Abstract
Aspergillus fumigatus is the leading causative agent of invasive aspergillosis (IA). The number of cases is on the rise, with mortality rates as high as 90% among immunocompromised patients. Molecular genetic studies in A. fumigatus could provide novel targets to potentially set the basis for antifungal therapies. In the current study, we investigated the role of the transcription factor gene mtfA in A. fumigatus. Our results revealed that mtfA plays a role in the growth and development of the fungus. Deletion or overexpression of mtfA leads to a slight reduction in colony growth, as well as a reduction in conidiation levels, in the overexpression strain compared to the wild-type strain. Furthermore, production of the secondary metabolite gliotoxin increased when mtfA was overexpressed, coinciding with an increase in the transcription levels of the gliotoxin genes gliZ and gliP with respect to the wild type. In addition, our study showed that mtfA is also necessary for normal protease activity in A. fumigatus; deletion of mtfA resulted in a reduction of protease activity compared to wild-type levels. Importantly, the absence of mtfA caused a decrease in virulence in the Galleria mellonella infection model, indicating that mtfA is necessary for A. fumigatus wild-type pathogenesis.
INTRODUCTION
Aspergillus fumigatus is a ubiquitous saprotrophic fungus that is also an opportunistic human pathogen. Inhalation of A. fumigatus conidia can cause allergic bronchopulmonary aspergillosis, an allergic response to the spores that occurs in hypersensitive patients (1, 2). In immunodepressed patients A. fumigatus is the leading causative agent of invasive aspergillosis (IA). This group of patients includes those infected with HIV, cancer patients undergoing chemotherapy, organ transplant patients, individuals with genetic immunodeficiencies, and patients with hematological malignancies (3–8). A number of factors are contributing to the increasing proportion of the population affected by this disease (9), for example, the rise in HIV cases, more advanced technology to perform organ transplants, and more effective therapies for cancer patients and autoimmune diseases (3, 9–11). Once the fungal infection has been established, IA has a mortality rate ranging from 40 to 90% in these patients.
The primary route of infection by A. fumigatus is inhalation. The small size (2.5 to 3.0 μm) of the asexual spores, called conidia, allows them to reach the lung alveoli (12). The first point of contact is with the bronchial epithelial cells (1). These cells have dectin-1 receptors that can recognize the β-1,3-glucan on the cell surfaces of conidia (1, 13, 14), activating the production of reactive oxygen species (ROS) and of antimicrobial peptides and cytokines as part of the initial immune response. In healthy individuals, conidia that are able to evade mucociliary clearance are quickly removed by either alveolar macrophages or epithelial cells via phagocytosis. A proinflammatory response also includes the recruiting neutrophils that have the capability to eliminate hyphae, preventing further fungal colonization. The identification of genetic mechanisms that regulate A. fumigatus conidiation could contribute to strategies to decrease the primary source of the fungal inoculum.
Fungal morphological development, such as conidiation, and secondary metabolism have been shown to be genetically linked (15–18). A. fumigatus possesses the capacity to produce a large range of secondary metabolites, also called natural products, which are important for environmental fitness (15, 19). Some of these secondary metabolites are virulence factors during A. fumigatus infection. The best known is gliotoxin, which has been shown to inhibit phagocytosis by macrophages and to kill neutrophils (20–34).
Knowledge of genetic links connecting morphogenesis and biosynthesis of natural products in A. fumigatus is still limited. The most studied genes are those encoding components of the global regulatory velvet protein complex (17, 18, 35), in which VeA might act as a scaffold. Previously reported work from our laboratory has shown that A. fumigatus veA plays a role in growth and development, as well as in regulating protease activity (17). Furthermore, we found that veA regulates the expression of secondary-metabolite gene clusters and concomitant biosynthesis of associated compounds, such as gliotoxin, fumagillin, fumitremorgin G, and fumigaclavine C (17, 35).
Components of the conserved velvet complex, particularly VeA, have been characterized in greatest depth in the model fungus Aspergillus nidulans (15–18, 36–39). Recently, in a mutagenesis screening, we identified mtfA, a veA-dependent gene encoding a novel C2H2 finger domain transcription factor in this model organism (40). A. nidulans mtfA regulates both morphological development and secondary metabolism. Deletion of A. nidulans mtfA decreases conidiation and sexual development (40). Furthermore, absence of mtfA also completely prevents the expression of genes involved in the production of the mycotoxin sterigmatocystin, the antitumoral compound terrequinone, and the beta-lactam antibiotic penicillin (40). Our previous studies also revealed that mtfA orthologs are present in many fungal species, including A. fumigatus (40). It is possible that mtfA could play similar roles in A. fumigatus that might affect virulence. In our current study, we characterized the role of this conserved master transcription factor gene in this opportunistic pathogen, particularly its roles in growth, conidiation, production of gliotoxin, and protease activity, as well as direct assessment of its role in pathogenicity.
MATERIALS AND METHODS
Strains and culture conditions.
The A. fumigatus strains used in this study are listed in Table 1. The strains were grown on Czapek-Dox medium (Difco) plus the necessary supplements (41) unless otherwise indicated. For solid medium, 10 g/liter of agar was added. Stocks were stored in 30% glycerol at −80°C.
TABLE 1.
Fungal strains used in the study
| Strain name | Pertinent genotype | Source |
|---|---|---|
| CEA10 | Wild type | Gift from Robert Cramer |
| CEA17 | pyrG1 | Gift from Robert Cramer |
| tTDS1 | pyrG1 gpdA(p)::mtfA::trpC(t)::pyrGA.fum | This study |
| tTDS4.1 | pyrG1 ΔmtfA::pyrGA.para | This study |
| tTDS10 | pyrG1 ΔmtfA::pyrGA.para mtfA::ptrA | This study |
| tTDS5.1 | pyrG1 mtfA::gfp::pyrGA fum | This study |
Generation of mtfA deletion, complementation, and overexpression mutant strains. (i) mtfA deletion strain.
The mtfA deletion (ΔmtfA) strain, tTDS4.1, was obtained by gene replacement using the A. fumigatus CEA17 strain, a pyrG auxotroph derived from the CEA10 isolate (Table 1). The deletion cassette was generated by fusion PCR as described by Szewczyk et al. (42). Briefly, 1.5-kb fragments corresponding to the mtfA 5′ and 3′ untranslated regions (UTRs) were PCR amplified from A. fumigatus genomic DNA using fumRM75p1/fumRM75p2, and fumRM73p3/fumRM73p4, respectively (Table 2). The intermediate fragment corresponding to Aspergillus parasiticus was PCR amplified from the genomic DNA of the fungus using primers Afumparapyrf2 and Afumparapyrr2 (Table 2). The three fragments were joined by fusion PCR using primers fumRM75p1 and fumRM73p4 (Table 2). Confirmation of double crossover and gene replacement was carried out by Southern blotting. quantitative reverse transcription (qRT)-PCR analysis was used to confirm the absence of mtfA expression in the deletion mutants (ΔmtfA) using primers AfumRM7qrtPCRF and AfumRM7qrtPCRR (Table 2). The resulting deletion strain was denoted tTDS4.1 (Table 1).
