Biochim. Biophys. Acta 1325:281–286. 10.1016/S0005-2736(96)00266-0 [DOI] [PubMed] [Google Scholar]
- 184.Valeva A, Weisser A, Walker B, Kehoe M, Bayley H, Bhakdi S, Palmer M. 1996. Molecular architecture of a toxin pore: a 15-residue sequence lines the transmembrane channel of staphylococcal alpha-toxin. EMBO J. 15:1857–1864 [PMC free article] [PubMed] [Google Scholar]
- 185.Walker B, Krishnasastry M, Zorn L, Bayley H. 1992. Assembly of the oligomeric membrane pore formed by staphylococcal alpha-hemolysin examined by truncation mutagenesis. J. Biol. Chem. 267:21782–21786 [PubMed] [Google Scholar]
- 186.Ozawa T, Kaneko J, Kamio Y. 1995. Essential binding of LukF of staphylococcal gamma-hemolysin followed by the binding of H gamma II for the hemolysis of human erythrocytes. Biosci. Biotechnol. Biochem. 59:1181–1183. 10.1271/bbb.59.1181 [DOI] [PubMed] [Google Scholar]
- 187.Yokota K, Kamio Y. 2000. Tyrosine72 residue at the bottom of rim domain in LukF crucial for the sequential binding of the staphylococcal gamma-hemolysin to human erythrocytes. Biosci. Biotechnol. Biochem. 64:2744–2747. 10.1271/bbb.64.2744 [DOI] [PubMed] [Google Scholar]
- 188.Aman MJ, Karauzum H, Bowden MG, Nguyen TL. 2010. Structural model of the pre-pore ring-like structure of Panton-Valentine leukocidin: providing dimensionality to biophysical and mutational data. J. Biomol. Struct. Dyn. 28:1–12. 10.1080/073911010010524952 [DOI] [PubMed] [Google Scholar]
- 189.Das SK, Darshi M, Cheley S, Wallace MI, Bayley H. 2007. Membrane protein stoichiometry determined from the step-wise photobleaching of dye-labelled subunits. Chembiochem 8:994–999. 10.1002/cbic.200600474 [DOI] [PubMed] [Google Scholar]
- 190.Yamashita K, Kawai Y, Tanaka Y, Hirano N, Kaneko J, Tomita N, Ohta M, Kamio Y, Yao M, Tanaka I. 2011. Crystal structure of the octameric pore of staphylococcal gamma-hemolysin reveals the beta-barrel pore formation mechanism by two components. Proc. Natl. Acad. Sci. U. S. A. 108:17314–17319. 10.1073/pnas.1110402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alessandrini A, Viero G, Dalla Serra M, Prevost G, Facci P. 2013. Gamma-hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: an AFM investigation. Biochim. Biophys. Acta 1828:405–411. 10.1016/j.bbamem.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 192.Galy R, Bergeret F, Keller D, Mourey L, Prevost G, Maveyraud L. 2012. Crystallization and preliminary crystallographic studies of both components of the staphylococcal LukE-LukD leukotoxin. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 68:663–667. 10.1107/S1744309112014662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dumont AL, Yoong P, Liu X, Day CJ, Chumbler NM, James DB, Alonzo F, III, Bode NJ, Lacy DB, Jennings MP, Torres VJ. 2014. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect. Immun. 82:1268–1276. 10.1128/IAI.01444-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Valentine FCO, Butler ECB. 1939. Specific immunity in acute staphylococcal osteomyelitis—with some observations on the causation of pyaemia. Lancet i:973–978 [Google Scholar]
- 195.Valentine F, Butler E. 1940. Toxoid treatment of recurrent infection after staphylococcal osteomyelitis. Lancet 235:914–917. 10.1016/S0140-6736(00)72373-7 [DOI] [Google Scholar]
- 196.Louria DB, Mudd S, Gladstone GP. 1969. Influence of immunization on chronic staphylococcal infections. Bull. N. Y. Acad. Med. 45:491 [Google Scholar]
- 197.Gladstone GP, Lenhart NA, Hochstein HD, Mudd S. 1962. Assay of anti-staphylococcal leucocidal components (F and S) in human serum. Br. J. Exp. Pathol. 43:295–312 [PMC free article] [PubMed] [Google Scholar]
- 198.Otto M. 2010. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64:143–162. 10.1146/annurev.micro.112408.134309 [DOI] [PubMed] [Google Scholar]
- 199.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, Lina G, van Strijp JA. 2013. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594. 10.1016/j.chom.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 200.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6:e1000715. 10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grojec PL, Jeljaszewicz J. 1981. Effect of staphylococcal leukocidin on mouse leukocyte system. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 250:446–455 [PubMed] [Google Scholar]
- 202.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J. Infect. Dis. 198:1166–1170. 10.1086/592053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. 2007. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315:1130–1133. 10.1126/science.1137165 [DOI] [PubMed] [Google Scholar]
- 204.Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. 2009. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin. Microbiol. Infect. 15:156–164. 10.1111/j.1469-0691.2008.02648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tseng CW, Kyme P, Low J, Rocha MA, Alsabeh R, Miller LG, Otto M, Arditi M, Diep BA, Nizet V, Doherty TM, Beenhouwer DO, Liu GY. 2009. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS One 4:e6387. 10.1371/journal.pone.0006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vandenesch F, Couzon F, Boisset S, Benito Y, Brown EL, Lina G, Etienne J, Bowden MG. 2010. The Panton-Valentine leukocidin is a virulence factor in a murine model of necrotizing pneumonia. J. Infect. Dis. 201:967–969. 10.1086/651026 (Reply, 201:969-970, doi:10.1086/651027.) [DOI] [PubMed] [Google Scholar]
- 207.Villaruz AE, Bubeck Wardenburg J, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. 2009. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J. Infect. Dis. 200:724–734. 10.1086/604728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761–1770. 10.1086/509506 [DOI] [PubMed] [Google Scholar]
- 209.Yoong P, Pier GB. 2010. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc. Natl. Acad. Sci. U. S. A. 107:2241–2246. 10.1073/pnas.0910344107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yoong P, Pier GB. 2012. Immune-activating properties of Panton-Valentine leukocidin improve the outcome in a model of methicillin-resistant Staphylococcus aureus pneumonia. Infect. Immun. 80:2894–2904. 10.1128/IAI.06360-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U. S. A. 107:5587–5592. 10.1073/pnas.0912403107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lipinska U, Hermans K, Meulemans L, Dumitrescu O, Badiou C, Duchateau L, Haesebrouck F, Etienne J, Lina G. 2011. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One 6:e22864. 10.1371/journal.pone.0022864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 204:937–941. 10.1093/infdis/jir441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Diep BA, Palazzolo-Ballance AM, Tattevin P, Basuino L, Braughton KR, Whitney AR, Chen L, Kreiswirth BN, Otto M, DeLeo FR, Chambers HF. 2008. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One 3:e3198. 10.1371/journal.pone.0003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cremieux AC, Dumitrescu O, Lina G, Vallee C, Cote JF, Muffat-Joly M, Lilin T, Etienne J, Vandenesch F, Saleh-Mghir A. 2009. Panton-Valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One 4:e7204. 10.1371/journal.pone.0007204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Olsen RJ, Kobayashi SD, Ayeras AA, Ashraf M, Graves SF, Ragasa W, Humbird T, Greaver JL, Cantu C, Swain JL, Jenkins L, Blasdel T, Cagle PT, Gardner DJ, DeLeo FR, Musser JM. 2010. Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am. J. Pathol. 176:1346–1354. 10.2353/ajpath.2010.090960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, Jones A, Palazzolo-Ballance AM, Perdreau-Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 197:1523–1530. 10.1086/587907 [DOI] [PubMed] [Google Scholar]
- 218.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:5883–5888. 10.1073/pnas.0900743106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Thurlow LR, Joshi GS, Clark JR, Spontak JS, Neely CJ, Maile R, Richardson AR. 2013. Functional modularity of the arginine catabolic mobile element contributes to the success of USA300 methicillin-resistant Staphylococcus aureus. Cell Host Microbe 13:100–107. 10.1016/j.chom.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lee SS, Kim YJ, Chung DR, Jung KS, Kim JS. 2010. Invasive infection caused by a community-associated methicillin-resistant Staphylococcus aureus strain not carrying Panton-Valentine leukocidin in South Korea. J. Clin. Microbiol. 48:311–313. 10.1128/JCM.00297-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, Shabb DW, Diep BA, Chambers HF, Otto M, DeLeo FR. 2011. Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 6:e18617. 10.1371/journal.pone.0018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nilsson IM, Hartford O, Foster T, Tarkowski A. 1999. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect. Immun. 67:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dajcs JJ, Thibodeaux BA, Girgis DO, O'Callaghan RJ. 2002. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 21:375–382. 10.1089/10445490260099656 [DOI] [PubMed] [Google Scholar]