TABLE 2.
Primers used in the study

(ii) Complementation strain.
A complementation strain was generated as follows. A 5.8-kb fragment containing the mtfA wild-type locus (including the mtfA coding region plus 3.3 kb of the 5′ UTR and 1.5 kb of the 3′ UTR) was PCR amplified with primers AfummtfAcompF and AfumRM7cp2 (Table 2). The primers contained engineered NotI sites to facilitate ligation to pTDS3, a PJET-based plasmid containing the fungal transformation marker ptrA. The fragment was digested with NotI and ligated into pTDS3 previously digested with the same enzyme to generate the complementation vector pTDS10. This plasmid was then transformed into the ΔmtfA strain, tTDS4.1 (Table 1). Integration of the plasmid was confirmed via PCR, and expression levels were evaluated using qRT-PCR. The resulting complementation strain was denoted tTDS10 (Table 1).
(iii) mtfA overexpression strain.
The mtfA overexpression (OEmtfA) strain, tTDS1, was generated by transforming A. fumigatus CEA17 with the plasmid pTDS1. This overexpression plasmid was generated first by PCR amplification of the mtfA coding region from A. fumigatus genomic DNA with primers fumRM7OEp1 and fumRM7OEp2 (Table 2), containing AscI and NotI sites. Then, the fragment was digested with AscI and NotI and ligated into pSD21 (17) previously digested with the same enzymes. pSD21 contains the A. nidulans gpdA promoter, gpdA(p), the A. nidulans trpC terminator, trpC(t), and the A. fumigatus pyrG transformation selection marker. Integration of the overexpression vector into the genome was confirmed by PCR using primers gdpApromoF and fumRM7OEp2 (Table 2). Overexpression of mtfA was confirmed by qRT-PCR using primers AfumRM7qrtPCRF and AfumRM7qrtPCRR (Table 2).
Morphological studies.
Fungal growth was evaluated as colony diameter. A. fumigatus wild-type (CEA10), ΔmtfA, complementation, and OEmtfA strains were point inoculated on Czapek-Dox medium and incubated at 37°C. The experiments were carried out with three replicates. The strains were grown for 5 days in the dark, and the colony diameter was measured.
To determine the role of mtfA in A. fumigatus conidiation, spores (1 × 107 spores/ml) of A. fumigatus CEA10, ΔmtfA, complementation, and OEmtfA strains were inoculated in 25 ml of liquid Czapek-Dox medium. The cultures were grown at 37°C. At 72 h, the mycelial mats were collected and homogenized in 10 ml of distilled water (dH2O). The conidia were counted using a hemacytometer under a light microscope.
Fluorescence microscopy.
The subcellular localization of the mtfA gene product was determined in A. fumigatus strain tTDS5.1, where the MtfA protein was tagged with the green fluorescent protein (GFP). This strain was generated by transforming CEA17 with an mtfA::gfp::pyrGA.fum cassette. The cassette was generated by fusion PCR as previously described by Szewczyk et al. (42). First, the 3′ end of the mtfA coding region was PCR amplified from genomic DNA with primers AfumRM7midF and AfumRM7rev (Table 2). The 3′ UTR of the mtfA gene was PCR amplified with primers fumTM73p3 and fumRM73p4, also from genomic DNA (Table 2). The plasmid p1439 (36) was used as the template to PCR amplify the intermediate DNA fragment containing gfp::pyrGA.fum. The three fragments were fused using primers AfumRM7midF and fumRM73p4 (Table 2). Correct integration of the cassette into the genome was verified by PCR with primers fumRM75p1 and fumRM73p4 (Table 2).
Conidia from tTDS5.1 were inoculated on coverslips submerged in Watch minimal medium (43) and incubated at 37°C in both light and dark for 16 h. Then, the slides were washed with 1× phosphate-buffered saline (PBS) and stained for 5 min with DAPI (4′, 6-diamidino-2-phenylindole) (1:1,000) under gentle agitation. Samples were then viewed using a Nikon Eclipse E-600 microscope equipped with Nomarski optics and fluorochromes for GFP, with excitation at 470 nm and emission at 525 nm and ×600 magnification. Micrographs were obtained using a Hamamatsu Orca-ER high-sensitivity monochrome digital charge-coupled-device (CCD) camera with Microsuite 5 image capture and optimization software. The exposure time was 500 ms for DAPI images and 2 s for GFP images.
Protease activity.
A. fumigatus wild-type, ΔmtfA, complementation, and OEmtfA strains were point inoculated on plates containing Czapek-Dox medium (1% agar) and 5% skim milk (Difco). The plates were incubated at 37°C in the dark. After 3 days, the cultures were blended in 25 ml distilled water and collected in a 50-ml Falcon tube. The tubes were centrifuged at 3,500 rpm at 4°C, and 1 ml of supernatant was transferred to an Eppendorf tube, where it was centrifuged again at 10,000 rpm for 10 min at 4°C. An azocasein assay was performed as previously described by Reichard et al. (44) with slight modifications. One-hundred microliters of supernatant was mixed with 400 μl of azocasein (Sigma) at a concentration of 5 mg/ml; dissolved in 50 mM Tris buffer (pH 7.5), 0.2 M NaCl, 5 mM CaCl2, 0.05% Brij 35, and 0.01% sodium azide; and incubated at 37°C for 90 min. One hundred and fifty microliters of 20% trichloroacetic acid was then added to stop the reaction, and the samples were left at room temperature for 30 min. The tubes were spun at 8,000 rpm for 3 min, and 500 μl of the supernatant was mixed with 500 μl 1 M NaOH. Two-hundred microliters from each sample was placed into a 96-well plate (BD Falcon) in duplicate, and the absorbance of the released azo group was read at 436 nm using a plate reader (Epoch by Biotek). A negative control was used with sterile distilled water mixed with azocasein.
In a separate experiment and to test for protease activity in cell-free culture supernatants, A. fumigatus CEA10, ΔmtfA, complementation, and OEmtfA strains were inoculated in 50 ml of liquid Czapek-Dox medium containing 2.5% skim milk (Difco). Cultures were grown at 37°C at 200 rpm. Culture supernatant was collected at 48 h and 72 h. The azocasein assay was performed on the culture supernatants as described above.
Gliotoxin analysis.
Plates containing 25 ml of liquid Czapek-Dox medium were inoculated with 1 × 107 conidia/ml of A. fumigatus wild-type, ΔmtfA, complementation, and OEmtfA strains. Liquid stationary-phase cultures were incubated at 37°C in the dark. The supernatants were collected by filtration at 120 h after inoculation, using Miracloth (Calbiochem) to remove the mycelium. Fifteen milliliters of supernatant was collected from each plate. An equal amount of chloroform was added to extract the gliotoxin. The gliotoxin extracts were then resuspended in 200 μl of methanol and filtered through a 0.22-μm-pore-size membrane. Quantification of gliotoxin was performed by high-performance liquid chromatography (HPLC), as previously described by Cramer et al. (45) with some modifications. A Waters 1525 HPLC apparatus was used for the analysis. Injections of 20 μl of the methanol extract were applied. The flow rate was 1 ml/min, using a mobile phase of water-acetonitrile-trifluoroacetic acid (65:34.9:0.1). UV detection was performed at 264 nm (Waters 2487 dual λ absorbance detector). Gliotoxin peak areas were compared to those of standard gliotoxin samples of known concentrations (Sigma-Aldrich, St. Louis, MO).