- 224.Dajcs JJ, Austin MS, Sloop GD, Moreau JM, Hume EB, Thompson HW, McAleese FM, Foster TJ, O'Callaghan RJ. 2002. Corneal pathogenesis of Staphylococcus aureus strain Newman. Invest. Ophthalmol. Vis. Sci. 43:1109–1115 http://www.iovs.org/content/43/4/1109.long [PubMed] [Google Scholar]
- 225.Siqueira JA, Speeg-Schatz C, Freitas FI, Sahel J, Monteil H, Prevost G. 1997. Channel-forming leucotoxins from Staphylococcus aureus cause severe inflammatory reactions in a rabbit eye model. J. Med. Microbiol. 46:486–494. 10.1099/00222615-46-6-486 [DOI] [PubMed] [Google Scholar]
- 226.Supersac G, Piemont Y, Kubina M, Prevost G, Foster TJ. 1998. Assessment of the role of gamma-toxin in experimental endophthalmitis using a hlg-deficient mutant of Staphylococcus aureus. Microb. Pathog. 24:241–251. 10.1006/mpat.1997.0192 [DOI] [PubMed] [Google Scholar]
- 227.Alonzo F, III, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. 2013. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493:51–55. 10.1038/nature11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Siwicki AK, Bownik A, Prevost G, Szmigielski S, Malaczewska J, Mikulska-Skupien E. 2003. In vitro effect of staphylococcal leukocidins (LukE, LukD) on the proliferative responses of blood lymphocytes in dog (Canis familiaris). Bull. Vet. Inst. Pulawy 47:395–401 http://bulletin.piwet.pulawy.pl/images/stories/pdf/20032/20032395402.pdf [Google Scholar]
- 229.Bownik A. 2006. In vitro effects of staphylococcal leukocidin LukE/LukD on the proliferative ability of lymphocytes isolated from common carp (Cyprinus carpio). Fish Shellfish Immunol. 20:656–659. 10.1016/j.fsi.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 230.Reyes-Robles T, Alonzo F, III, Kozhaya L, Lacy DB, Unutmaz D, Torres VJ. 2013. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14:453–459. 10.1016/j.chom.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gravet A, Couppie P, Meunier O, Clyti E, Moreau B, Pradinaud R, Monteil H, Prevost G. 2001. Staphylococcus aureus isolated in cases of impetigo produces both epidermolysin A or B and LukE-LukD in 78% of 131 retrospective and prospective cases. J. Clin. Microbiol. 39:4349–4356. 10.1128/JCM.39.12.4349-4356.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gravet A, Rondeau M, Harf-Monteil C, Grunenberger F, Monteil H, Scheftel JM, Prevost G. 1999. Predominant Staphylococcus aureus isolated from antibiotic-associated diarrhea is clinically relevant and produces enterotoxin A and the bicomponent toxin LukE-LukD. J. Clin. Microbiol. 37:4012–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J. Infect. Dis. 206:1185–1193. 10.1093/infdis/jis495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. 2013. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect. Immun. 81:1830–1841. 10.1128/IAI.00095-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.DuMont AL, Yoong P, Day CJ, Alonzo F, III, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. U. S. A. 110:10794–10799. 10.1073/pnas.1305121110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR, Otto M, van Strijp JA, Nijland R. 2013. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell. Microbiol. 15:1427–1437. 10.1111/cmi.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fromageau A, Gilbert FB, Prevost G, Rainard P. 2010. Binding of the Staphylococcus aureus leucotoxin LukM to its leucocyte targets. Microb. Pathog. 49:354–362. 10.1016/j.micpath.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 238.Barrio MB, Rainard P, Prevost G. 2006. LukM/LukF′-PV is the most active Staphylococcus aureus leukotoxin on bovine neutrophils. Microbes Infect. 8:2068–2074. 10.1016/j.micinf.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 239.Rainard P. 2007. Staphylococcus aureus leucotoxin LukM/F′ is secreted and stimulates neutralising antibody response in the course of intramammary infection. Vet. Res. 38:685–696. 10.1051/vetres:2007026 [DOI] [PubMed] [Google Scholar]