Gene expression analysis.
Conidia (1 × 107 spores/ml) of A. fumigatus wild-type, ΔmtfA, complementation, and OEmtfA strains were inoculated in 25 ml of liquid Czapek-Dox medium and incubated in the dark at 37°C. Total RNA was collected at 72 h using Pig-B, as described by Weber and collaborators (46).
cDNA was generated from the extracted total RNA samples, and qRT-PCR was performed using an Mx3000p thermocycler (Agilent Technologies) with SYBR green Jumpstart Taq Ready mix (Sigma). Expression of brlA, wetA, gliP, and gliZ was examined. The PCR primers used to analyze the expression of these genes are listed in Table 2.
Pathogenesis analysis using G. mellonella as an infection model.
Spore suspensions of A. fumigatus wild-type, ΔmtfA, complementation, and OEmtfA stains were generated in 1× PBS (with 0.1% Tween). The spore suspensions were quantified using a hemacytometer and diluted in 1× PBS to a concentration of 1 × 106 spores/10 μl. The infection procedure was done as previously described by Fuchs et al. (47). Briefly, G. mellonella larvae with a weight range between 275 and 300 mg and lacking gray markings were selected for the experiment. Groups of 30 larvae were selected for each A. fumigatus strain, and PBS injections and noninjected larvae were used as a control. The larvae were injected behind the last left proleg with 1 × 106 spores, using a Hamilton syringe. The larvae were transferred into a petri dish (90 mm by 15 mm) wrapped in aluminum foil. The plates were placed at 37°C in the dark. After 16 h, the larvae were checked every 2 h for mortality. Mortality curves were generated using PRISM software. Statistical analysis was performed using a log rank test to generate pairwise comparisons of the survival of the larvae infected with different strains.
RESULTS
mtfA affects growth rate and conidiation in A. fumigatus.
In order to elucidate the role of mtfA (accession number XP_747808.1) in A. fumigatus, deletion (ΔmtfA), complementation, and overexpression (OEmtfA) strains were generated as specified in Materials and Methods. The strains were confirmed by Southern blotting or diagnostic PCR (Fig. 1 and 2). mtfA expression levels in wild-type, deletion, complementation, and overexpression analysis were also analyzed (Fig. 1E and 2C). As expected, the ΔmtfA strain did not show expression. Expression of mtfA in the OEmtfA strain was approximately 10 times greater than in the wild-type strain, indicating that the overexpression cassette was functional in the strain (Fig. 2).
FIG 1.
Generation of A. fumigatus mtfA deletion and complementation strains. Confirmation of mtfA deletion (ΔmtfA) and complementation (com) strains by Southern blotting, PCR, and qRT-PCR. (A) Diagram showing the replacement of mtfA with the marker gene pyrG by a double-crossover event. EcoRI restrictions sites (E) and probe template are shown. (B) X-ray image showing the Southern blot results confirming the deletion of mtfA. Genomic DNA samples were digested with EcoRI. The expected band sizes were 1.8 kb (wild type [WT]) and 6.1 kb (ΔmtfA). (C) Linear representation of plasmid pTDS10 used for complementation. (D) PCR confirmation of the mtfA wild-type allele integration in the ΔmtfA genome. The wild-type strain was used as a control. The PCR yielded the predicted 1-kb PCR product. (E) qRT-PCR expression analysis of mtfA with primers AfumRM7qrtPCRF and AfumRM7qrtPCRR (Table 2). mtfA expression is recovered in the complementation strain. The relative expression was calculated using the 2−ΔΔCT method, as described by Livak and Schmittgen (70). Expression of 18S rRNA was used as an internal reference gene. Values were normalized to the expression levels in the wild type, considered 1. The error bars represent standard errors.
FIG 2.
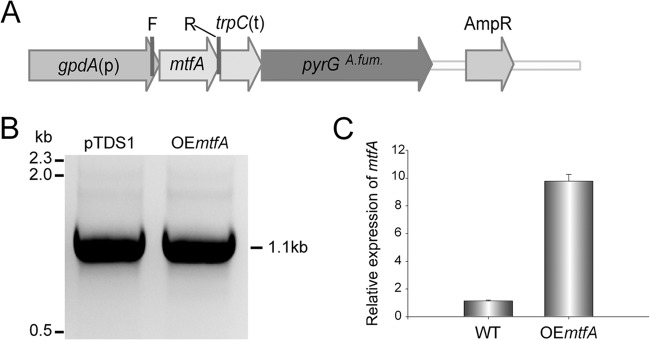
Generation of the mtfA overexpression strain. The mtfA overexpression (OEmtfA) strain was confirmed by PCR and qRT-PCR analyses. (A) Linear representation of the overexpression plasmid pTDS1. The primers utilized for the diagnostic PCR are shown: F (gdpApromoF) and R (fumRM7OEp2) (Table 2). (B) PCR results, indicating integration of the overexpression plasmid into the genome, obtaining the expected 1.1-kb PCR product. pTDS1 was used as a positive control. (C) Expression of mtfA analyzed by qRT-PCR, showing greater accumulation of mtfA transcripts in the OE::mtfA strain than in the wild type. The qRT-PCR primers used were AfumRM7qrtPCRF and AfumRM7qrtPCRR (Table 2). The relative expression was calculated using the 2−ΔΔCT method, as described by Livak and Schmittgen (70). The expression of 18S rRNA was used as an internal reference gene. Values were normalized to the expression levels in the wild type, considered 1. The error bars represent standard errors.
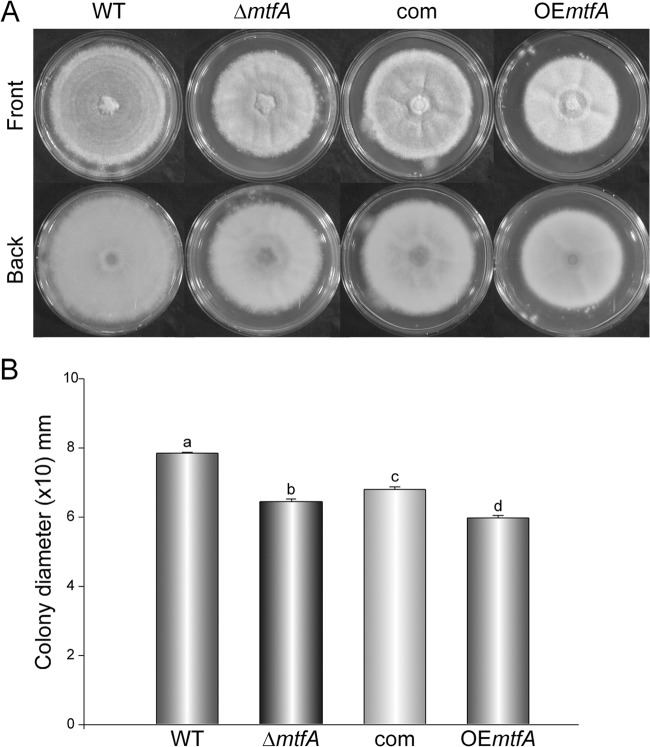
Our results showed a slight reduction in colony growth in the ΔmtfA strain (18%) compared to the wild-type control (Fig. 3A and B). Complementation with the mtfA wild-type allele was able to partially recover radial growth. Overexpression of mtfA resulted in a more pronounced reduction in colony diameter (Fig. 3). The indicated differences in colony growth were statistically significant (Fig. 3).