- 240.Fromageau A, Cunha P, Gilbert FB, Rainard P. 2011. Purified Staphylococcus aureus leukotoxin LukM/F′ does not trigger inflammation in the bovine mammary gland. Microb. Pathog. 51:396–401. 10.1016/j.micpath.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 241.Simpson V, Davison N, Kearns A, Pichon B, Hudson L, Koylass M, Blackett T, Butler H, Rasigade J, Whatmore A. 2013. Association of a lukM-positive clone of Staphylococcus aureus with fatal exudative dermatitis in red squirrels (Sciurus vulgaris). Vet. Microbiol. 162(2-4):987–991. 10.1016/j.vetmic.2012.10.025 [DOI] [PubMed] [Google Scholar]
- 242.Potrich C, Bastiani H, Colin DA, Huck S, Prevost G, Dalla Serra M. 2009. The influence of membrane lipids in Staphylococcus aureus gamma-hemolysins pore formation. J. Membr. Biol. 227:13–24. 10.1007/s00232-008-9140-6 [DOI] [PubMed] [Google Scholar]
- 243.Monma N, Nguyen VT, Kaneko J, Higuchi H, Kamio Y. 2004. Essential residues, W177 and R198, of LukF for phosphatidylcholine-binding and pore-formation by staphylococcal gamma-hemolysin on human erythrocyte membranes. J. Biochem. 136:427–431. 10.1093/jb/mvh140 [DOI] [PubMed] [Google Scholar]
- 244.Meyer F, Girardot R, Piemont Y, Prevost G, Colin DA. 2009. Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding. Infect. Immun. 77:266–273. 10.1128/IAI.00402-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Alonzo F, Torres VJ. 2013. Staphylococcus aureus and CCR5: unveiling commonalities in host-pathogen interactions and potential treatment strategies. Future Microbiol. 8:425–428. 10.2217/fmb.13.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu ZJ, Yang YJ, Jiang L, Xu YC, Wang AX, Du GH, Gao JM. 2011. Tyrosine sulfation in N-terminal domain of human C5a receptor is necessary for binding of chemotaxis inhibitory protein of Staphylococcus aureus. Acta Pharmacol. Sin. 32:1038–1044. 10.1038/aps.2011.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bunschoten A, Feitsma LJ, Kruijtzer JA, de Haas CJ, Liskamp RM, Kemmink J. 2010. CHIPS binds to the phosphorylated N-terminus of the C5a-receptor. Bioorg. Med. Chem. Lett. 20:3338–3340. 10.1016/j.bmcl.2010.04.028 [DOI] [PubMed] [Google Scholar]
- 248.Nikiforovich GV, Baranski TJ. 2009. Structural models for the complex of chemotaxis inhibitory protein of Staphylococcus aureus with the C5a receptor. Biochem. Biophys. Res. Commun. 390:481–484. 10.1016/j.bbrc.2009.09.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ippel JH, de Haas CJ, Bunschoten A, van Strijp JA, Kruijtzer JA, Liskamp RM, Kemmink J. 2009. Structure of the tyrosine-sulfated C5a receptor N terminus in complex with chemotaxis inhibitory protein of Staphylococcus aureus. J. Biol. Chem. 284:12363–12372. 10.1074/jbc.M808179200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Postma B, Poppelier MJ, van Galen JC, Prossnitz ER, van Strijp JA, de Haas CJ, van Kessel KP. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the C5a and formylated peptide receptor. J. Immunol. 172:6994–7001 http://www.jimmunol.org/content/172/11/6994.long [DOI] [PubMed] [Google Scholar]
- 251.Postma B, Kleibeuker W, Poppelier MJ, Boonstra M, Van Kessel KP, Van Strijp JA, de Haas CJ. 2005. Residues 10-18 within the C5a receptor N terminus compose a binding domain for chemotaxis inhibitory protein of Staphylococcus aureus. J. Biol. Chem. 280:2020–2027. 10.1074/jbc.M412230200 [DOI] [PubMed] [Google Scholar]
- 252.Graves SF, Kobayashi SD, Braughton KR, Whitney AR, Sturdevant DE, Rasmussen DL, Kirpotina LN, Quinn MT, DeLeo FR. 2012. Sublytic concentrations of Staphylococcus aureus Panton-Valentine leukocidin alter human PMN gene expression and enhance bactericidal capacity. J. Leukoc. Biol. 92:361–374. 10.1189/jlb.1111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA, Broglie PM, Marketon K, Austermann J, Vogl T, Foell D, Niemann S, Peters G, Roth J, Loffler B. 2012. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 92:1069–1081. 10.1189/jlb.0112014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nariya H, Kamio Y. 1995. Identification of the essential regions for LukS- and H gamma II-specific functions of staphylococcal leukocidin and gamma-hemolysin. Biosci. Biotechnol. Biochem. 59:1603–1604. 10.1271/bbb.59.1603 [DOI] [PubMed] [Google Scholar]