FIG 3.
Effect of mtfA on A. fumigatus colony growth. (A) A. fumigatus wild-type, ΔmtfA, complementation (com), and mtfA overexpression (OEmtfA) strains were point inoculated on solid Czapek-Dox medium, and the cultures were incubated at 37°C in the dark for 5 days. The experiment was carried out with three replicates. (B) Quantification of colony diameters in 5-day-old cultures. The error bars represent standard errors. Different letters above the bars represent significantly different values (P ≤ 0.05).
In addition to its role in vegetative growth, our study revealed that mtfA also influences morphological differentiation. Overexpression of mtfA resulted in a significant reduction in conidiation in comparison to the wild type (68% reduction) (Fig. 4). Two genes in the asexual development pathway, bristle A (brlA), which encodes a known transcription factor essential for the initiation of conidiophore formation (48), and wetA, the last gene in this signaling pathway leading to conidiation, were both drastically downregulated in the overexpression strain (Fig. 4).
FIG 4.
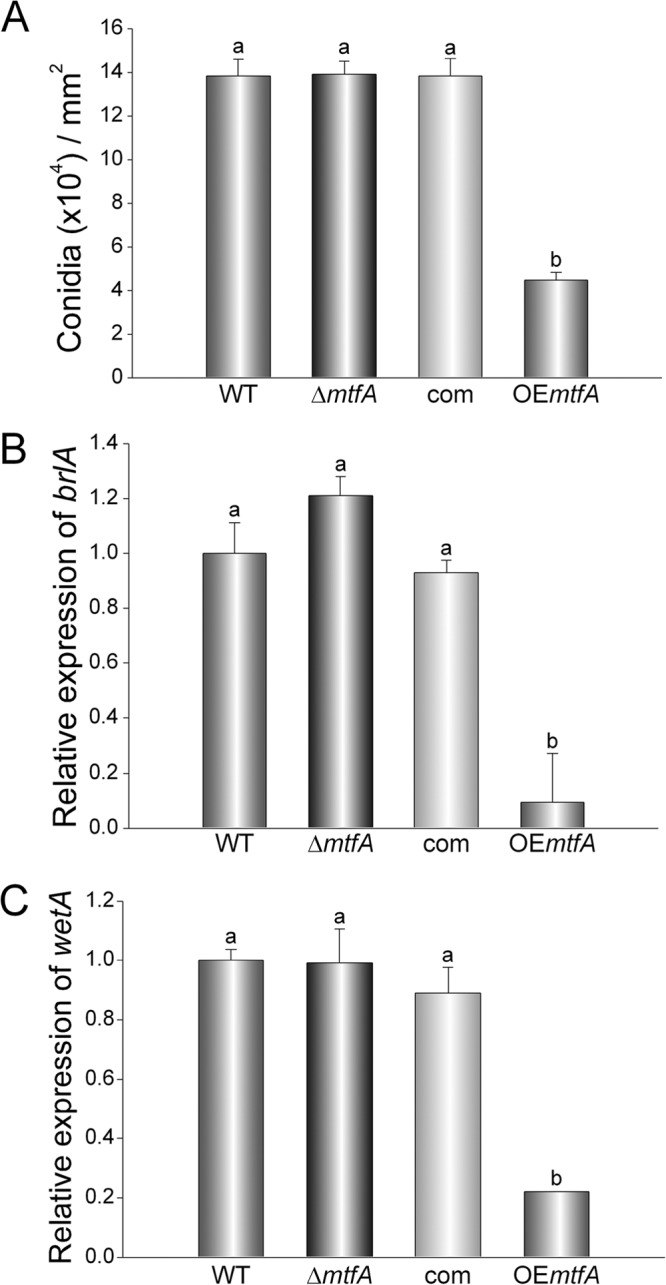
Effect of mtfA on A. fumigatus asexual development. A. fumigatus wild-type, ΔmtfA, complementation (com), and mtfA overexpression (OEmtfA) strains were inoculated in Czapek-Dox liquid medium (1 × 107 spores/ml), and the cultures were grown for 72 h at 37°C. (A) Mycelial mats were homogenized in water, and conidia were quantified using a hemacytometer. (B and C) Expression analysis of brlA (B) and wetA (C). The error bars represent standard errors. Different letters above the bars represent significantly different values (P ≤ 0.05).
MtfA localizes in the nucleus.
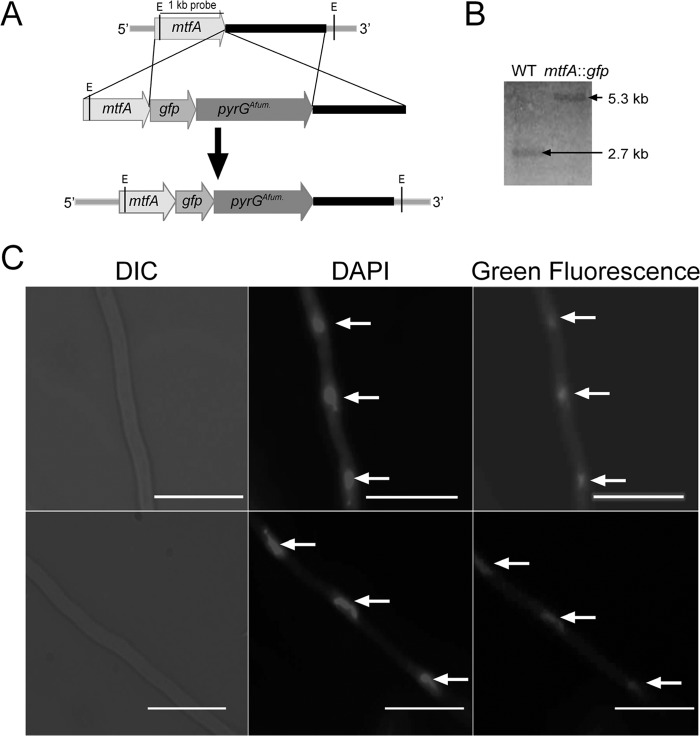
Our previous work showed that in A. nidulans the MtfA ortholog localizes in the nucleus. In the current study, we investigated whether the subcellular localization of MtfA is conserved in A. fumigatus. With this goal, a strain with mtfA fused to gfp was generated (Fig. 5A and B). A single copy of the fusion cassette was integrated at the mtfA locus. The resulting mtfA::gfp strain presented a wild-type phenotype (data not shown). Our results verified that MtfA also localizes in the nuclei when grown in the dark, as determined by comparison of the green fluorescent images with those from DAPI staining (Fig. 5C). A similar experiment carried out under white light yielded similar results, indicating that the localization of MtfA is not light dependent (see Fig. S1 in the supplemental material).