- 255.Yu RK, Tsai YT, Ariga T, Yanagisawa M. 2011. Structures, biosynthesis, and functions of gangliosides—an overview. J. Oleo Sci. 60:537–544. 10.5650/jos.60.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nariya H, Izaki K, Kamio Y. 1993. The C-terminal region of the S component of staphylococcal leukocidin is essential for the biological activity of the toxin. FEBS Lett. 329:219–222. 10.1016/0014-5793(93)80225-J [DOI] [PubMed] [Google Scholar]
- 257.Ozawa T, Kaneko J, Nariya H, Izaki K, Kamio Y. 1994. Inactivation of gamma-hemolysin H gamma II component by addition of monosialoganglioside GM1 to human erythrocyte. Biosci. Biotechnol. Biochem. 58:602–605. 10.1271/bbb.58.602 [DOI] [PubMed] [Google Scholar]
- 258.Tomita N, Abe K, Kamio Y, Ohta M. 2011. Cluster-forming property correlated with hemolytic activity by staphylococcal gamma-hemolysin transmembrane pores. FEBS Lett. 585:3452–3456. 10.1016/j.febslet.2011.09.041 [DOI] [PubMed] [Google Scholar]
- 259.Konig B, Prevost G, Piemont Y, Konig W. 1995. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 171:607–613. 10.1093/infdis/171.3.607 [DOI] [PubMed] [Google Scholar]
- 260.Hensler T, Konig B, Prevost G, Piemont Y, Koller M, Konig W. 1994. Leukotriene B4 generation and DNA fragmentation induced by leukocidin from Staphylococcus aureus: protective role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF for human neutrophils. Infect. Immun. 62:2529–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kobayashi Y. 2008. The role of chemokines in neutrophil biology. Front. Biosci. 13:2400–2407. 10.2741/2853 [DOI] [PubMed] [Google Scholar]
- 262.Ferstl R, Akdis CA, O'Mahony L. 2012. Histamine regulation of innate and adaptive immunity. Front. Biosci. 17:40–53. 10.2741/3914 [DOI] [PubMed] [Google Scholar]
- 263.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. 2005. Leukotrienes: underappreciated mediators of innate immune responses. J. Immunol. 174:589–594 http://www.jimmunol.org/content/174/2/589.long [DOI] [PubMed] [Google Scholar]
- 264.Konig B, Koller M, Prevost G, Piemont Y, Alouf JE, Schreiner A, Konig W. 1994. Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, and erythrogenic toxin A): generation of interleukin-8. Infect. Immun. 62:4831–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Colin DA, Monteil H. 2003. Control of the oxidative burst of human neutrophils by staphylococcal leukotoxins. Infect. Immun. 71:3724–3729. 10.1128/IAI.71.7.3724-3729.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Perret M, Badiou C, Lina G, Burbaud S, Benito Y, Bes M, Cottin V, Couzon F, Juruj C, Dauwalder O, Goutagny N, Diep BA, Vandenesch F, Henry T. 2012. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell. Microbiol. 14:1019–1036. 10.1111/j.1462-5822.2012.01772.x [DOI] [PubMed] [Google Scholar]
- 267.Granstrom E. 1984. The arachidonic acid cascade. The prostaglandins, thromboxanes and leukotrienes. Inflammation 8(Suppl):S15–S25 [DOI] [PubMed] [Google Scholar]
- 268.Ricciotti E, FitzGerald GA. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31:986–1000. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Malachowa N, Kobayashi SD, Freedman B, Dorward DW, Deleo FR. 2013. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J. Immunol. 191:6022–6029. 10.4049/jimmunol.1301821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zivkovic A, Sharif O, Stich K, Doninger B, Biaggio M, Colinge J, Bilban M, Mesteri I, Hazemi P, Lemmens-Gruber R, Knapp S. 2011. TLR2 and CD14 mediate innate immunity and lung inflammation to staphylococcal Panton-Valentine leukocidin in vivo. J. Immunol. 186:1608–1617. 10.4049/jimmunol.1001665 [DOI] [PubMed] [Google Scholar]
- 271.Hayden MS, Ghosh S. 2004. Signaling to NF-kappab. Genes Dev. 18:2195–2224. 10.1101/gad.1228704 [DOI] [PubMed] [Google Scholar]
- 272.Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42:145–151. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 273.Inden K, Kaneko J, Miyazato A, Yamamoto N, Mouri S, Shibuya Y, Nakamura K, Aoyagi T, Hatta M, Kunishima H, Hirakata Y, Itoh Y, Kaku M, Kawakami K. 2009. Toll-like receptor 4-dependent activation of myeloid dendritic cells by leukocidin of Staphylococcus aureus. Microbes Infect. 11:245–253. 10.1016/j.micinf.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 274.Latz E, Xiao TS, Stutz A. 2013. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13:397–411. 10.1038/nri3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bauernfeind F, Hornung V. 2013. Of inflammasomes and pathogens—sensing of microbes by the inflammasome. EMBO Mol. Med. 5:814–826. 10.1002/emmm.201201771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E. 2013. Inflammasomes and host defenses against bacterial infections. Curr. Opin. Microbiol. 16:23–31. 10.1016/j.mib.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ciraci C, Janczy JR, Sutterwala FS, Cassel SL. 2012. Control of innate and adaptive immunity by the inflammasome. Microbes Infect. 14:1263–1270. 10.1016/j.micinf.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Franchi L, Munoz-Planillo R, Nunez G. 2012. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13:325–332. 10.1038/ni.2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. 2009. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the NLRP3 inflammasome. J. Immunol. 183:3942–3948. 10.4049/jimmunol.0900729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. 10.1371/journal.pone.0007446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205:807–817. 10.1093/infdis/jir846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hanamsagar R, Torres V, Kielian T. 2011. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J. Neurochem. 119:736–748. 10.1111/j.1471-4159.2011.07481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Aslam R, Laventie BJ, Marban C, Prevost G, Keller D, Strub JM, Dorsselaer A, Haikel Y, Taddei C, Metz-Boutigue MH. 2013. Activation of neutrophils by the two-component leukotoxin LukE/D from Staphylococcus aureus: proteomic analysis of the secretions. J. Proteome Res. 12:3667–3678. 10.1021/pr400199x [DOI] [PubMed] [Google Scholar]
- 284.Genestier AL, Michallet MC, Prevost G, Bellot G, Chalabreysse L, Peyrol S, Thivolet F, Etienne J, Lina G, Vallette FM, Vandenesch F, Genestier L. 2005. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115:3117–3127. 10.1172/JCI22684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jover E, Tawk MY, Laventie BJ, Poulain B, Prevost G. 2013. Staphylococcal leukotoxins trigger free intracellular Ca(2+) rise in neurones, signalling through acidic stores and activation of store-operated channels. Cell. Microbiol. 15:742–758. 10.1111/cmi.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–57. 10.1038/nature12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gladstone GP, Glencross EJG. 1960. Growth and toxin production of staphylococci in cellophane sacs in vivo. Br. J. Exp. Pathol. 41:313–333 [PMC free article] [PubMed] [Google Scholar]
- 288.Graves SF, Kobayashi SD, Braughton KR, Diep BA, Chambers HF, Otto M, Deleo FR. 2010. Relative contribution of Panton-Valentine leukocidin to PMN plasma membrane permeability and lysis caused by USA300 and USA400 culture supernatants. Microbes Infect. 12:446–456. 10.1016/j.micinf.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Finck-Barbancon V, Prevost G, Piemont Y. 1991. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res. Microbiol. 142:75–85. 10.1016/0923-2508(91)90099-V [DOI] [PubMed] [Google Scholar]
- 290.Donahue JA, Baldwin JN. 1966. Hemolysin and leukocidin production by 80/81 strains of Staphylococcus aureus. J. Infect. Dis. 116:324–328. 10.1093/infdis/116.3.324 [DOI] [PubMed] [Google Scholar]
- 291.Bronner S, Stoessel P, Gravet A, Monteil H, Prevost G. 2000. Variable expressions of Staphylococcus aureus bicomponent leucotoxins semiquantified by competitive reverse transcription-PCR. Appl. Environ. Microbiol. 66:3931–3938. 10.1128/AEM.66.9.3931-3938.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu. Rev. Genet. 42:541–564. 10.1146/annurev.genet.42.110807.091640 [DOI] [PubMed] [Google Scholar]
- 293.Lyon GJ, Novick RP. 2004. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides 25:1389–1403. 10.1016/j.peptides.2003.11.026 [DOI] [PubMed] [Google Scholar]