FIG 5.
Nuclear localization of A. fumigatus MtfA. (A) Diagram showing the strategy used to fuse mtfA with gfp. The tag was integrated into the mtfA locus via double-crossover events, using pyrG as the selection marker. (B) Southern analysis results confirming the integration of the mtfA::gfp cassette at the mtfA locus. Genomic DNA samples were digested with EcoRI. The expected bands corresponded to 2.7-kb (WT) and 5.3-kb (mtfA::gfp) DNA fragments. (C) Micrographs showing the nuclear localization of MtfA::GFP grown in the dark. From left to right are Nomarski images (differential interference contrast [DIC]), DAPI images, and green fluorescent images. The scale bars represent 20 μm. The arrows indicate nuclei.
MtfA acts as a positive regulator of protease activity in A. fumigatus.
The ability to secrete hydrolytic enzymes into its environment contributes to the success of A. fumigatus in colonizing its natural niches (49). Our results showed that mtfA acts as a positive regulator of protease production in A. fumigatus. The quantitative azocasein assay showed a significant decrease in protease activity in the deletion mutant colonies, with reduction of 50% compared to the wild type (Fig. 6). Furthermore, analysis of protease activity in culture supernatants showed a pattern similar to that observed in solid cultures, indicating a reduction in secreted protease activity in the absence of mtfA (see Fig. S2 in the supplemental material).
FIG 6.

Protease activity is controlled by mtfA. A. fumigatus wild-type, ΔmtfA, complementation (com), and mtfA overexpression (OEmtfA) strains were point inoculated on solid Czapek-Dox medium supplemented with 5% skim milk (Difco), and the cultures were incubated for 4 days at 37°C in the dark. Proteolytic activity was quantified by using an azocasein assay as described in Materials and Methods. The experiment was carried out with three replicates. Standard errors are shown. Different letters above the bars represent significantly different values (P ≤ 0.05).
Gliotoxin production is dependent on mtfA.
Gliotoxin is a secondary metabolite produced by A. fumigatus that has been shown to have immunosuppressive properties. The mycotoxin has been associated with pathogenicity and has been isolated from the lungs of infected mice (20–34). In the model fungus A. nidulans, it was shown that mtfA controlled the production of several secondary metabolites, such as penicillin and the mycotoxin sterigmatocystin (40). For this reason, we investigated whether mtfA controls gliotoxin production in A. fumigatus. Cultures of A. fumigatus wild-type, ΔmtfA, complementation, and OEmtfA strains were analyzed for gliotoxin content by HPLC after 120 h of incubation time. Our results indicated an increase in the production of the toxin in the OEmtfA strain (Fig. 7A). At 120 h, the overexpression strain showed a 4-fold increase in the production of gliotoxin.
FIG 7.
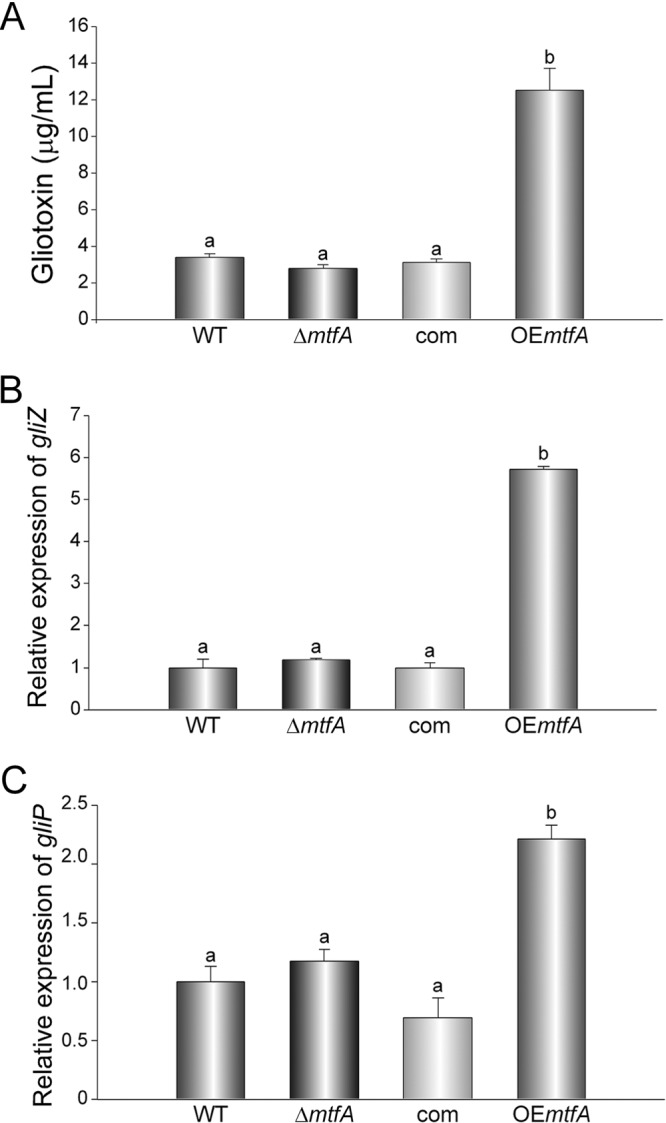
Gliotoxin production is regulated by mtfA. A. fumigatus wild-type, ΔmtfA, complementation (com), and mtfA overexpression (OEmtfA) strains were grown in liquid Czapek-Dox stationary-phase cultures, and mycelial samples were collected at 72 h for RNA analysis. qRT-PCR was used to determine the expression levels of gliZ (A) and gliP (B) using primer pairs gliZ_qRTPCR_F838 and gliZ_qRTPCR_R839, and gliP_qRTPCR_F840 and gliP_qRTPCR_R841, respectively (Table 2). (C) Quantification of gliotoxin in filtrates from 120-h liquid Czapek-Dox stationary-phase cultures by HPLC. The bars represent the averages of three replicates. The error bars represent standard errors. Different letters above the bars represent significantly different values (P ≤ 0.05).
The gene cluster responsible for the production of gliotoxin has been identified (50). This gene cluster includes a putative Zn2Cys6 binuclear transcription factor gene, gliZ (21), and a non-ribosomal peptide synthase gene, gliP (45, 51–53), both necessary for gliotoxin production. We investigated whether mtfA affects gliotoxin production in A. fumigatus by regulating the transcription of gliZ and gliP. The overexpression mutant showed an increase in gliZ and gliP expression at 72 h (Fig. 7B and C). The increase in the transcription of both of these genes is concomitant with the increase in the production of gliotoxin observed in the OEmtfA strain.
mtfA is necessary for normal virulence in a G. mellonella infection model.
G. mellonella larvae are commonly used as a nonvertebrate host for virulence studies of fungal species (54–58). The insect presents both cellular and humoral responses to the invading fungal pathogen (59–62). Our pathogenicity assay indicated that absence of mtfA results in a decrease in virulence (Fig. 8). Thirty-three percent of the larvae infected with the ΔmtfA strain survived the infection at the end of the 30-h monitoring time. Overexpression of mtfA showed no differences in virulence with respect to the wild-type control.
FIG 8.
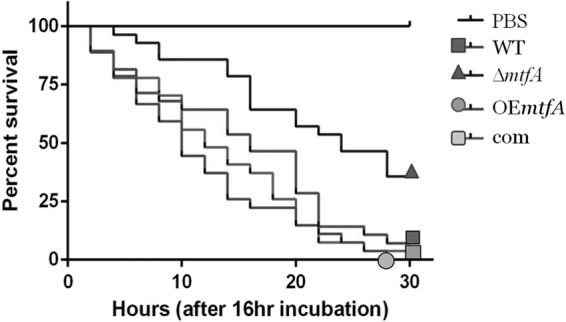
Deletion of mtfA decreases virulence in a Galleria model of infection. Larvae of G. mellonella were infected with A. fumigatus wild-type, ΔmtfA, complementation (com), and mtfA overexpression (OEmtfA) strains. After a 16-h incubation period, survival was monitored every 2 h up to 30 h. Survival rates are shown. The experiments included 30 larvae per group. Statistical analysis was carried out by pairwise comparison using a log rank test.
DISCUSSION
The filamentous fungus A. fumigatus, the leading causative agent of IA, efficiently disseminates by producing abundant conidia. The small size of the asexual spores allows them to reach the lung alveoli, resulting in devastating infections in immunosuppressed hosts (12, 63). Our current study revealed that conidiation in A. fumigatus is regulated by the mtfA gene. mtfA encodes a putative C2H2 zinc finger domain-type transcription factor first described in the model organism A. nidulans (40), where this master transcription factor gene was shown to regulate both development and secondary metabolism (40). Similarly to the case in A. nidulans, absence of mtfA in the opportunistic pathogen A. fumigatus resulted in a slight decrease in colony growth, and forced overexpression of mtfA resulted in a greater decrease in colony growth, along with severe reduction in conidiation. The decrease in conidial formation in the mtfA overexpression strain coincided with a significant decrease in the expression of brlA with respect to the wild-type strain. BrlA is another C2H2 zinc finger transcription factor that acts as a primary regulator of asexual development, controlling the developmental switch from vegetative growth to conidiophore formation (48, 64). Expression of the wetA gene is also downregulated in the A. fumigatus mtfA overexpression strain. WetA, also present during conidiogenesis, is required for the synthesis of a layer in the cell wall that results in impermeable conidia (65). Additionally, it is known that WetA also regulates the expression of additional genes expressed at a late stage in the formation of the asexual spores (65).
A. fumigatus MtfA was found to localize mainly in nuclei, similar to the case in A. nidulans (40), where mtfA was also shown to control the expression of biosynthetic genes involved in the production of secondary metabolites, some with beneficial properties, such as penicillin, and others detrimental, such as the mycotoxin sterigmatocystin (40). For this reason, we evaluated whether A. fumigatus mtfA influences the production of gliotoxin, a compound known for its negative impact on the immune system, including inhibition of phagocytosis and killing of neutrophils (20–34), as well as acting as an antioxidant (66), facilitating A. fumigatus infection in the lung (67). Overexpression of A. fumigatus mtfA resulted in an increase in gliotoxin production with respect to the control strains. Furthermore, our study indicated that expression of gliZ, encoding a putative Zn2Cys6 binuclear transcription factor that controls the expression of the gliotoxin gene cluster (21), and gliP, encoding a non-ribosomal peptide synthase essential for gliotoxin biosynthesis (45, 51–53), were upregulated when mtfA was overexpressed, suggesting that the observed increase in gliotoxin production could, at least in part, result from the mtfA-dependent increase in gliZ expression, leading to greater expression of gliotoxin biosynthetic genes. Based on our previous studies in A. nidulans, it is possible that the regulatory scope of mtfA on A. fumigatus could be broader, including the biosynthesis of other natural products. Future studies in our laboratory will focus on elucidating whether mtfA regulates the biosynthesis of other secondary metabolites produced by the fungus.
In addition to fungal secondary metabolites, the activities of hydrolytic enzymes could also influence fungal infection. For example, an association between the production of fungal proteases and pathogenicity has been previously shown (49, 68). Secreted proteases could cause cytokine release, mucin secretion, and cell peeling (release of epithelial cells), which may contribute to increasing the ability of A. fumigatus to colonize the lung environment (1). Recently, our group also demonstrated that protease activity is regulated by veA in A. fumigatus (17) and other fungi, such as Aspergillus flavus (69). Due to the fact that in A. nidulans mtfA and veA are functionally connected (40), during the course of the present work we also examined the possible role of mtfA in regulating protease activity. Cultures of the ΔmtfA strain showed a decrease of protease activity compared to the wild type. These results provide evidence that mtfA is a positive regulator of protease activity in A. fumigatus. Additional experiments examining cell-free culture supernatants yielded similar results, with significant differences in the protease activity in the secreted proteins, depending on the presence or absence of mtfA.
Because mtfA plays a role in growth, conidiation, gliotoxin biosynthesis, and protease activity, it is possible that mtfA could also affect A. fumigatus pathogenesis. Using a G. mellonella infection model, we found that deletion of mtfA resulted in a decrease in the mortality rate. This indicates that mtfA is a virulence factor necessary to achieve normal pathogenesis levels in this commonly used infection system, showing potential as a promising target against A. fumigatus infection.
In conclusion, our studies revealed that mtfA affects several important cellular processes in the opportunistic pathogen A. fumigatus, such as growth and conidiation, the most important form of dissemination in this fungal species, affecting the expression of developmental genes. We also demonstrated that mtfA regulates the expression of gliotoxin genes, gliZ and gliP, and concomitant gliotoxin biosynthesis, as well as protease activity in the secretome. Importantly, deletion of mtfA resulted in a reduction in virulence in a G. mellonella infection model, suggesting that mtfA and mtfA-dependent factors may be used as possible genetic targets against invasive aspergillosis.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by Northern Illinois University.
We thank Robert Cramer for providing us with A. fumigatus strains CEA10 and CEA17.
Footnotes
Published ahead of print 11 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00075-14.