- 294.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. 2011. Peptide signaling in the staphylococci. Chem. Rev. 111:117–151. 10.1021/cr100370n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thoendel M, Horswill AR. 2013. Random mutagenesis and topology analysis of the autoinducing peptide biosynthesis proteins in Staphylococcus aureus. Mol. Microbiol. 87:318–337. 10.1111/mmi.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Geisinger E, George EA, Chen J, Muir TW, Novick RP. 2008. Identification of ligand specificity determinants in AgrC, the Staphylococcus aureus quorum-sensing receptor. J. Biol. Chem. 283:8930–8938. 10.1074/jbc.M710227200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Balaban N, Novick RP. 1995. Translation of RNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3′-end deletion. FEMS Microbiol. Lett. 133:155–161. 10.1111/j.1574-6968.1995.tb07877.x [DOI] [PubMed] [Google Scholar]
- 298.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK. 2004. Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol. 186:7549–7555. 10.1128/JB.186.22.7549-7555.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341–7353. 10.1128/JB.183.24.7341-7353.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Said-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, Arvidson S, Foster TJ, Projan SJ, Kreiswirth BN. 2003. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 185:610–619. 10.1128/JB.185.2.610-619.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.McNamara PJ, Milligan-Monroe KC, Khalili S, Proctor RA. 2000. Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. J. Bacteriol. 182:3197–3203. 10.1128/JB.182.11.3197-3203.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Benson MA, Lilo S, Wasserman GA, Thoendel M, Smith A, Horswill AR, Fraser J, Novick RP, Shopsin B, Torres VJ. 2011. Staphylococcus aureus regulates the expression and production of the staphylococcal superantigen-like secreted proteins in a Rot-dependent manner. Mol. Microbiol. 81:659–675. 10.1111/j.1365-2958.2011.07720.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Benson MA, Lilo S, Nygaard T, Voyich JM, Torres VJ. 2012. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J. Bacteriol. 194:4355–4365. 10.1128/JB.00706-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. 2006. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol. Microbiol. 61:1038–1048. 10.1111/j.1365-2958.2006.05292.x [DOI] [PubMed] [Google Scholar]
- 306.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, Gaspin C, Vandenesch F, Romby P. 2007. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 21:1353–1366. 10.1101/gad.423507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.McNamara PJ, Bayer AS. 2005. A rot mutation restores parental virulence to an agr-null Staphylococcus aureus strain in a rabbit model of endocarditis. Infect. Immun. 73:3806–3809. 10.1128/IAI.73.6.3806-3809.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR, Whitney AR, Otto M, DeLeo FR. 2009. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 199:1698–1706. 10.1086/598967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. 2010. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201:241–254. 10.1086/649570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zurek OW, Nygaard TK, Watkins RL, Pallister KB, Torres VJ, Horswill AR, Voyich JM. 2014. The role of innate immunity in promoting SaeR/S-mediated virulence in Staphylococcus aureus. J. Innate Immun. 6:21–30. 10.1159/000351200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Reyes D, Andrey DO, Monod A, Kelley WL, Zhang G, Cheung AL. 2011. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 193:6020–6031. 10.1128/JB.05436-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chien Y, Manna AC, Projan SJ, Cheung AL. 1999. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J. Biol. Chem. 274:37169–37176. 10.1074/jbc.274.52.37169 [DOI] [PubMed] [Google Scholar]
- 313.Rechtin TM, Gillaspy AF, Schumacher MA, Brennan RG, Smeltzer MS, Hurlburt BK. 1999. Characterization of the SarA virulence gene regulator of Staphylococcus aureus. Mol. Microbiol. 33:307–316. 10.1046/j.1365-2958.1999.01474.x [DOI] [PubMed] [Google Scholar]
- 314.Chien Y, Cheung AL. 1998. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J. Biol. Chem. 273:2645–2652. 10.1074/jbc.273.5.2645 [DOI] [PubMed] [Google Scholar]
- 315.Yamazaki K, Kato F, Kamio Y, Kaneko J. 2006. Expression of gamma-hemolysin regulated by sae in Staphylococcus aureus strain Smith 5R. FEMS Microbiol. Lett. 259:174–180. 10.1111/j.1574-6968.2006.00236.x [DOI] [PubMed] [Google Scholar]
- 316.Novick RP, Jiang D. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709–2717. 10.1099/mic.0.26575-0 [DOI] [PubMed] [Google Scholar]