REFERENCES
- 1.Osherov N. 2012. Interaction of the pathogenic mold fungus Aspergillus fumigatus with lung epithelial cells. Front. Microbiol. 3:346. 10.3389/fmicb.2012.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutsen AP, Slavin RG. 2011. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clin. Dev. Immunol. 2011:843763. 10.1155/2011/843763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning DW. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781–803. 10.1086/513943 [DOI] [PubMed] [Google Scholar]
- 4.Kliasova GA, Petrova NA, Parovichnikova EN, Gotman LN, Isaev VG, Mikhailova EA, Ustinova EN, Khoroshko ND, Vishnevskaia ES, Kremenetskaia AM, Kravchenko SK, Kaplanskaia IB, Kokhno AA, Ptitsin SA, Liubimova LS, Mendeleeva LP, Mitish NE, Galstian GM, Ryzhko W, Tochenov AV, Savchenko VG. 2005. Invasive pulmonary aspergillosis. Ter. Ark. 77:65–71 [PubMed] [Google Scholar]
- 5.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100:4358–4366. 10.1182/blood-2002-05-1496 [DOI] [PubMed] [Google Scholar]
- 6.Pagano L, Girmenia C, Mele L, Ricci P, Tosti ME, Nosari A, Buelli M, Picardi M, Allione B, Corvatta L, D'Antonio D, Montillo M, Melillo L, Chierichini A, Cenacchi A, Tonso A, Cudillo L, Candoni A, Savignano C, Bonini A, Martino P, Del Favero A. 2001. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica 86:862–870 [PubMed] [Google Scholar]
- 7.Post MJ, Lass-Floerl C, Gastl G, Nachbaur D. 2007. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl. Infect. Dis. 9:189–195. 10.1111/j.1399-3062.2007.00219.x [DOI] [PubMed] [Google Scholar]
- 8.Wiederhold NP, Lewis RE. 2003. Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy 23:1592–1610. 10.1592/phco.23.15.1592.31965 [DOI] [PubMed] [Google Scholar]
- 9.Latge JP. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohl TM, Feldmesser M. 2007. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot. Cell 6:1953–1963. 10.1128/EC.00274-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherif R, Segal BH. 2010. Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr. Opin. Pulm. Med. 16:242–250. 10.1097/MCP.0b013e328337d6de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruchel R, Reichard U. 1999. Pathogenesis and clinical presentation of aspergillosis. Contrib. Microbiol. 2:21–43. 10.1159/000060302 [DOI] [PubMed] [Google Scholar]
- 13.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latge JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. 2010. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 116:5394–5402. 10.1182/blood-2010-04-279307 [DOI] [PubMed] [Google Scholar]
- 14.Sun WK, Lu X, Li X, Sun QY, Su X, Song Y, Sun HM, Shi Y. 6 May 2012. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur. J. Clin. Microbiol. Infect. Dis. 10.1007/s10096-012-1624-8 [DOI] [PubMed] [Google Scholar]
- 15.Calvo AM, Wilson RA, Bok JW, Keller NP. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447–459. 10.1128/MMBR.66.3.447-459.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo AM. 2008. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45:1053–1061. 10.1016/j.fgb.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 17.Dhingra S, Andes D, Calvo AM. 2012. VeA regulates conidiation, gliotoxin production, and protease activity in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot. Cell 11:1531–1543. 10.1128/EC.00222-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H, Bayram O, Braus G, Kim SC, Yu J. 2012. Characterization of the velvet regulators in Aspergillus fumigatus. Mol. Microbiol. 86:937–953. 10.1111/mmi.12032 [DOI] [PubMed] [Google Scholar]
- 19.Tekaia F, Latge JP. 2005. Aspergillus fumigatus: saprophyte or pathogen? Curr. Opin. Microbiol. 8:385–392. 10.1016/j.mib.2005.06.017 [DOI] [PubMed] [Google Scholar]
- 20.Amitani R, Taylor G, Elezis EN, Llewellyn-Jones C, Mitchell J, Kuze F, Cole PJ, Wilson R. 1995. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 63:3266–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bok JW, Chung D, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Kirby KA, Keller NP. 2006. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 74:6761–6768. 10.1128/IAI.00780-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comera C, André K, Laffitte J, Collet X, Galtier P, Maridonneau-Parini I. 2007. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signaling pathways in human neutrophils. Microbes Infect. 9:47–54. 10.1016/j.micinf.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 23.Frame R, Carlton WW. 1988. Acute toxicity of gliotoxin in hamsters. Toxicol. Lett. 40:269–273. 10.1016/0378-4274(88)90050-1 [DOI] [PubMed] [Google Scholar]
- 24.Mullbacher A, Eichner RD. 1984. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc. Natl. Acad. Sci. U. S. A. 81:3835–3837. 10.1073/pnas.81.12.3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan XQ, Harday J. 2007. Electromicroscopic observations on gliotoxin-induced apoptosis of cancer cells in culture and human cancer xenografts in transplanted SCID mice. In Vivo 21:259–265 [PubMed] [Google Scholar]
- 26.Piva TJ. 1994. Gliotoxin induces apoptosis in mouse l929 fibroblast cells. Biochem. Mol. Biol. Int. 33:411–419 [PubMed] [Google Scholar]
- 27.Stanzani M, Orciuolo E, Lewis R, Kontoyiannis DP, Martins SL, St John LS, Komanduri KV. 2005. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 105:2258–2265. 10.1182/blood-2004-09-3421 [DOI] [PubMed] [Google Scholar]
- 28.Suen YK, Fung KP, Lee CY, Kong SK. 2001. Gliotoxin induces apoptosis in cultured macrophages via production of reactive oxygen species and cytochrome c release without mitochondrial depolarization. Free Radic. Res. 35:1–10. 10.1080/10715760100300541 [DOI] [PubMed] [Google Scholar]
- 29.Tsunawaki S, Yoshida LS, Nishida S, Kobayashi T, Shimoyama T. 2004. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 72:3373–3382. 10.1128/IAI.72.6.3373-3382.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waring P. 1990. DNA fragmentation induced in macrophages by gliotoxin does not require protein-synthesis and is preceded by raised inositol triphosphate levels. J. Biol. Chem. 265:14476–14480 [PubMed] [Google Scholar]
- 31.Yamada A, Kataoka T, Nagai K. 2000. The fungal metabolite gliotoxin: immunosuppressive activity on CTL-mediated cytotoxicity. Immunol. Lett. 71:27–32. 10.1016/S0165-2478(99)00155-8 [DOI] [PubMed] [Google Scholar]
- 32.Yoshida LS, Abe S, Tsunawaki S. 2000. Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem. Biophys. Res. Commun. 268:716–723. 10.1006/bbrc.2000.2192 [DOI] [PubMed] [Google Scholar]
- 33.Eichner RD, Waring P, Geue AM, Braithwaite AW, Mullbacher A. 1988. Gliotoxin causes oxidative damage to plasmid and cellular DNA. J. Biol. Chem. 263:3772–3777 [PubMed] [Google Scholar]
- 34.Richard JL, Dvorak TJ, Ross PF. 1996. Natural occurrence of gliotoxin in turkeys infected with Aspergillus fumigatus, Fresenius. Mycopathologia 134:167–170. 10.1007/BF00436725 [DOI] [PubMed] [Google Scholar]
- 35.Dhingra S, Lind AL, Lin HC, Tang Y, Rokas A, Calvo AM. 2013. The fumagillin gene cluster, an example of hundreds of genes under veA control in Aspergillus fumigatus. PLoS One 8:e77147. 10.1371/journal.pone.0077147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stinnett SM, Espeso EA, Cobeno L, Araujo-Bazan L, Calvo AM. 2007. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol. Microbiol. 63:242–255. 10.1111/j.1365-2958.2006.05506.x [DOI] [PubMed] [Google Scholar]
- 37.Kato N, Brooks W, Calvo AM. 2003. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell 2:1178–1186. 10.1128/EC.2.6.1178-1186.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayram O, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, Braus-Stromeyer S, Kwon NJ, Keller NP, Yu JH, Braus GH. 2008. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504–1506. 10.1126/science.1155888 [DOI] [PubMed] [Google Scholar]
- 39.Bayram O, Braus GH. 2012. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol. Rev. 36:1–24. 10.1111/j.1574-6976.2011.00285.x [DOI] [PubMed] [Google Scholar]
- 40.Ramamoorthy V, Dhingra S, Kincaid A, Shantappa S, Feng X, Calvo AM. 2013. The putative C2H2 transcription factor MtfA is a novel regulator of secondary metabolism and morphogenesis in Aspergillus nidulans. PLoS One 8:e74122. 10.1371/journal.pone.0074122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Käfer E. 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19:33–131. 10.1016/S0065-2660(08)60245-X [DOI] [PubMed] [Google Scholar]
- 42.Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1:3111–3120. 10.1038/nprot.2006.405 [DOI] [PubMed] [Google Scholar]
- 43.Peñalva MA. 2005. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 42:963–975. 10.1016/j.fgb.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 44.Reichard U, Monod M, Odds F, Ruchel R. 1997. Virulence of an aspergillopepsin-deficient mutant of Aspergillus fumigatus and evidence for another aspartic proteinase linked to the fungal cell wall. J. Med. Vet. Mycol. 35:189–196. 10.1080/02681219780001131 [DOI] [PubMed] [Google Scholar]
- 45.Cramer RA, Gamcsik MP, Brooking RM, Najvar LK, Kirkpatrick WR, Patterson TF, Balibar CJ, Graybill JR, Perfect JR, Abraham SN, Steinbach WJ. 2006. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5:972–980. 10.1128/EC.00049-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber K, Bolander M, Sarkar G. 1998. PIG-B: a homemade monophasic cocktail for the extraction of RNA. Mol. Biotechnol. 9:73–77. 10.1007/BF02752699 [DOI] [PubMed] [Google Scholar]
- 47.Fuchs B, O'Brien E, Khoury J, Mylonakis E. 2010. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1:475–482. 10.4161/viru.1.6.12985 [DOI] [PubMed] [Google Scholar]
- 48.Adams TH, Boylan MT, Timberlake WE. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353–362. 10.1016/0092-8674(88)90198-5 [DOI] [PubMed] [Google Scholar]
- 49.Richie DL, Hartl L, Aimanianda V, Winters MS, Fuller KK, Miley MD, White S, McCarthy JW, Latge PJ, Feldmesser M, Rhodes JC, Askew DS. 2009. A role for the unfolded protein response (upr) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog. 5:e1000258. 10.1371/journal.ppat.1000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardiner D, Howlett BJ. 2005. Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol. Lett. 248:241–248. 10.1016/j.femsle.2005.05.046 [DOI] [PubMed] [Google Scholar]
- 51.Kupfahl C, Heinekamp T, Geginat G, Ruppert T, Hartl A, Hof H, Brakhage AA. 2006. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol. Microbiol. 62:292–302. 10.1111/j.1365-2958.2006.05373.x [DOI] [PubMed] [Google Scholar]
- 52.Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. 2008. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197:479–486. 10.1086/525044 [DOI] [PubMed] [Google Scholar]
- 53.Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Mullbacher A, Gallin JI, Simon MM, Kwon-Chung KJ. 2007. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 6:1562–1569. 10.1128/EC.00141-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reeves EP, Messina CG, Doyle S, Kavanagh K. 2004. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 158:73–79. 10.1023/B:MYCO.0000038434.55764.16 [DOI] [PubMed] [Google Scholar]
- 55.Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73:3842–3850. 10.1128/IAI.73.7.3842-3850.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mylonakis E. 2008. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia 165:1–3. 10.1007/s11046-007-9082-z [DOI] [PubMed] [Google Scholar]
- 57.Dunphy GB, Oberholzer U, Whiteway M, Zakarian RJ, Boomer I. 2003. Virulence of Candida albicans mutants toward larval Galleria mellonella (Insecta, Lepidoptera, Galleridae). Can. J. Microbiol. 49:514–524. 10.1139/w03-064 [DOI] [PubMed] [Google Scholar]
- 58.Brennan M, Thomas DY, Whiteway M, Kavanagh K. 2002. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 34:153–157. 10.1111/j.1574-695X.2002.tb00617.x [DOI] [PubMed] [Google Scholar]
- 59.Jackson JC, Higgins LA, Lin X. 2009. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One 4:e4224. 10.1371/journal.pone.0004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kavanagh K, Reeves EP. 2004. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 28:101–112. 10.1016/j.femsre.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 61.Ratcliffe NA. 1985. Invertebrate immunity—a primer for the non-specialist. Immunol. Lett. 10:253–270. 10.1016/0165-2478(85)90100-2 [DOI] [PubMed] [Google Scholar]
- 62.Tojo S, Naganuma F, Arakawa K, Yokoo S. 2000. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J. Insect. Physiol. 46:1129–1135. 10.1016/S0022-1910(99)00223-1 [DOI] [PubMed] [Google Scholar]
- 63.Samson RA. 1999. The genus Aspergillus with special regard to the Aspergillus fumigatus group. Contrib. Microbiol. 2:5–20. 10.1159/000060298 [DOI] [PubMed] [Google Scholar]
- 64.Han S, Adams TH. 2001. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genomics 266:260–270. 10.1007/s004380100552 [DOI] [PubMed] [Google Scholar]
- 65.Marshall MA, Timberlake WE. 1991. Aspergillus nidulans wetA activates spore-specific gene expression. Mol. Cell. Biol. 11:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi HS, Shim JS, Kim JA, Kang SW, Kwon HJ. 2007. Discovery of gliotoxin as a new small molecule targeting thioredoxin redox system. Biochem. Biophys. Res. Commun. 359:523–528. 10.1016/j.bbrc.2007.05.139 [DOI] [PubMed] [Google Scholar]
- 67.Scharf DH, Heinekamp T, Remme N, Hortschansky P, Brakhage AA, Hertweck C. 2012. Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 93:467–472. 10.1007/s00253-011-3689-1 [DOI] [PubMed] [Google Scholar]
- 68.Kolattukudy PE, Lee JD, Rogers LM, Zimmerman P, Ceselski S, Fox B, Stein B, Copelan EA. 1993. Evidence for possible involvement of an elastolytic serine-protease in aspergillosis. Infect. Immun. 61:2357–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duran RM, Gregersen S, Smith TD, Bhetariya PJ, Cary JW, Harris-Coward PY, Mattison CP, Grimm C, Calvo AM. 4 March 2014. The role of Aspergillus flavus veA in the production of extracellular proteins during growth on starch substrates. Appl. Microbiol. Biotechnol. 10.1007/s00253-014-5598-6 [DOI] [PubMed] [Google Scholar]
- 70.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 24:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.