- 317.Giraudo AT, Raspanti CG, Calzolari A, Nagel R. 1994. Characterization of a Tn551-mutant of Staphylococcus aureus defective in the production of several exoproteins. Can. J. Microbiol. 40:677–681. 10.1139/m94-107 [DOI] [PubMed] [Google Scholar]
- 318.Giraudo AT, Cheung AL, Nagel R. 1997. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch. Microbiol. 168:53–58. 10.1007/s002030050469 [DOI] [PubMed] [Google Scholar]
- 319.Harraghy N, Kormanec J, Wolz C, Homerova D, Goerke C, Ohlsen K, Qazi S, Hill P, Herrmann M. 2005. sae is essential for expression of the staphylococcal adhesins Eap and Emp. Microbiology 151:1789–1800. 10.1099/mic.0.27902-0 [DOI] [PubMed] [Google Scholar]
- 320.Geiger T, Goerke C, Mainiero M, Kraus D, Wolz C. 2008. The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis-related signals. J. Bacteriol. 190:3419–3428. 10.1128/JB.01927-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sun F, Li C, Jeong D, Sohn C, He C, Bae T. 2010. In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J. Bacteriol. 192:2111–2127. 10.1128/JB.01524-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, Friedman DB, Heinrichs DE, Dunman PM, Skaar EP. 2010. Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect. Immun. 78:1618–1628. 10.1128/IAI.01423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kolar SL, Ibarra JA, Rivera FE, Mootz JM, Davenport JE, Stevens SM, Horswill AR, Shaw LN. 2013. Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2:18–34. 10.1002/mbo3.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Panton PN, Valentine FCO, Dix VW. 1931. Staphylococcal infection and antitoxin treatment. Lancet ii:1180–1183 [Google Scholar]
- 325.Adlam C, Ward PD, Turner WH. 1980. Effect of immunization with highly purified Panton-Valentine leucocidin and delta-toxin on staphylococcal mastitis in rabbits. J. Comp. Pathol. 90:265–274. 10.1016/0021-9975(80)90063-8 [DOI] [PubMed] [Google Scholar]
- 326.Gauduchon V, Cozon G, Vandenesch F, Genestier AL, Eyssade N, Peyrol S, Etienne J, Lina G. 2004. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J. Infect. Dis. 189:346–353. 10.1086/380909 [DOI] [PubMed] [Google Scholar]
- 327.Zaidi T, Zaidi T, Yoong P, Pier GB. 2013. Staphylococcus aureus corneal infections: effect of the Panton-Valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest. Ophthalmol. Vis. Sci. 54:4430–4438. 10.1167/iovs.13-11701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Karauzum H, Adhikari RP, Sarwar J, Devi VS, Abaandou L, Haudenschild C, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Warfield KL, Shulenin S, Aman MJ. 2013. Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS One 8:e65384. 10.1371/journal.pone.0065384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hermos CR, Yoong P, Pier GB. 2010. High levels of antibody to Panton-Valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin. Infect. Dis. 51:1138–1146. 10.1086/656742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Thomsen IP, Dumont AL, James DB, Yoong P, Saville BR, Soper N, Torres VJ, Creech CB. 2014. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect. Immun. 82:1234–1242. 10.1128/IAI.01558-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Laventie BJ, Potrich C, Atmanene C, Saleh M, Joubert O, Viero G, Bachmeyer C, Antonini V, Mancini I, Cianferani-Sanglier S, Keller D, Colin DA, Bourcier T, Anderluh G, van Dorsselaer A, Dalla Serra M, Prevost G. 2013. P-sulfonato-calix[n]arenes inhibit staphylococcal bicomponent leukotoxins by supramolecular interactions. Biochem. J. 450:559–571. 10.1042/BJ20121628 [DOI] [PubMed] [Google Scholar]
- 332.Laventie BJ, Rademaker HJ, Saleh M, de Boer E, Janssens R, Bourcier T, Subilia A, Marcellin L, van Haperen R, Lebbink JH, Chen T, Prevost G, Grosveld F, Drabek D. 2011. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc. Natl. Acad. Sci. U. S. A. 108:16404–16409. 10.1073/pnas.1102265108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Perret F, Lazar AN, Coleman AW. 2006. Biochemistry of the para-sulfonato-calix[n]arenes. Chem. Commun. (Camb.) 2006:2425–2438. 10.1039/B600720C [DOI] [PubMed] [Google Scholar]
- 334.Sadik CD, Kim ND, Luster AD. 2011. Neutrophils cascading their way to inflammation. Trends Immunol. 32:452–460. 10.1016/j.it.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]